Fluorescence-Based Multiplex Protein Detection Using Optically Encoded Microbeads
Abstract
:1. Introduction
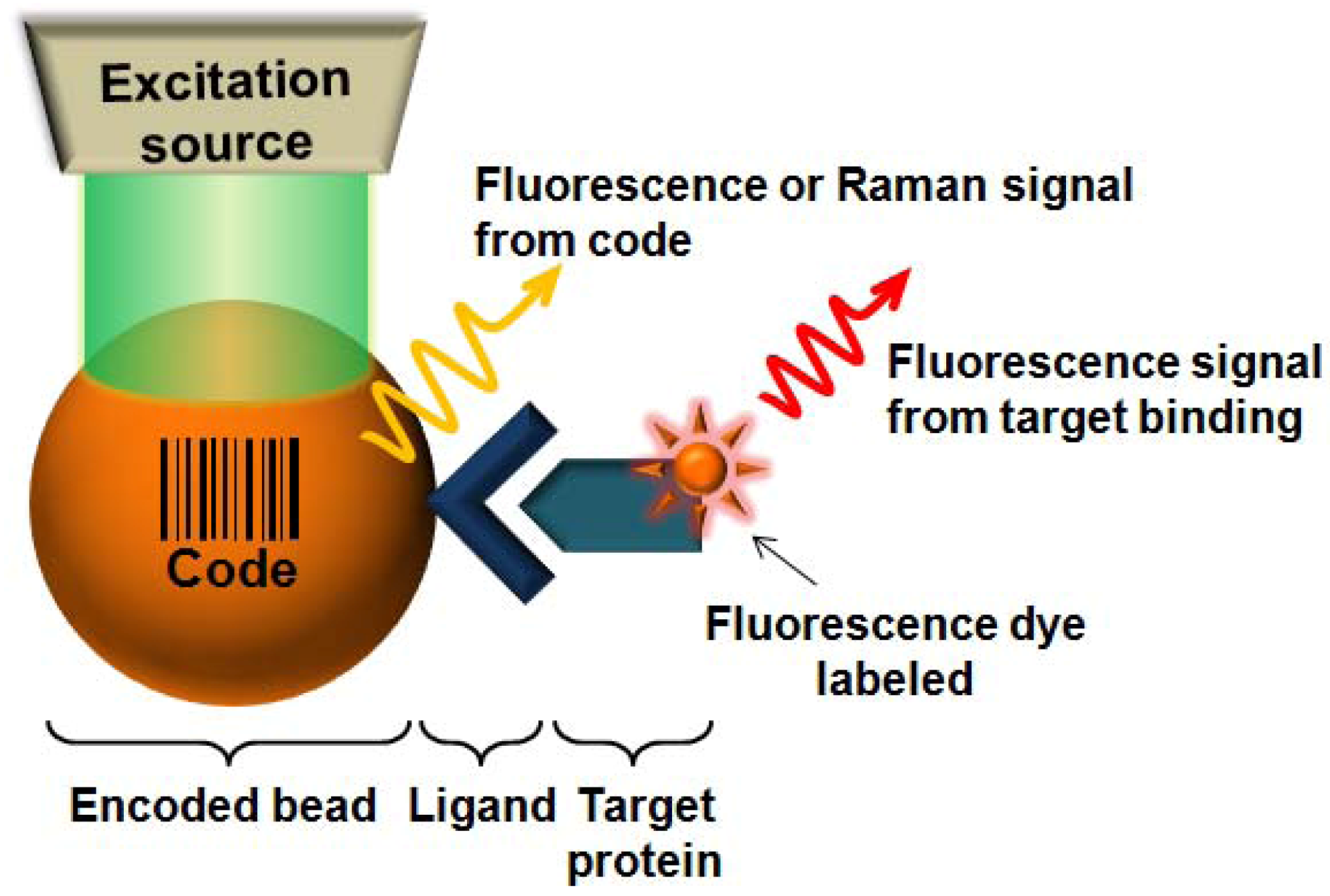
2. Fluorescence-Encoded Beads for Protein Detection
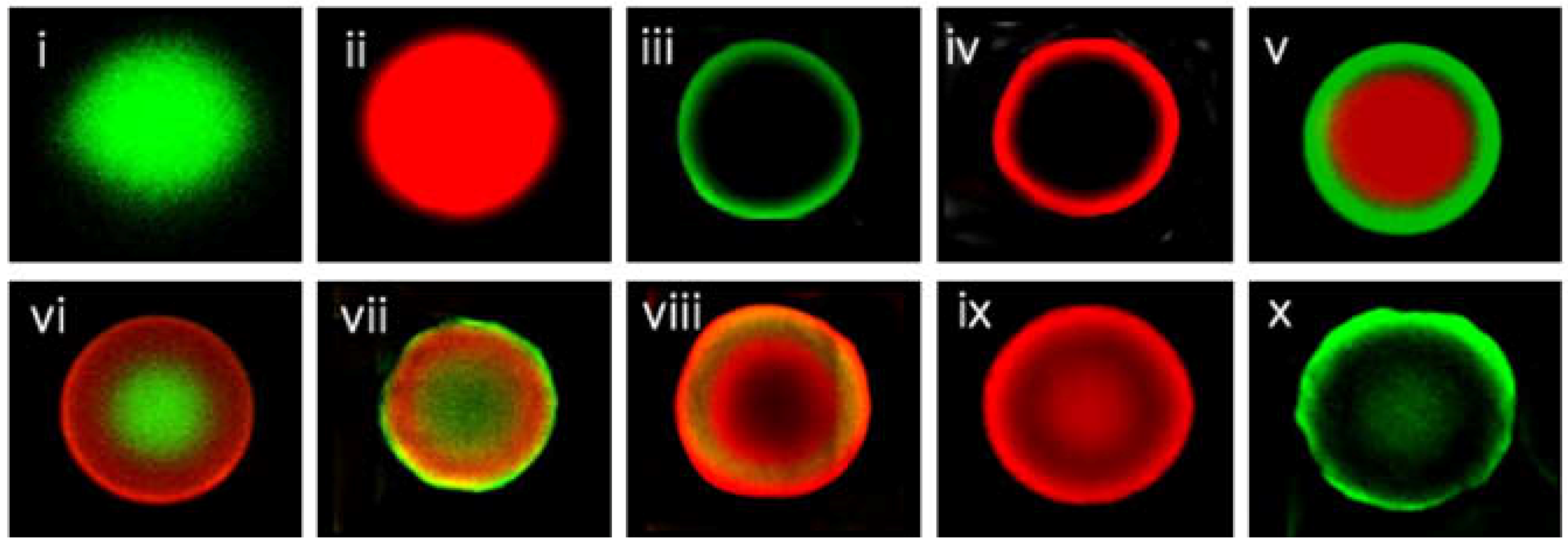
2.1. Fluorescence-Encoded Beads
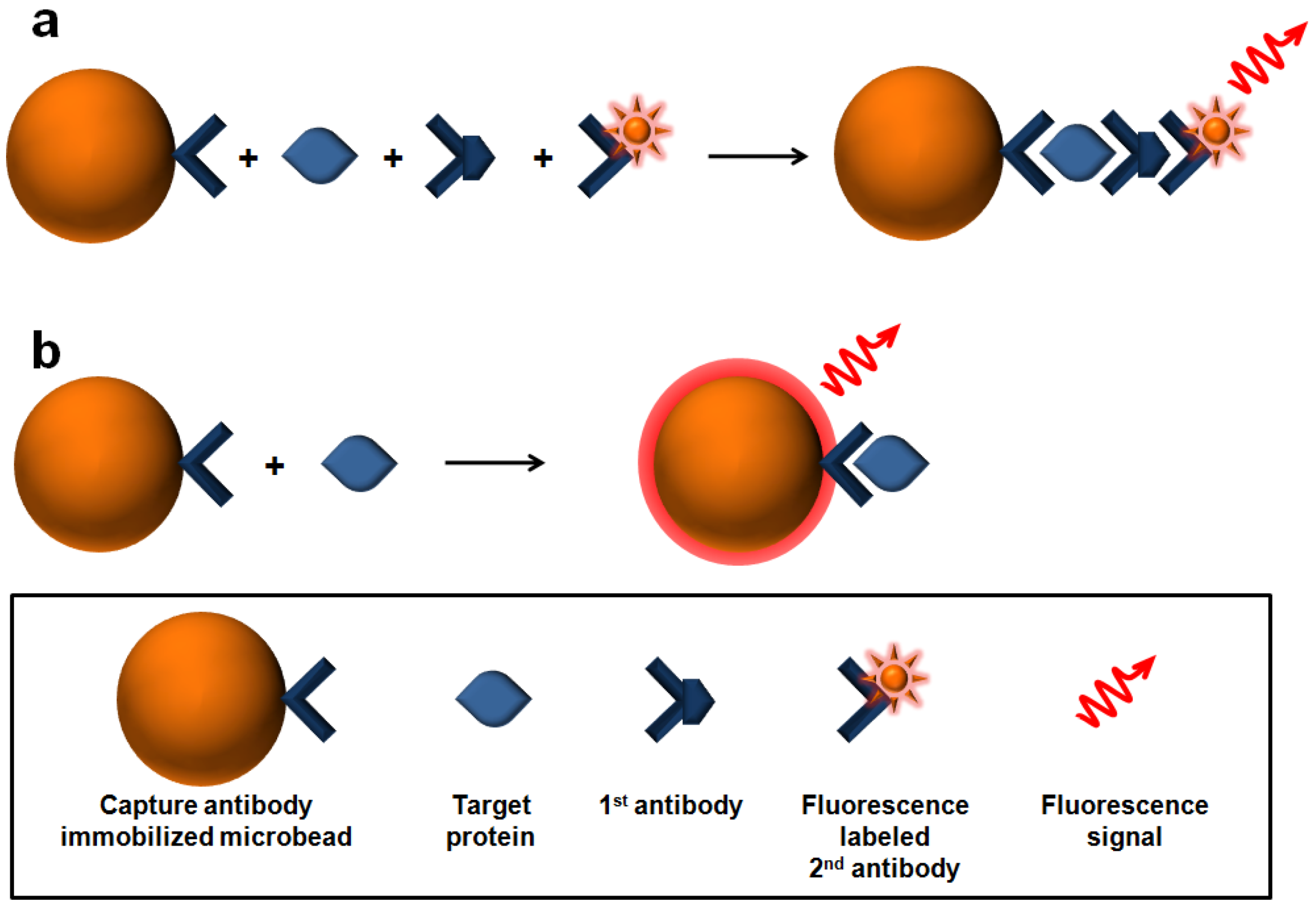
2.2. Label-Free Protein Detection Using Optically-Encoded Beads
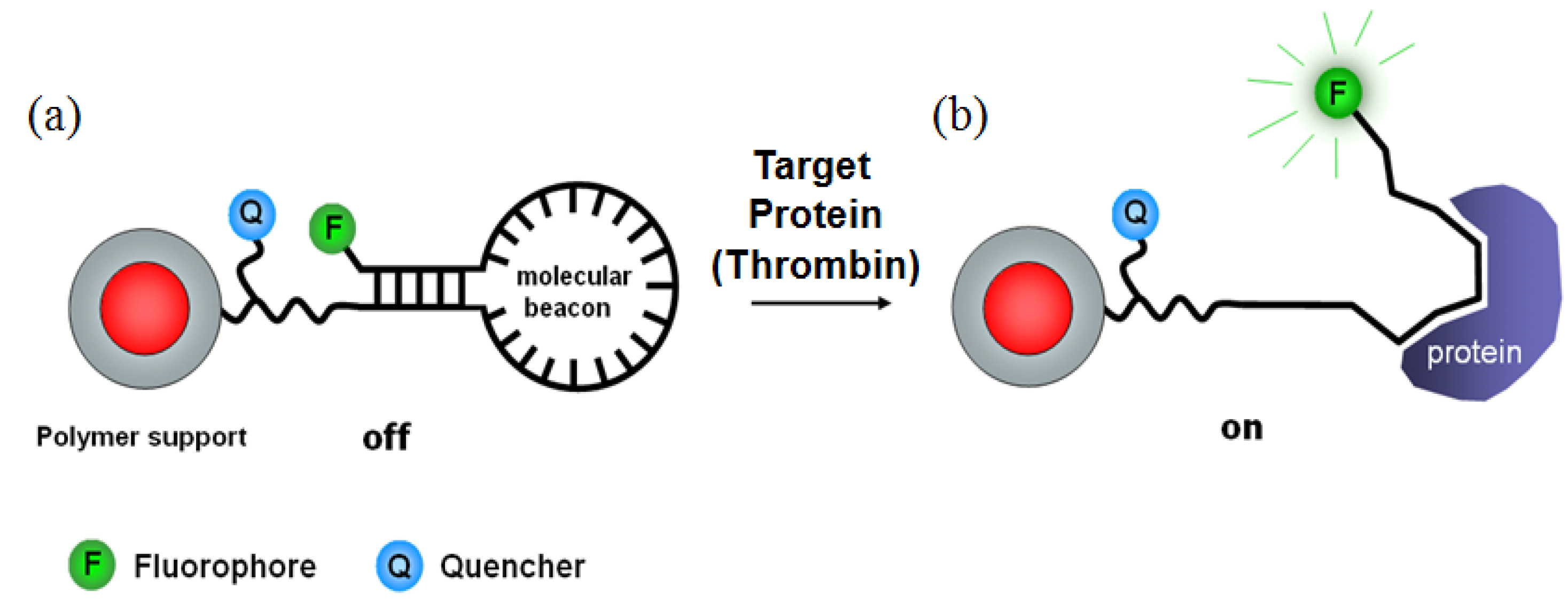
2.2.1. Molecular Beacon-Based Protein Detection Methods
2.2.2. Polydiacetylene-Coated Coding Beads

3. SERS-Encoded Beads for Protein Detection
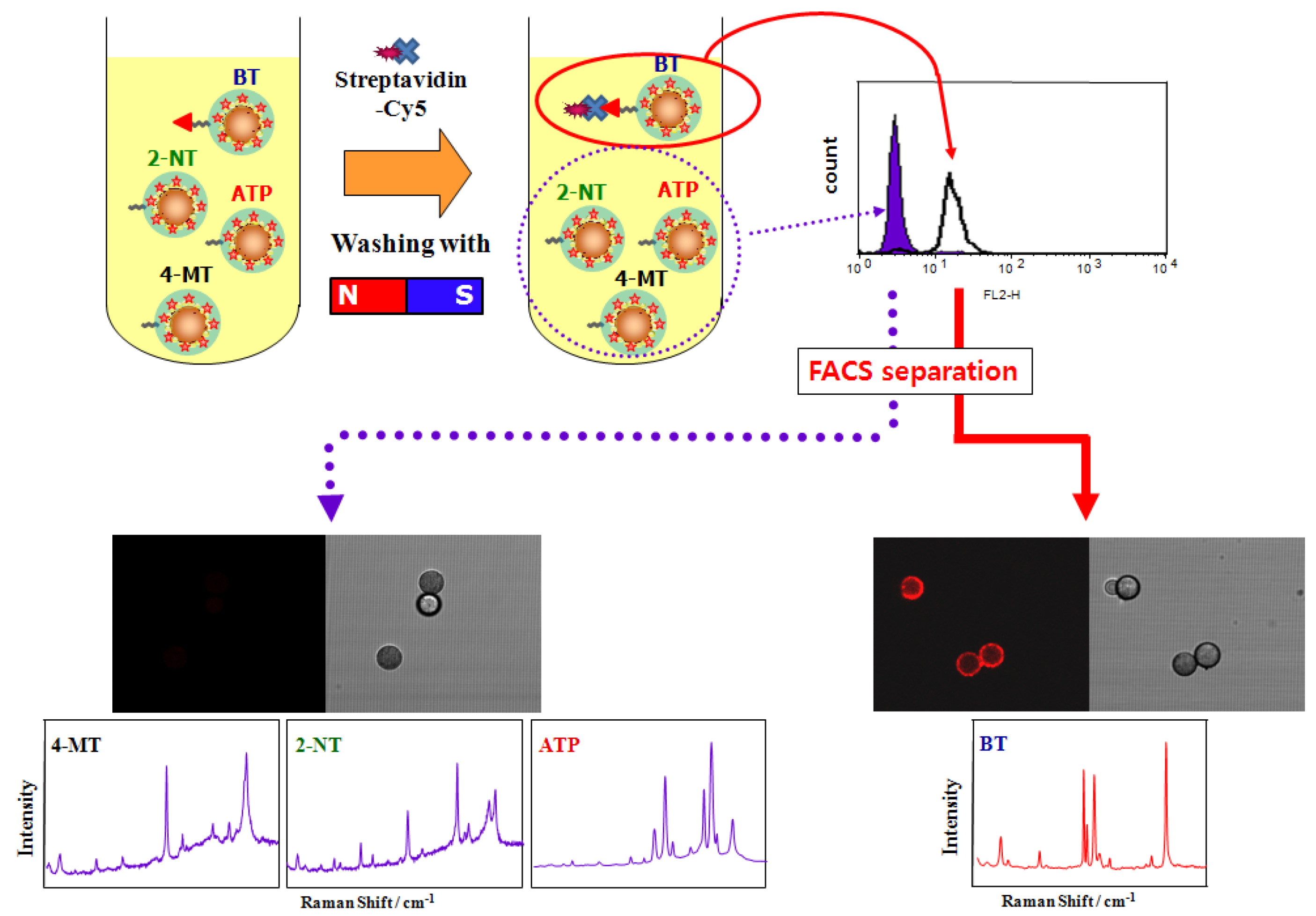
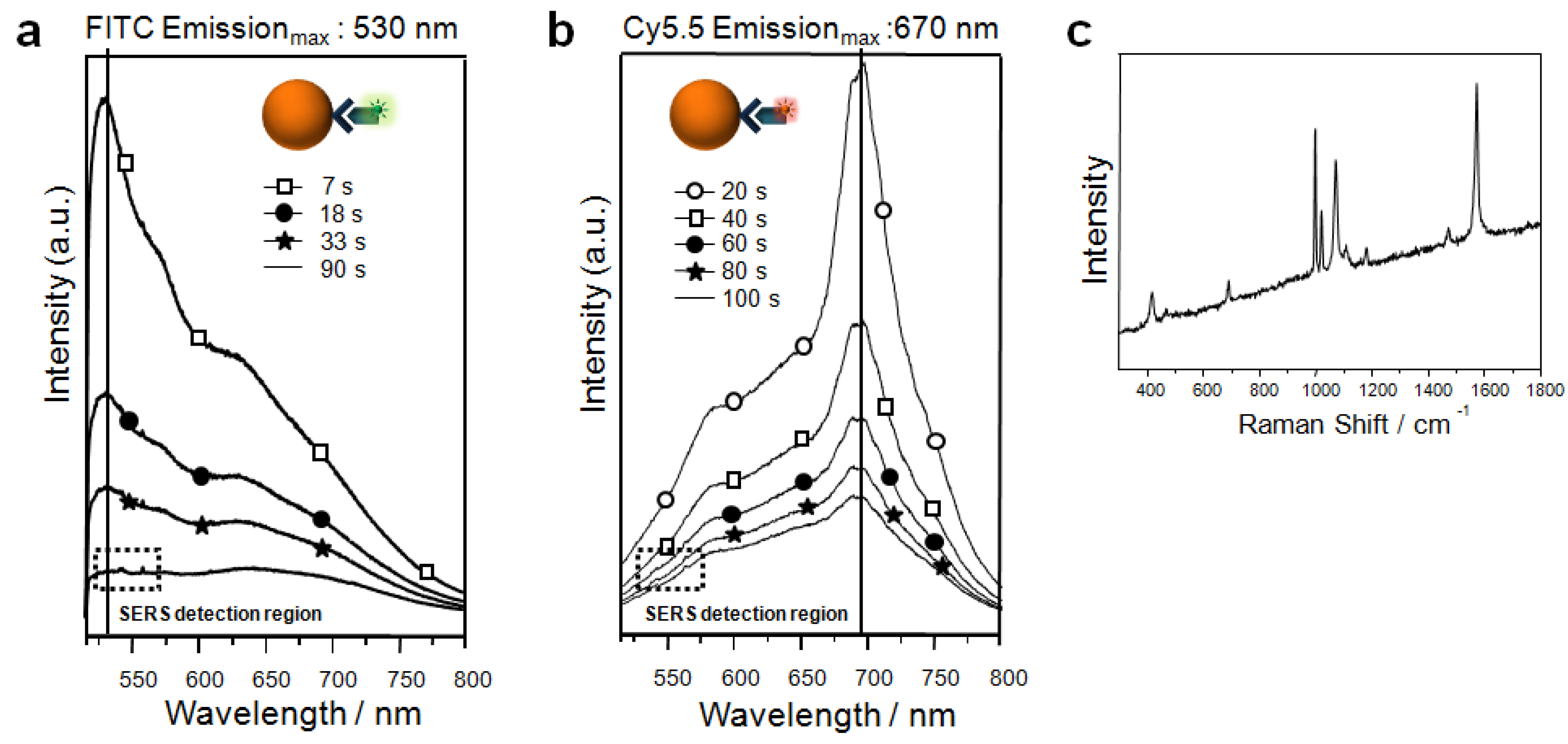
4. Conclusions and Perspectives
- (1) With regard to coding materials, unlimited coding number has not been fully accomplished. Fluorescence-based beads can be limited in their number and/or toxicity. Although the coding number of SERS beads has great potential in respect of coding numbers, they have not been completely established. So far, only several to dozens of Raman dyes are widely used, and different signal intensity and signal complexity sometimes limit their practical coding number.
- (2) For detection of protein binding, on-beads label-free detection is still at the beginning stage. Development of more smart and practical detection method is necessary to multiplex, fast and sensitive detection.
- (3) The great advantages of optically-encoded beads came from well developed decoding and sorting system. So far decoding and sorting with flow cytometry seem to give best performance and can be immediately applicable and promising way.
Acknowledgements
Conflict of Interest
References
- Hsu, H.Y.; Joos, T.O.; Koga, H. Multiplex microsphere-based flow cytometric platforms for protein analysis and their application in clinical proteomics—From assays to results. Electrophoresis 2009, 30, 4008–4019. [Google Scholar] [CrossRef]
- Tessler, L.A.; Reifenberger, J.G.; Mitra, R.D. Protein quantification in complex mixtures by solid phase single-molecule counting. Anal. Chem. 2009, 81, 7141–7148. [Google Scholar]
