Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-Cyclodextrin Inclusion Complex
Abstract
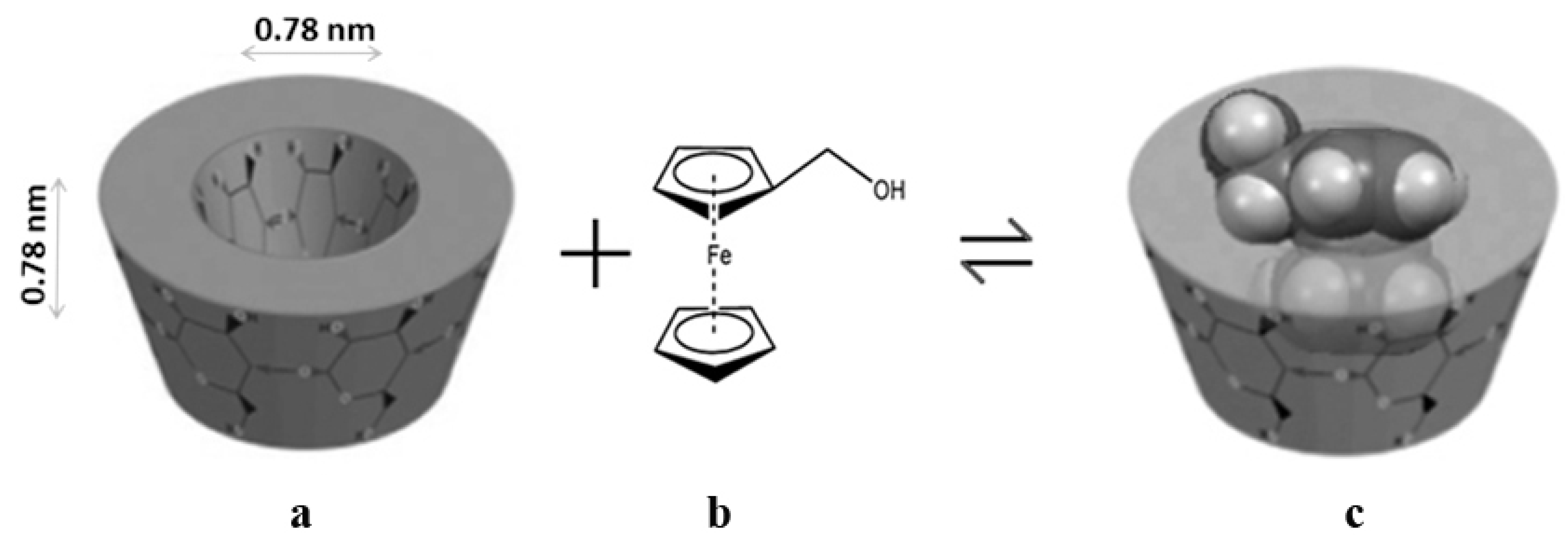
:1. Introduction
2. Results and Discussion
- solid state: XRD and FT-IR;
- solution: Solubility studies, NMR, UV-Vis spectroscopy.
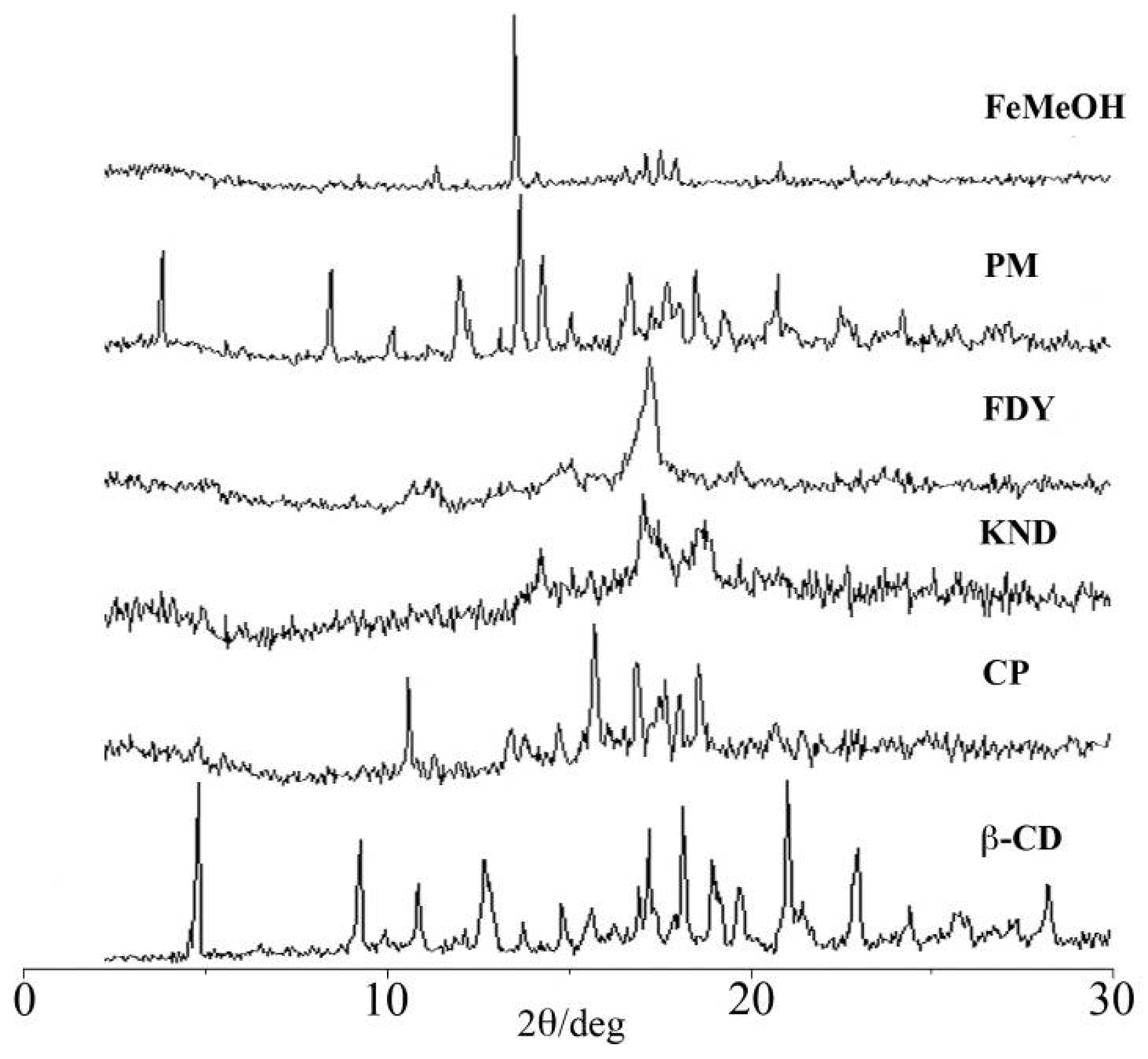
2.1. X-ray Powder Diffractometry (XRD)
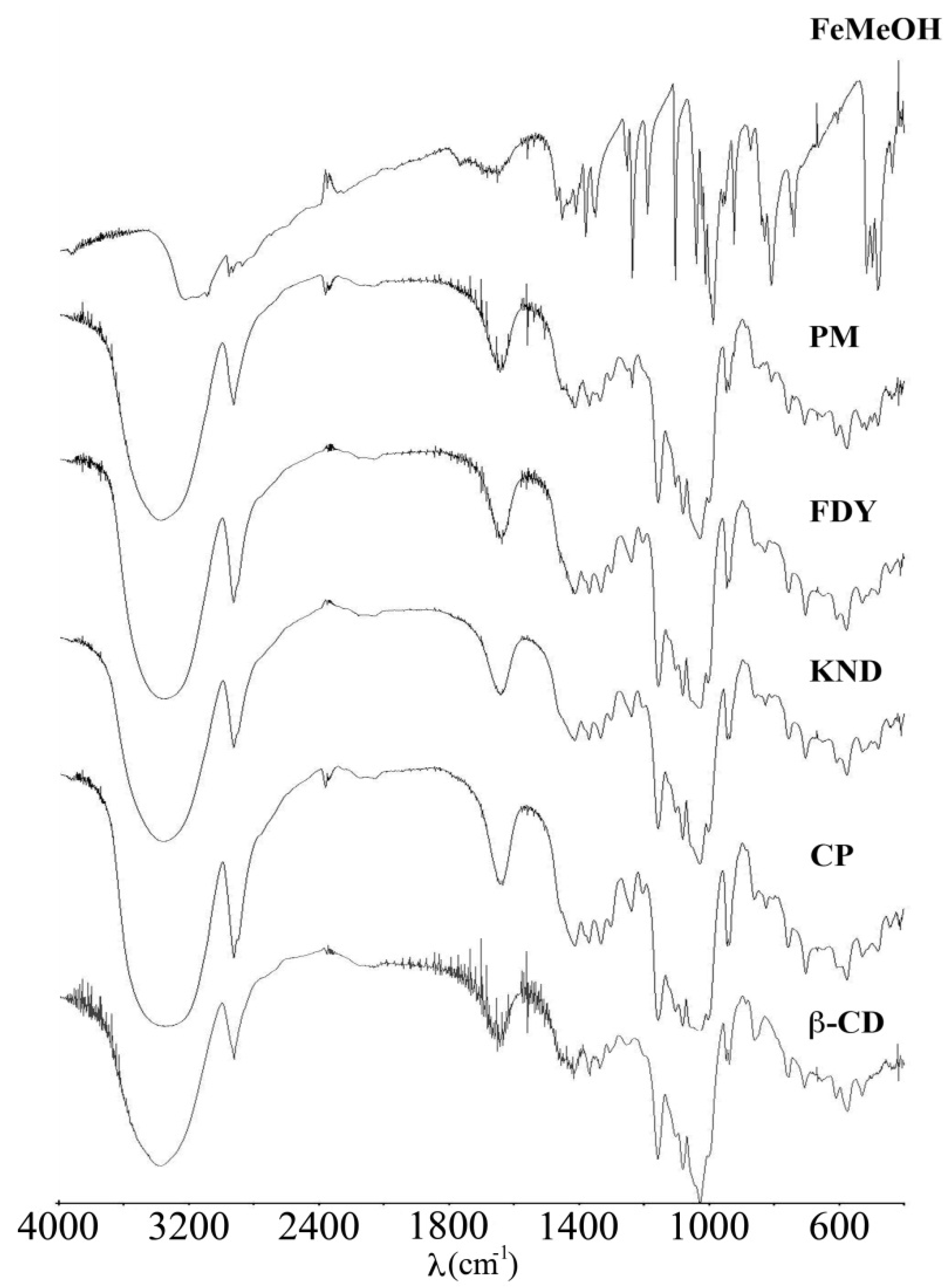
2.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3. Ultraviolet-Visible (UV-Vis) Spectroscopy
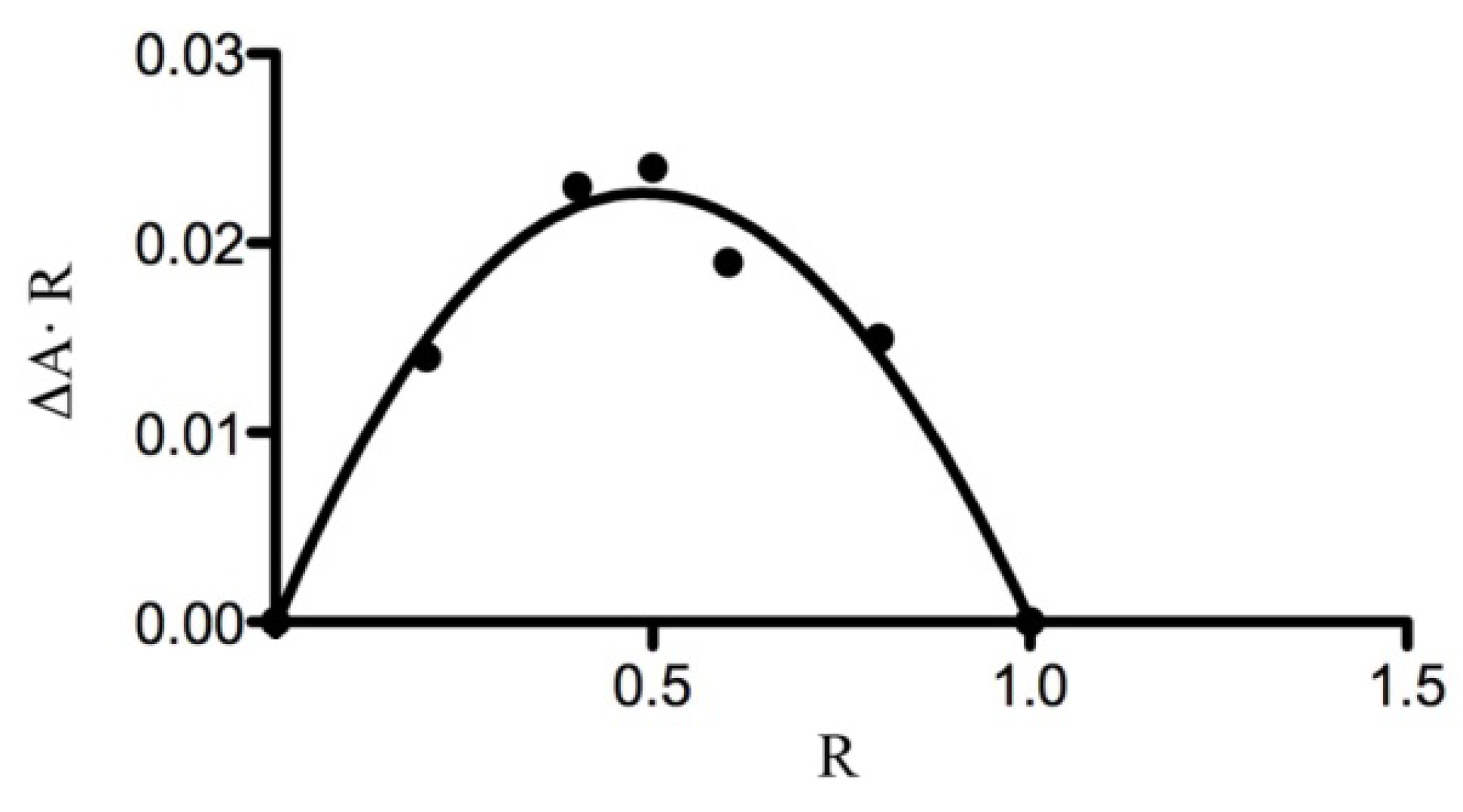
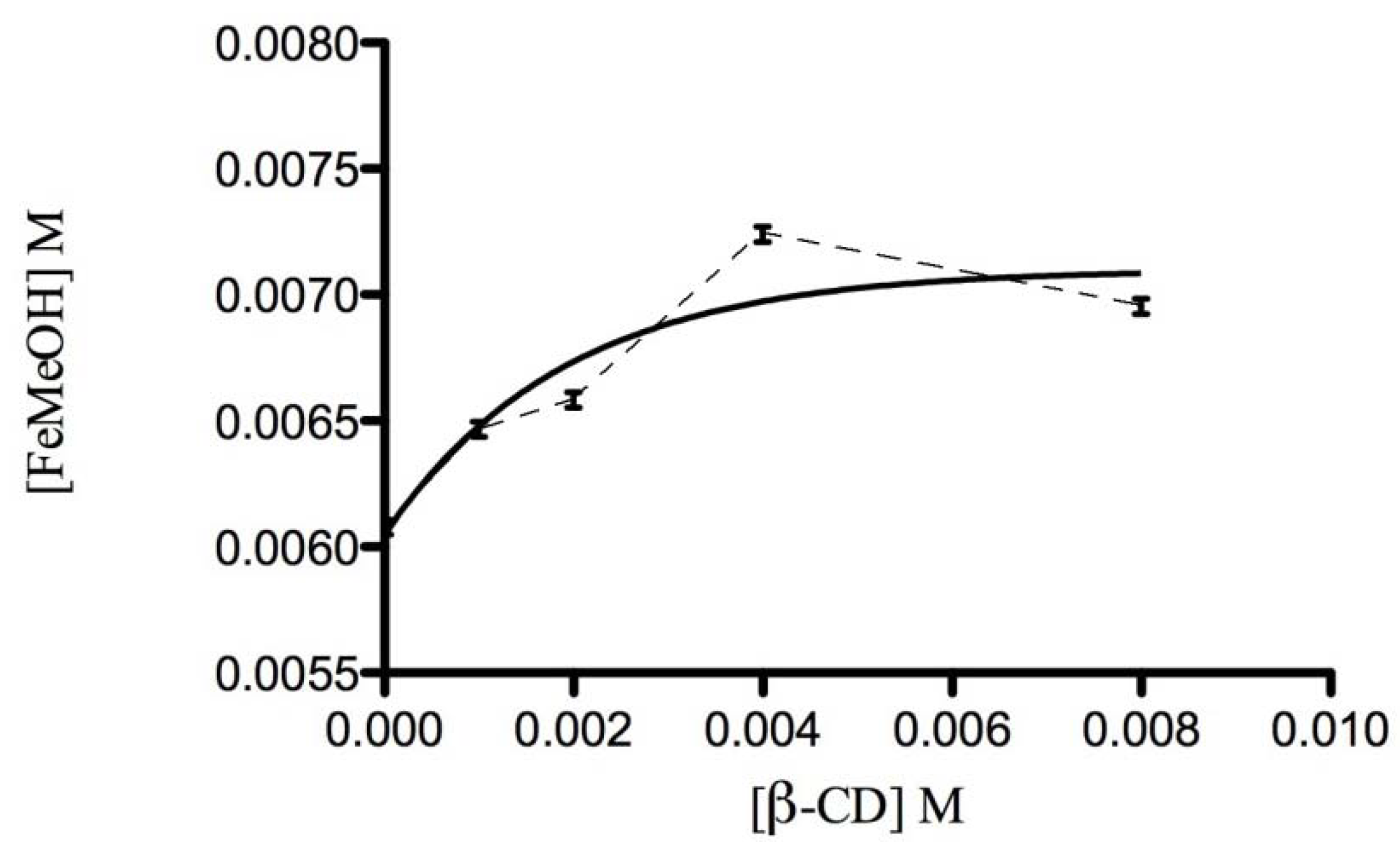
2.3.1. Phase Solubility Studies
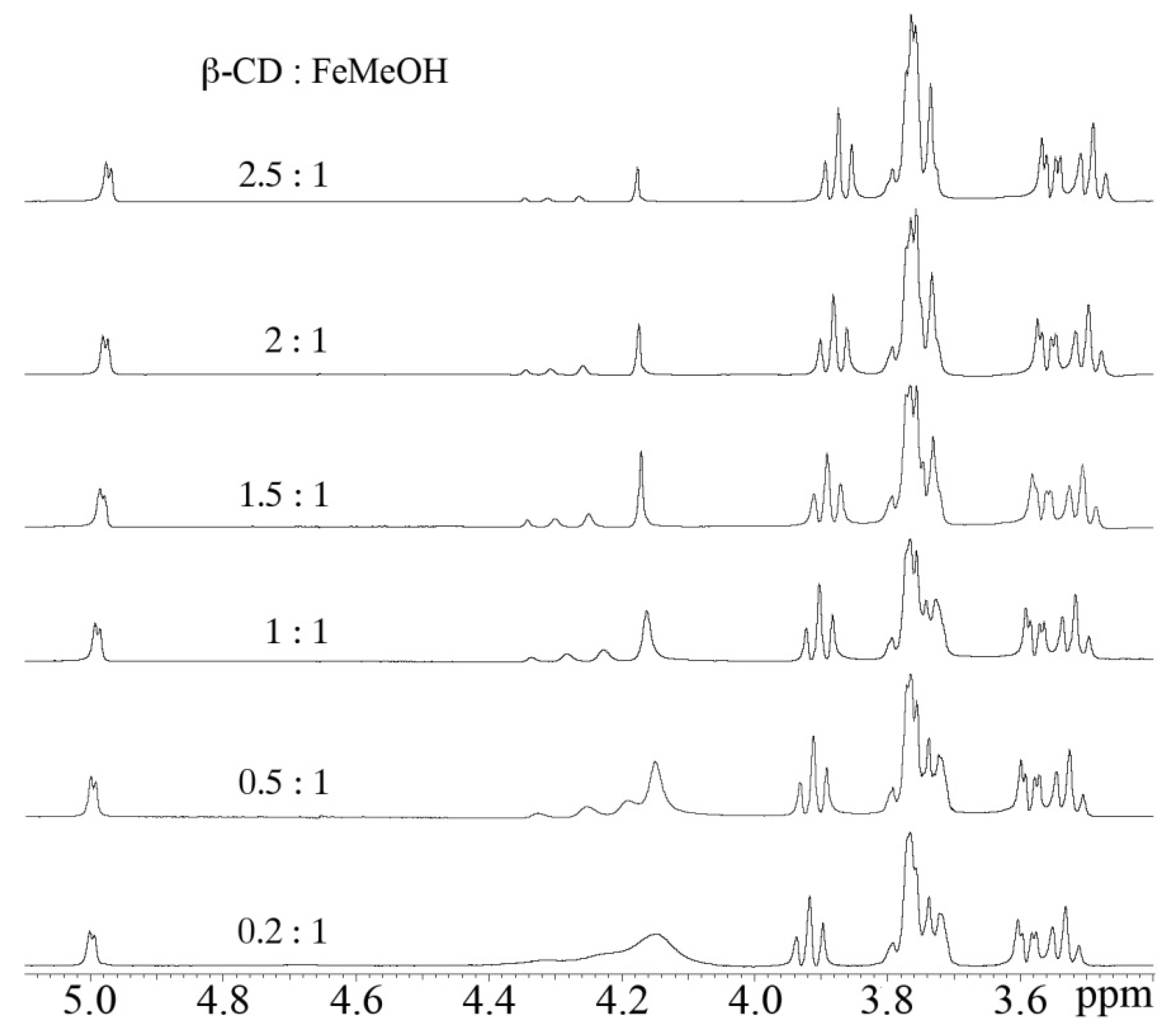
2.4. Nuclear Magnetic Resonance (NMR)
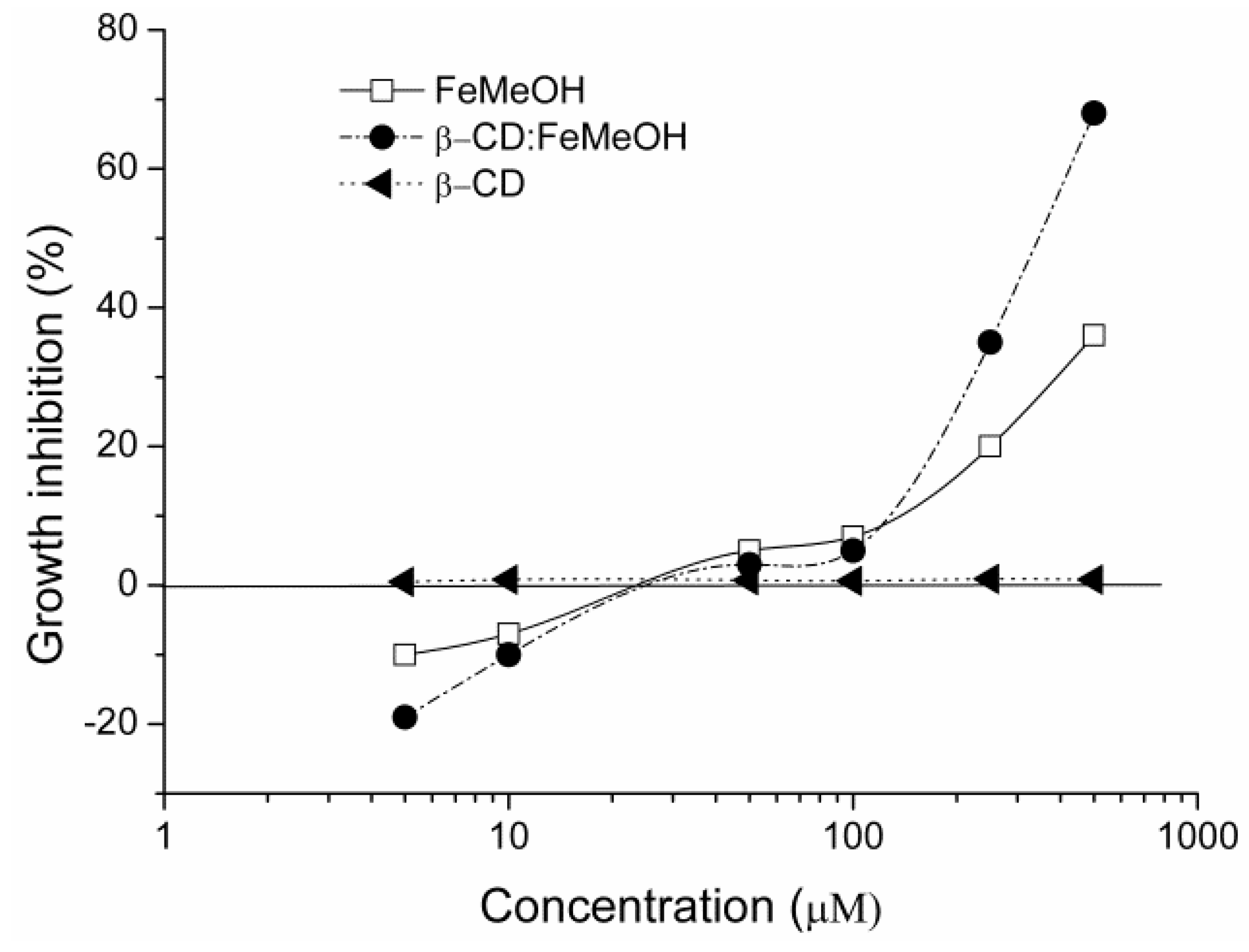
2.5. Cytotoxicity Test
3. Experimental
3.1. Materials
3.2. Preparation of Solid Binary Systems
3.2.1. Physical Mixing
3.2.2. Kneading Method
3.2.3. Coprecipitation Method
3.2.4. Freeze-Drying Method
3.3. X-ray Powder Diffraction (XRD)
3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5. Ultraviolet-Visible (UV-Vis) Spectroscopy
3.5.1. Phase Solubility Study
3.6. Nuclear Magnetic Resonance (NMR)
3.7. Cytotoxicity Test
4. Conclusions
Acknowledgments
References and Notes
- Szejtli, J. Cyclodextrin Technology; Kluwer Academic: Budapest, Hungary, 1988; pp. 43–45. [Google Scholar]
