Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components
Abstract
:1. Introduction
2. Results and Discussion
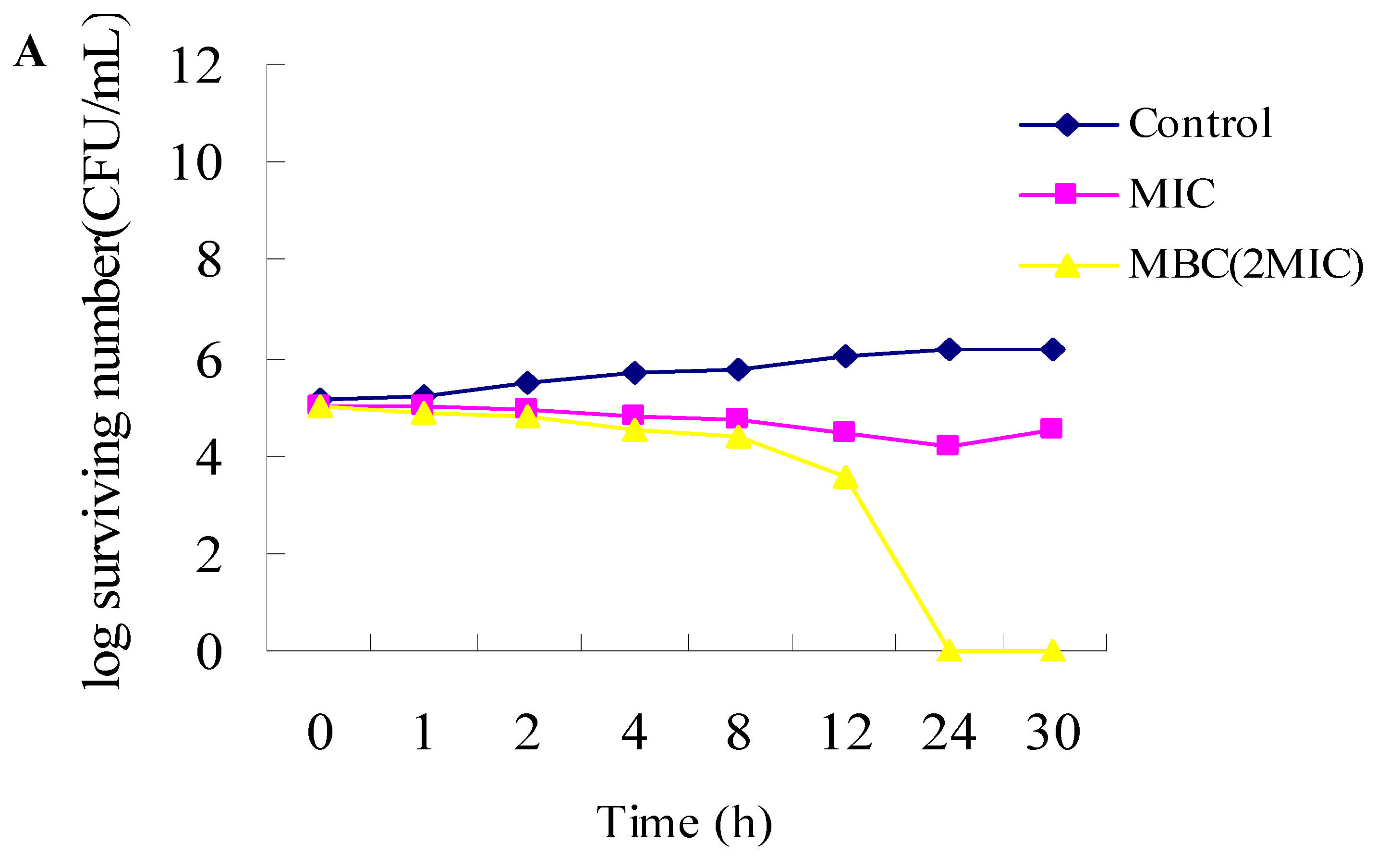
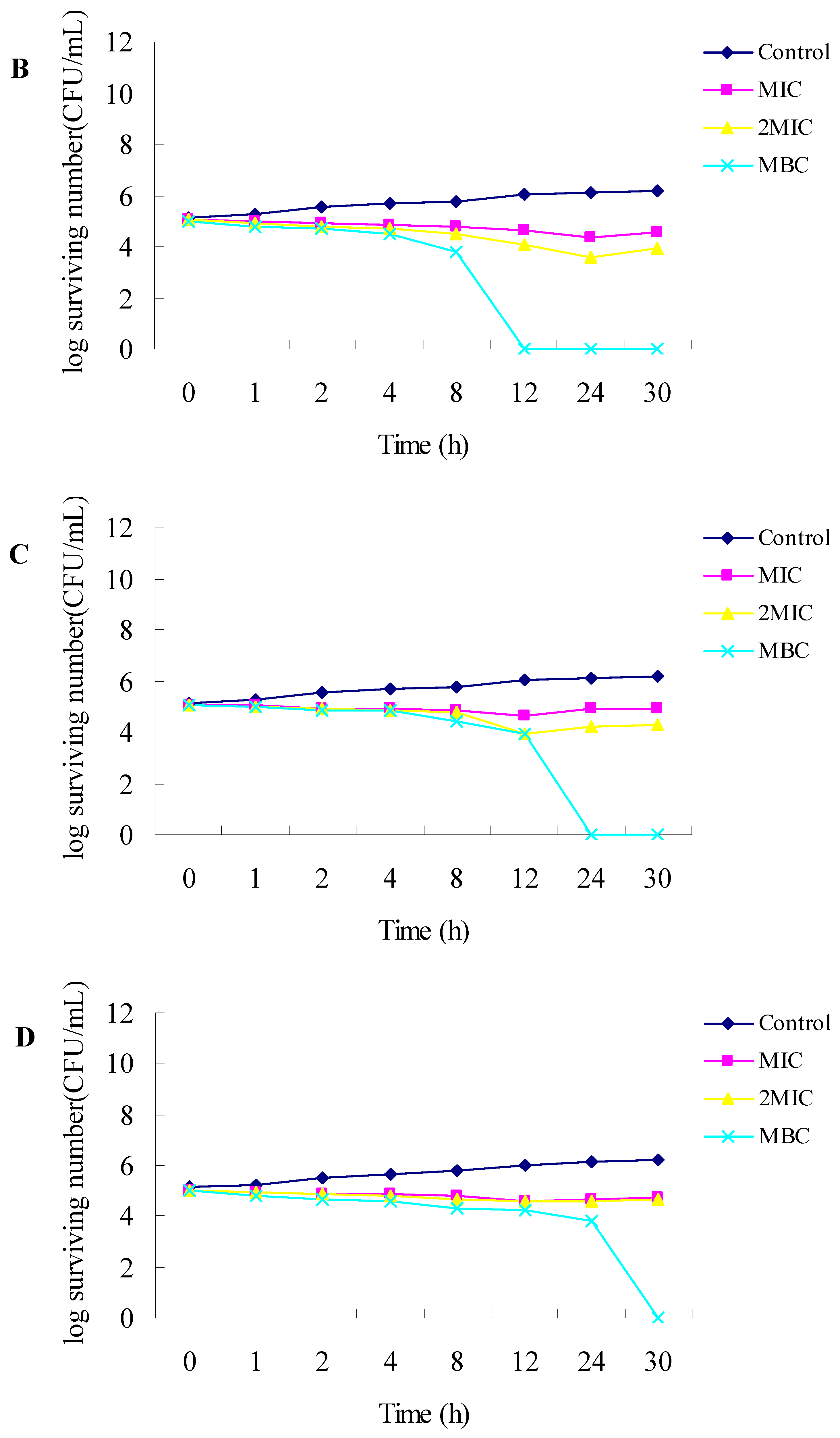
2.1. Antibacterial Activities
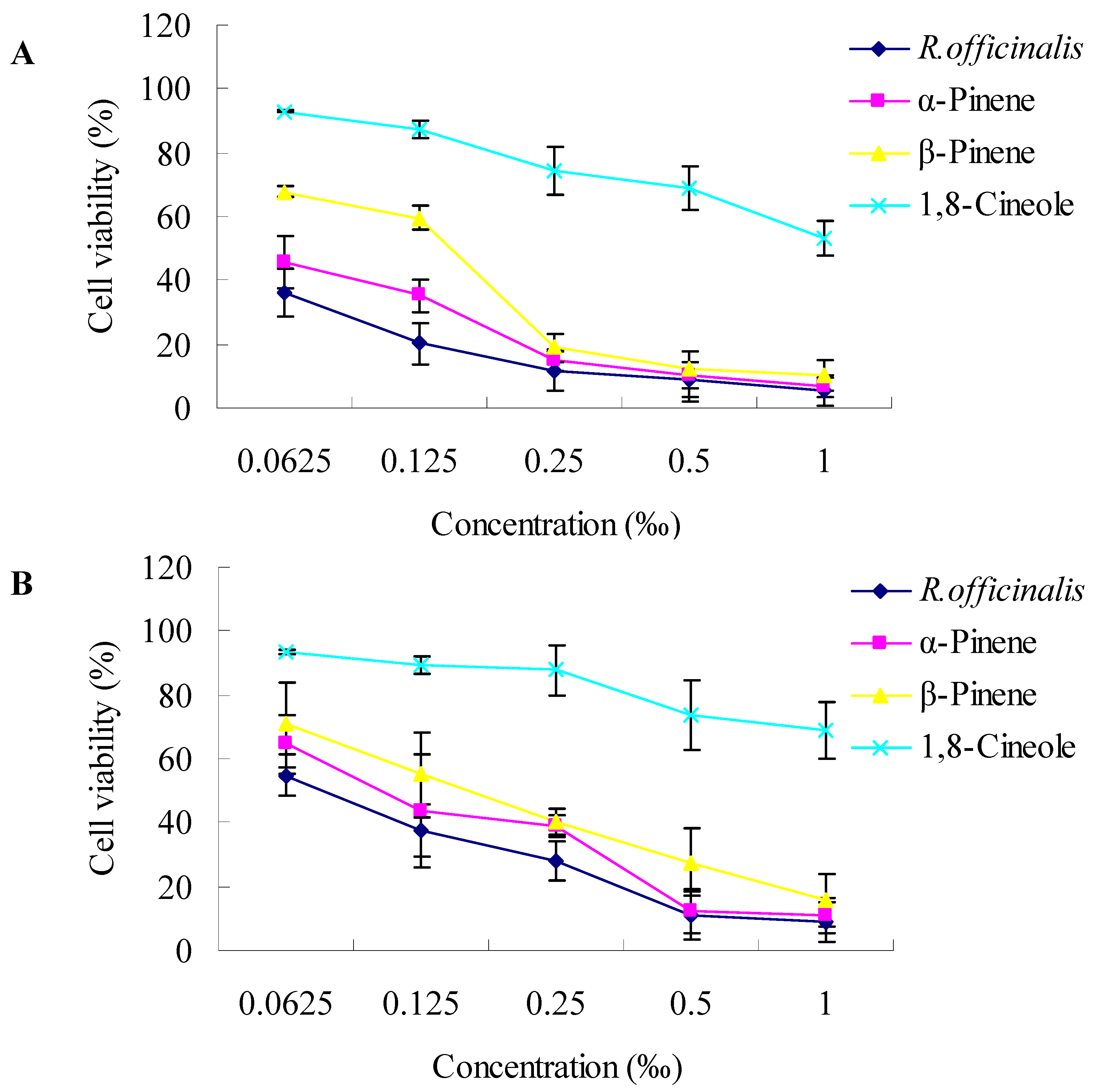
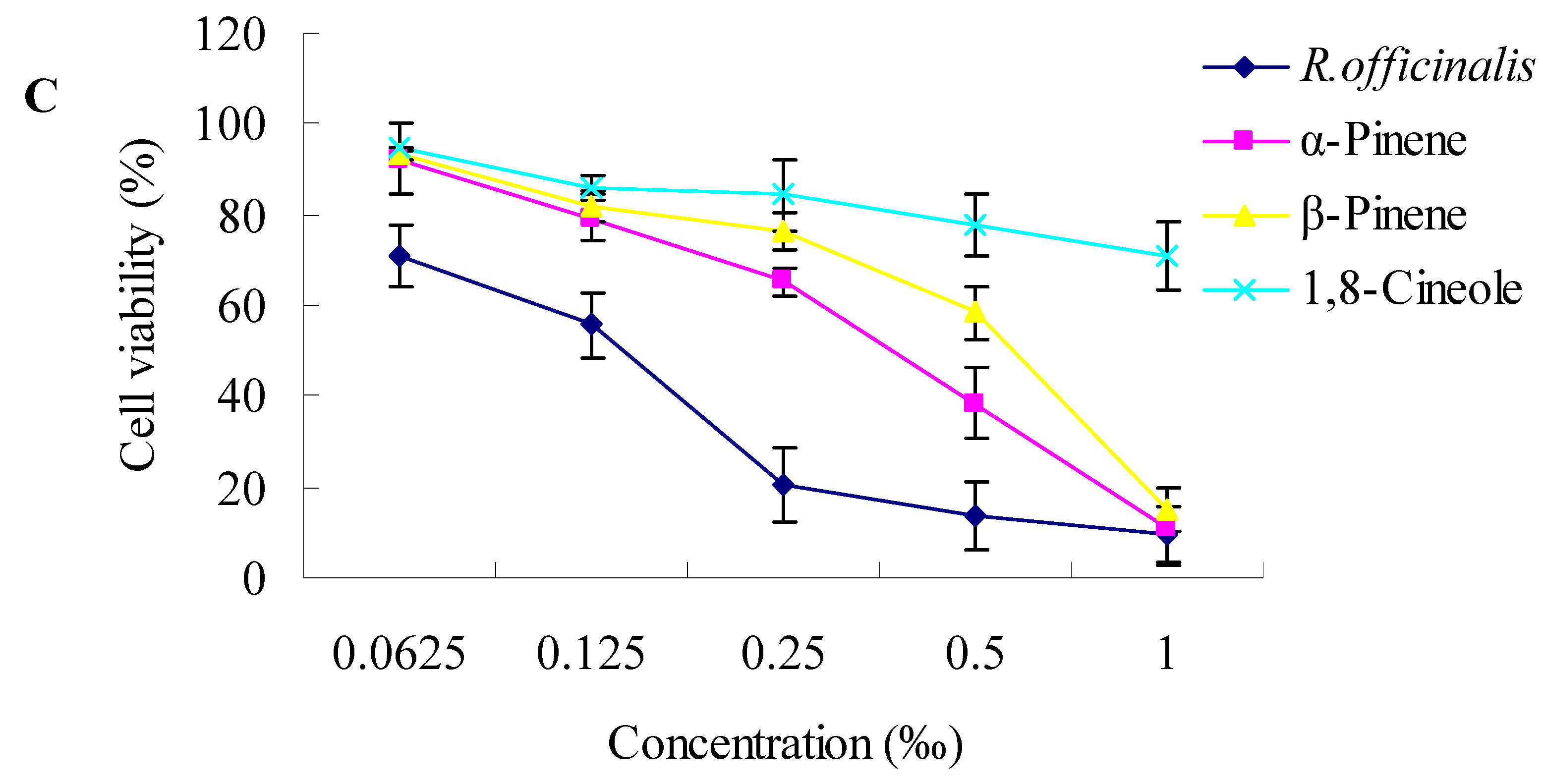
2.2. Cytotoxic Activity towards Cancer Cells
3. Experimental
3.1. Essential Oils
3.2. Antimicrobial Activity
3.2.1. Microorganisms
3.2.2. Determinition of MICs and MBCs Values
3.2.3. Time-Kill Dynamic Curves
3.3. Cytotoxicity Assay
3.3.1. Maintenance of Human Cancer Cell Lines
3.3.2. Cytotoxicity Assay
4. Conclusions
Acknowledgements
References and Notes
- Grayer, R.J.; Harborne, J.B. Survey of antigungal compounds from higher plants. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Takahashi, H.; Aoyagi, K.; Nakanishi, Y.; Sasaki, H.; Yoshida, T.; Honda, H. Classification of intramural metastases and lymph node metastases of esophageal cancer from gene expression based on boosting and projective adaptive resonance theory. J. Biosci. Bioeng. 2006, 102, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, A.; Wakitani, S.; Takagi, M. Noninvasive discrimination of human normal cells and malignant tumor cells by phase-shifting laser microscopy. J. Biosci. Bioeng. 2010, 109, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, W.C.; Ho, T.F.; Wu, H.S.; Wei, Y.H. Development of natural anti-tumor drugs by microorganisms. J. Biosci. Bioeng. 2011, 111, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Z. Anticancer drug discovery in the future: An evolutionary perspective. Drug Discov. Today 2009, 14, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.G.; Yu, H.M.; Liang, L.; Fu, Y.J.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharm. 2006, 103, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, M.; Legault, J.; Dufour, D.; Pichette, A. Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 2005, 12, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yuan, J.; Liu, F.; Ye, J. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2005, 39, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Lei, J.C.; Yu, H.D.; Cai, X.; Zou, G.L. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.G.; de Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; de Vivar, A.R. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharm. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of Rosmarinus officinalis L. essntial oil, 1,8-cineole, α-pinene and β-pinene are available from the authors. |
| R.officinalis L. | α-Pinene | β-Pinene | 1,8-Cineole | Streptomycin (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Bs | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 1.25 | 2.5 | 0.0313 | 0.125 |
| Sa | 0.0313 | 0.0625 | 0.0313 | 0.125 | 0.0313 | 0.125 | 1.25 | 5 | 0.0313 | >0.125 |
| Se | 0.0313 | 0.0625 | 0.0313 | 0.0625 | 0.0625 | 0.125 | 5 | >5 | 0.00781 | 0.125 |
| Ec | 0.0625 | 0.125 | 0.0625 | 0.125 | 0.0625 | 0.125 | 1.25 | 5 | 0.0625 | >0.125 |
| Pa | 0.0625 | 0.25 | 0.0625 | 0.25 | 0.0625 | 0.25 | 2.5 | 2.5 | 0.125 | >0.125 |
| R. officinalis L. | α-Pinene | β-Pinene | 1,8-Cineole | |
|---|---|---|---|---|
| SK-OV-3 | 0.025 | 0.052 | 0.12 | 1.10 |
| HO-8910 | 0.076 | 0.11 | 0.16 | 2.90 |
| Bel-7402 | 0.13 | 0.32 | 0.43 | 3.47 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704-2713. https://doi.org/10.3390/molecules17032704
Wang W, Li N, Luo M, Zu Y, Efferth T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules. 2012; 17(3):2704-2713. https://doi.org/10.3390/molecules17032704
Chicago/Turabian StyleWang, Wei, Nan Li, Meng Luo, Yuangang Zu, and Thomas Efferth. 2012. "Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components" Molecules 17, no. 3: 2704-2713. https://doi.org/10.3390/molecules17032704
APA StyleWang, W., Li, N., Luo, M., Zu, Y., & Efferth, T. (2012). Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules, 17(3), 2704-2713. https://doi.org/10.3390/molecules17032704