- Kingsmore, S.F. Multiplexed protein measurement: Technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006, 5, 310–320. [Google Scholar] [CrossRef]
- Krutzik, P.O.; Nolan, G.P. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat. Methods 2006, 3, 361–368. [Google Scholar] [CrossRef]
- Maecker, H.T.; Nolan, G.P.; Fathman, C.G. New technologies for autoimmune disease monitoring. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 322–328. [Google Scholar] [CrossRef]
- Nolan, G.P. What’s wrong with drug screening today. Nat. Chem. Biol. 2007, 3, 187–191. [Google Scholar] [CrossRef]
- Schulz, K.R.; Danna, E.A.; Krutzik, P.O.; Nolan, G.P. Single-cell phospho-protein analysis by flow cytometry. Curr. Protoc. Immunol. 2007, 8, 1–20. [Google Scholar]
- Armstrong, E.G.; Ehrlich, P.H.; Birken, S.; Schlatterer, J.P.; Siris, E.; Hembree, W.C.; Canfield, R.E. Use of a highly sensitive and specific immunoradiometric assay for detection of human chorionic-gonadotropin in urine of normal nonpregnant, and pregnant individuals. J. Clin. Endocrinol. Metab. 1984, 59, 867–874. [Google Scholar] [CrossRef]
- Grossman, H.B.; Messing, E.; Soloway, M.; Tomera, K.; Katz, G.; Berger, Y.; Shen, Y. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005, 293, 810–816. [Google Scholar]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Gstaiger, M.; Aebersold, R. Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nat. Rev. Genet. 2009, 10, 617–627. [Google Scholar] [CrossRef]
- Han, X.M.; Aslanian, A.; Yates, J.R. Mass spectrometry for proteomics. Curr. Opin. Chem. Biol. 2008, 12, 483–490. [Google Scholar] [CrossRef]
- Pan, S.; Aebersold, R.; Chen, R.; Rush, J.; Goodlett, D.R.; McIntosh, M.W.; Zhang, J.; Brentnall, T.A. Mass spectrometry based targeted protein quantification: Methods and applications. J. Proteome Res. 2009, 8, 787–797. [Google Scholar]
- Elshal, M.F.; McCoy, J.P. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef]
- Zhu, H.; Snyder, M. Protein chip technology. Curr. Opin. Chem. Biol. 2003, 7, 55–63. [Google Scholar] [CrossRef]