- Szejtli, J. Cyclodextrin Technology; Kluwer Academic: Dordecht, The Netherlands, 1989; pp. 8–72. [Google Scholar]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany, 1978. [Google Scholar]
- de Matos Jensen, C.E.; dos Santos, R.A.S.; Denadai, A.M.L.; Santos, C.F.F.; Braga, A.N.G.; Sinisterra, R.D. Pharmaceutical composition of valsartan: β-Cyclodextrin: Physico-chemical characterization and anti-hypertensive evaluation. Molecules 2010, 15, 4067–4084. [Google Scholar] [CrossRef] [PubMed]
- Baboota, S.; Khanna, R.; Karmarkar, R.; Agarwal, S.P. Cyclodextrin Based Drug Delivery Systems. In Progress in Controlled and Novel Drug Delivery System, 1st ed.; Jain, N.K., Ed.; CBS Publishers & Distributors: New Delhi, India, 2004; pp. 384–400. [Google Scholar]
- dos Santos, C.; Buera, M.P.; Mazzobre, M.F. Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes. J. Sci. Food Agric. 2011, 91, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Redenti, E.; Szente, L.; Szejtli, J. Cyclodextrin complexes of salts of acidic drugs thermodynamic properties, structural features, and pharmaceutical applications. J. Pharm. Sci. 2001, 90, 979–986. [Google Scholar] [CrossRef]