- Li, Y.W.; Reichert, W.M. Adapting cDNA microarray format to cytokine detection protein arrays. Langmuir 2003, 19, 1557–1566. [Google Scholar] [CrossRef]
- Peluso, P.; Wilson, D.S.; Do, D.; Tran, H.; Venkatasubbaiah, M.; Quincy, D.; Heidecker, B.; Poindexter, K.; Tolani, N.; Phelan, M.; et al. Optimizing antibody immobilization strategies for the construction of protein microarrays. Anal. Biochem. 2003, 312, 113–124. [Google Scholar]
- Qiu, J.; Madoz-Gurpide, J.; Misek, D.E.; Kuick, R.; Brenner, D.E.; Michailidis, G.; Haab, B.B.; Omenn, G.S.; Hanash, S. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J. Proteome Res. 2004, 3, 261–267. [Google Scholar] [CrossRef]
- Levit-Binnun, N.; Lindner, A.B.; Zik, O.; Eshhar, Z.; Moses, E. Quantitative detection of protein arrays. Anal. Chem. 2003, 75, 1436–1441. [Google Scholar] [CrossRef]
- Verpoorte, E. Beads and chips: new recipes for analysis. Lab Chip 2003, 3, 60N–68N. [Google Scholar] [CrossRef]
- Morgan, E.; Varro, R.; Sepulveda, H.; Ember, J.A.; Apgar, J.; Wilson, J.; Lowe, L.; Chen, R.; Shivraj, L.; Agadir, A.; et al. Cytometric bead array: A multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 2004, 110, 252–266. [Google Scholar] [CrossRef]
- Sanchez-Martin, R.M.; Muzerelle, M.; Chitkul, N.; How, S.E.; Mittoo, S.; Bradley, M. Bead-based cellular analysis, sorting and multiplexing. Chembiochem 2005, 6, 1341–1345. [Google Scholar] [CrossRef]
- Templin, M.F.; Stoll, D.; Bachmann, J.; Joos, T.O. Protein microarrays and multiplexed sandwich immunoassays: What beats the beads? Comb. Chem. High T. Scr. 2004, 7, 223–229. [Google Scholar]
- Nolan, J.P.; Mandy, F. Multiplexed and microparticle-based analyses: Quantitative tools for the large-scale analysis of biological systems. Cytom. Part A 2006, 69A, 318–325. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sklar, L.A. Suspension array technology: Evolution of the flat-array paradigm. Trends Biotechnol. 2002, 20, 9–12. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.Y.; Tan, W.H. Dual-luminophore-doped silica nanoparticles for multiplexed signaling. Nano Lett. 2005, 5, 37–43. [Google Scholar]
- Pickering, J.W.; Martins, T.B.; Schroder, M.C.; Hill, H.R. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immun. 2002, 9, 872–876. [Google Scholar]
- Martins, T.B. Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin. Diagn. Lab. Immun. 2002, 9, 41–45. [Google Scholar]
- Braeckmans, K.; De Smedt, S.C.; Leblans, M.; Pauwels, R.; Demeester, J. Encoding microcarriers: Present and future technologies. Nat. Rev. Drug Discov. 2002, 1, 447–456. [Google Scholar] [CrossRef]
- Finkel, N.H.; Lou, X.H.; Wang, C.Y.; He, L. Barcoding the microworld. Anal. Chem. 2004, 76, 353A–359A. [Google Scholar]
- Wilson, R.; Cossins, A.R.; Spiller, D.G. Encoded microcarriers for high-throughput multiplexed detection. Angew. Chem. Int. Ed. 2006, 45, 6104–6117. [Google Scholar]
- Telford, W.G. Analysis of UV-excited fluorochromes by flow cytometry using near-ultraviolet laser diodes. Cytom. Part A 2004, 61A, 9–17. [Google Scholar] [CrossRef]
- Jun, B.H.; Noh, M.S.; Kim, G.; Kang, H.; Kim, J.H.; Chung, W.J.; Kim, M.S.; Kim, Y.K.; Cho, M.H.; Jeong, D.H.; Lee, Y.S. Protein separation and identification using magnetic beads encoded with surface-enhanced Raman spectroscopy. Anal. Biochem. 2009, 391, 24–30. [Google Scholar]
- Jun, B.H.; Rho, C.; Byun, J.W.; Kim, J.H.; Chung, W.J.; Kang, H.; Park, J.; Cho, S.H.; Kim, B.G.; Lee, Y.S. Multilayer fluorescence optically encoded beads for protein detection. Anal. Biochem. 2010, 396, 313–315. [Google Scholar]
- Yingyongnarongkul, B.E.; How, S.E.; Diaz-Mochon, J.J.; Muzerelle, M.; Bradley, M. Parallel and multiplexed bead-based assays and encoding strategies. Comb. Chem. High T. Scr. 2003, 6, 577–587. [Google Scholar] [Green Version]
- Jun, B.H.; Kim, J.E.; Rho, C.; Byun, J.W.; Kim, Y.H.; Kang, H.; Kim, J.H.; Kang, T.; Cho, M.H.; Lee, Y.S. Immobilization of aptamer-based molecular beacons onto optically-encoded micro-sized beads. J. Nanosci. Nanotechnol. 2011, 11, 6249–6252. [Google Scholar] [Green Version]