- Duchene, D.; Vaution, C.; Glomot, F. Cyclodextrin, their value in pharmaceutical technology. Drug Dev. Ind. Pharm. 1988, 12, 2193–2215. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F. Methods of Investigating and Preparing Inclusion Compounds. In Cyclodextrins and Their Industrial Uses; Editions de Santé: Paris, France, 1987; pp. 131–172. [Google Scholar]
- Sapkal, N.P.; Kilor, V.A.; Bhusari, K.P.; Daud, A.S. Evaluation of some methods for preparing gliclazide-β-cyclodextrin inclusion complexes. Trop. J. Pharm. Res. 2007, 6, 833–840. [Google Scholar] [CrossRef]
- Singh, R.; Bharti, N.; Madan, J.; Hiremath, S.N. Characterization of cyclodextrin inclusion complexes—A review. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]
- Pires, M.A.S.; dos Santos, R.A.S.; Sinisterra, R.D. Pharmaceutical composition of hydrochlorothiazide:β-cyclodextrin: Preparation by three different methods, physico-chemical characterization and in vivo diuretic activity evaluation. Molecules 2011, 16, 4482–4499. [Google Scholar] [CrossRef] [PubMed]
- van Staveren, D.R.; Metzler-Nolte, N. The bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.F.R.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613. [Google Scholar] [CrossRef]
- Snegur, L.V.; Babin, V.N.; Simenel, A.A.; Nekrasov, Y.S.; Ostrovskaya, L.A.; Sergeevac, N.S. Antitumor activities of ferrocene compounds. Russ. Chem. Bull. 2010, 59, 2167–2168. [Google Scholar] [CrossRef]