- Jun, B.H.; Kim, J.H.; Park, H.; Kim, J.S.; Yu, K.N.; Lee, S.M.; Choi, H.; Kwak, S.Y.; Kim, Y.K.; Jeong, D.H.; Cho, M.H.; Lee, Y.S. Surface-enhanced Raman spectroscopic-encoded beads for multiplex immunoassay. J. Comb. Chem. 2007, 9, 237–244. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Wittemann, S.; Schneider, E.M.; Weiss, M.; Joos, T.O. Suspension microarrays for the identification of the response patterns in hyperinfiammatory diseases. Med. Eng. Phys. 2008, 30, 976–983. [Google Scholar] [CrossRef]
- Schwenk, J.M.; Lindberg, J.; Sundberg, M.; Uhlen, M.; Nilsson, P. Determination of binding specificities in highly multiplexed bead-based assays for antibody proteomics. Mol. Cell. Proteomics 2007, 6, 125–132. [Google Scholar]
- Martins, T.B.; Burlingame, R.; von Muhlen, C.A.; Jaskowski, T.D.; Litwin, C.M.; Hill, H.R. Evaluation of multiplexed fluorescent microsphere immunoassay for detection of autoantibodies to nuclear antigens. Clin. Diagn. Lab. Immun. 2004, 11, 1054–1059. [Google Scholar]
- Vignali, D.A.A. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 2000, 243, 243–255. [Google Scholar] [CrossRef]
- Slaastad, H.; Wu, W.; Goullart, L.; Kanderova, V.; Tjønnfjord, G.; Stuchly, J.; Kalina, T.; Holm, A.; Lund-Johansen, F. Multiplexed immuno-precipitation with 1725 commercially available antibodies to cellular proteins. Proteomics 2011, 11, 4578–4582. [Google Scholar]
- Li, H.T.; Ying, L.M.; Green, J.J.; Balasubramanian, S.; Klenerman, D. Ultrasensitive coincidence fluorescence detection of single DNA molecules. Anal. Chem. 2003, 75, 1664–1670. [Google Scholar]
- Gao, X.H.; Nie, S.M. Quantum dot-encoded mesoporous beads with high brightness and uniformity: Rapid readout using flow cytometry. Anal. Chem. 2004, 76, 2406–2410. [Google Scholar] [CrossRef]
- Han, M.Y.; Gao, X.H.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef]
- Bradley, M.; Bruno, N.; Vincent, B. Distribution of CdSe quantum dots within swollen polystyrene microgel particles using confocal microscopy. Langmuir 2005, 21, 2750–2753. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Xiang, Q.; Farcas, G.A.; Lee, J.A.; Rhee, A.; Lafferty, E.I.; Perrault, S.D.; Kain, K.C.; Chan, W.C.W. Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett. 2007, 7, 2812–2818. [Google Scholar]
- Fournier‐Bidoz, S.; Jennings, T.L.; Klostranec, J.M.; Fung, W.; Rhee, A.; Li, D.; Chan, W.C.W. Facile and rapid one-step mass preparation of quantum-dot barcodes. Angew. Chem. 2008, 120, 5659–5663. [Google Scholar]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H.; et al. Ultra-sensitive, biocompatible, quantum dot-embedded silica nanoparticles for bio-imaging. Adv. Funct. Mater. 2011. [Google Scholar] [CrossRef]
- Tan, W.H.; Wang, K.M.; He, X.X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med. Res. Rev. 2004, 24, 621–638. [Google Scholar] [CrossRef]
- Zhao, X.J.; Bagwe, R.P.; Tan, W.H. Development of organic-dye-doped silica nanoparticles in a reverse microemulsion. Adv. Mater. 2004, 16, 173–176. [Google Scholar] [CrossRef]
- Zhao, X.J.; Hilliard, L.R.; Mechery, S.J.; Wang, Y.P.; Bagwe, R.P.; Jin, S.G.; Tan, W.H. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 15027–15032. [Google Scholar]
- Zhao, X.J.; Tapec-Dytioco, R.; Tan, W.H. Ultrasensitive DNA detection using highly fluorescent bioconjugated nanoparticles. J. Am. Chem. Soc. 2003, 125, 11474–11475. [Google Scholar]
- Ray, S.; Mehta, G.; Srivastava, S. Label-free detection techniques for protein microarrays: Prospects, merits and challenges. Proteomics 2010, 10, 731–748. [Google Scholar] [CrossRef]
- Suzuki, T.; Matsuzaki, T.; Hagiwara, H.; Aoki, T.; Takata, K. Recent advances in fluorescent labeling techniques for fluorescence microscopy. Acta Histochem. Cytochem. 2007, 40, 131–137. [Google Scholar]
- Ray, S.; Mehta, G.; Srivastava, S. Label-free detection techniques for protein microarrays: Prospects, merits and challenges. Proteomics 2010, 10, 731–748. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Hu, J.; Xu, M.; Zhao, W.; Sun, L.; Zhu, C.; Xu, H.; Gu, Z. Encoded porous beads for label-free multiplex detection of tumor markers. Adv. Mater. 2009, 21, 569–572. [Google Scholar]