- Guan, L.; Shi, Z.; Li, M.; Gu, Z. Ferrocene-filled single-walled carbon nanotubes. Carbon 2005, 43, 2780–2785. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D. Macrocycles and Cryptands Containing the Ferrocene Unit. In Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science; Hayashi, T., Togni, A., Eds.; VCH: Weinheim, Germany, 1995; pp. 279–316. [Google Scholar]
- Köpf-Maier, P.; Köpf, H.; Neuse, E. Ferricenium complexes: A new type of water-soluble antitumor agent. Cancer Res. Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Harada, A.; Takahashi, S. Preparation and Properties of Cyclodextrin-Ferrocene Inclusion Complexes. J. Chem. Soc. Chem. Commun. 1984, 10, 645–646. [Google Scholar] [CrossRef]
- Petrovski, Ž.; Norton de Matos, M.R.P.; Braga, S.S.; Pereira, C.C.L.; Matos, M.L.; Gonçalves, I.S.; Pillinger, M.; Alves, P.M.; Romão, C.C. Synthesis, characterization and antitumor activity of 1,2-disubstituted ferrocenes and cyclodextrin inclusion complexes. J. Organomet. Chem. 2008, 693, 675–684. [Google Scholar] [CrossRef]
- Shago, R.F.; Swarts, J.C.; Kreft, E.; van Rensburg, C.E.J. Antineoplastic activity of a series of ferrocene-containing alcohols. Anticancer Res. 2007, 27, 3431–3434. [Google Scholar] [PubMed]
- Atkinson, R.C.J.; Long, N.J. Monodentate Ferrocene Donor Ligands. In Ferrocenes Ligands, Materials and Biomolecules; Stepnicka, P., Ed.; Wiley: New York, NY, USA, 2008; pp. 3–32. [Google Scholar]
- Haga, N.; Mineralogical Institute, Faculty of Science University of Tokyo, Tokyo, Japan. ICDD Grant-in-Aid. Unpublished work. 1984. [Google Scholar]
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Figueiras, A.; Ribeiro, L.; Vieira, M.T.; Veiga, E.F. Preparation and physicochemical characterization of omeprazole: methylbeta-cyclodextrin inclusion complex in solid state. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 173–177. [Google Scholar] [CrossRef]
- Spulber, M.; Pinteala, M.; Fifere, A.; Harabagiu, V.; Simionescu, B.C. Inclusion complexes of 5-flucytosine with β-cyclodextrin and hydroxypropyl-β-cyclodextrin: Characterization in aqueous solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 117–125. [Google Scholar] [CrossRef]
- Rosenblum, M.; Woodward, R.B. The Structure and Chemistry of Ferrocene. III. Evidence Pertaining to the Ring Rotational Barrier. J. Am. Chem. Soc. 1958, 80, 5443. [Google Scholar] [CrossRef]
- Lippincott, E.R.; Nelson, R.D. The vibrational spectra and structure of ferrocene and ruthenocene. Spectrochimica Acta 1958, 10, 307–329. [Google Scholar] [CrossRef]