- Kim, M.S.; Kim, J.H.; Lee, Y.S.; Lim, G.G.; Lee, H.B.; Park, J.H.; Kim, Y.K. Experimental and theoretical analysis of DEP-based particle deflection for the separation of protein-bound particles. J. Micromech. Microeng. 2009, 19, 015029. [Google Scholar] [CrossRef]
- Jun, B.H.; Baek, J.; Kang, H.; Park, Y.J.; Jeong, D.H.; Lee, Y.S. Preparation of polydiacetylene immobilized optically encoded beads. J. Colloid Interf. Sci. 2011, 355, 29–34. [Google Scholar] [CrossRef]
- Tyagi, S.; Kramer, F.R. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar] [CrossRef]
- Maxwell, D.J.; Taylor, J.R.; Nie, S.M. Self-assembled nanoparticle probes for recognition and detection of biomolecules. J. Am. Chem. Soc. 2002, 124, 9606–9612. [Google Scholar]
- Horejsh, D.; Martini, F.; Poccia, F.; Ippolito, G.; Di Caro, A.; Capobianchi, M.R. A molecular beacon, bead-based assay for the detection of nucleic acids by flow cytometry. Nucleic Acids Res. 2005, 33, e13–e13. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Araujo, H.; Wilson, E.G. Polymerization of Langmuir-Blodgett films of diacetylenes. Surf. Sci. 2000, 460, 182–189. [Google Scholar] [CrossRef]
- Morigaki, K.; Baumgart, T.; Offenhausser, A.; Knoll, W. Patterning solid-supported lipid bilayer membranes by lithographic polymerization of a diacetylene lipid. Angew. Chem. Int. Ed. 2001, 40, 172–174. [Google Scholar] [CrossRef]
- Yamanaka, S.A.; Charych, D.H.; Loy, D.A.; Sasaki, D.Y. Solid phase immobilization of optically responsive liposomes in sol-gel materials for chemical and biological sensing. Langmuir 1997, 13, 5049–5053. [Google Scholar] [CrossRef]
- Namgung, J.Y.; Jun, B.H.; Lee, Y.S. Synthesis of alkyne-terminated PCDA linker for applying click chemistry on PDA layers. Synlett 2010, 449–452. [Google Scholar]