- Rawat, S.; Jain, S.K. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm. 2004, 57, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Studies on the formation of complex minerals in solution and on their stability. Ann. Chimie France 1928, 9, 113–203. [Google Scholar]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef] [PubMed]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Zughul, M.B. Rigorous nonlinear regression analysis of phase solubility diagrams to obtain complex stoichiometry and true thermodynamic drug-cyclodextrin complexation parameters. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 525–530. [Google Scholar] [CrossRef]
- Zughul, M.B.; Al Omari, M.; Badwan, A.A. Thermodynamics of propylparaben/β-cyclodextrin inclusion complexes. Pharm. Develop. Technol. 1998, 3, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: effects on drug permeation trough biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Pitha, J.; Szente, I.; Szejtli, J. Molecular Encapsulation of Drugs by Cyclodextrins and Congeners. In Controlled Drug Delivery; Bruck, S.D., Ed.; CRC: Boca Raton, FL, USA, 1983; Volume 1, pp. 126–148. [Google Scholar]
- Connors, K.A. Mesurements of Cyclodextrin Complex Stability Constants. In Cyclodextrins. Comprehensive Supramolecular Chemistry; Szejtli, J., Osa, T., Eds.; Elsevier: New York, NY, USA, 1996; Volume 3, pp. 205–241. [Google Scholar]
- Leyva, E.; Moctezuma, E.; Strouse, J.; Garcìa-Garibay, M.A. Spectrometric and 2D NMR studies on the complexation of chlorophenols with cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 41–46. [Google Scholar] [CrossRef]
- Osella, D.; Ferrali, M.; Zanello, P.; Laschi, F.; Fontani, M.; Nervi, C.; Cavigiolio, G. On the mecchanism of the antitumor activity of ferrocenium derivatives. Inorg. Chim. Acta 2000, 306, 42–48. [Google Scholar] [CrossRef]
- Moriwaki, C.; Costa, G.L.; Ferracini, C.N.; de Moraes, F.F.; Zanin, G.M.; Pineda, E.A.G.; Matioli, G. Enhancement of solubility of albendazole by complexation with β-cyclodextrin. Braz. J. Chem. Eng. 2008, 25, 255–267. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.; Palmer, A.G.; Skelton, N.J. Protein NMR Spectroscopy: Principles and Practice; Academic Press, Inc.: San Diego, CA, USA, 1996. [Google Scholar]
- Hwang, T.L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary wave forms and pulsed field gradients. J. Magn. Reson. A 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Bartels, C.; Xia, T.; Billeter, M.; Güntert, P.; Wüthrich, K. The program XEASY for computer supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 1995, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Chen, D.; Jiang, J.; Zhou, Q. Growth inhibiton of human breast cancer cell line MDA-MB-231 by rosiglitazone through activation of PPARγ. Chin. J. Clin. Oncol. 2008, 5, 407–412. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-Cyclodextrin Inclusion Complex. Molecules 2012, 17, 6056-6070. https://doi.org/10.3390/molecules17056056
Iacovino R, Caso JV, Rapuano F, Russo A, Isidori M, Lavorgna M, Malgieri G, Isernia C. Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-Cyclodextrin Inclusion Complex. Molecules. 2012; 17(5):6056-6070. https://doi.org/10.3390/molecules17056056
Chicago/Turabian StyleIacovino, Rosa, Jolanda Valentina Caso, Filomena Rapuano, Agostino Russo, Marina Isidori, Margherita Lavorgna, Gaetano Malgieri, and Carla Isernia. 2012. "Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-Cyclodextrin Inclusion Complex" Molecules 17, no. 5: 6056-6070. https://doi.org/10.3390/molecules17056056
APA StyleIacovino, R., Caso, J. V., Rapuano, F., Russo, A., Isidori, M., Lavorgna, M., Malgieri, G., & Isernia, C. (2012). Physicochemical Characterization and Cytotoxic Activity Evaluation of Hydroxymethylferrocene:β-Cyclodextrin Inclusion Complex. Molecules, 17(5), 6056-6070. https://doi.org/10.3390/molecules17056056