- Doering, W.E.; Nie, S.M. Spectroscopic tags using dye-embedded nanoparticles and surface-enhanced Raman scattering. Anal. Chem. 2003, 75, 6171–6176. [Google Scholar] [CrossRef]
- Driskell, J.D.; Kwarta, K.M.; Lipert, R.J.; Porter, M.D.; Neill, J.D.; Ridpath, J.F. Low-level detection of viral pathogens by a surface-enhanced Raman scattering based immunoassay. Anal. Chem. 2005, 77, 6147–6154. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Sun, L.; Koo, T.W.; Chan, S.; Sundararajan, N.; Yamakawa, M.; Berlin, A.A. Composite organic-inorganic nanoparticles (COINs) with chemically encoded optical signatures. Nano Lett. 2005, 5, 49–54. [Google Scholar] [CrossRef]
- Wabuyele, M.B.; Vo-Dinh, T. Detection of human immunodeficiency virus type 1 DNA sequence using plasmonics nanoprobes. Anal. Chem. 2005, 77, 7810–7815. [Google Scholar] [CrossRef]
- Jun, B.H.; Kim, G.; Noh, M.S.; Kang, H.; Kim, Y.K.; Cho, M.H.; Jeong, D.H.; Lee, Y.S. Surface-enhanced Raman scattering-active nanostructures and strategies for bioassays. Nanomedicine 2011, 6, 1463–1480. [Google Scholar] [CrossRef]
- Jun, B.H.; Kim, G.; Baek, J.; Kang, H.; Kim, T.; Hyeon, T.; Jeong, D.H.; Lee, Y.S. Magnetic field induced aggregation of nanoparticles for sensitive molecular detection. Phys. Chem. Chem. Phys. 2011, 13, 7298–7303. [Google Scholar]
- Kim, J.H.; Kang, H.; Kim, S.; Jun, B.H.; Kang, T.; Chae, J.; Jeong, S.; Kim, J.; Jeong, D.H.; Lee, Y.S. Encoding peptide sequences with surface-enhanced Raman spectroscopic nanoparticles. Chem. Commun. 2011, 47, 2306–2308. [Google Scholar]
- Jun, B.H.; Noh, M.S.; Kim, J.; Kim, G.; Kang, H.; Kim, M.S.; Seo, Y.T.; Baek, J.; Kim, J.H.; Park, J.; et al. Multifunctional silver-embedded magnetic nanoparticles as SERS nanoprobes and their applications. Small 2010, 6, 119–125. [Google Scholar] [CrossRef]
- Noh, M.S.; Jun, B.H.; Kim, S.; Kang, H.; Woo, M.A.; Minai-Tehrani, A.; Kim, J.E.; Kim, J.; Park, J.; Lim, H.T.; et al. Magnetic surface-enhanced Raman spectroscopic (M-SERS) dots for the identification of bronchioalveolar stem cells in normal and lung cancer mice. Biomaterials 2009, 30, 3915–3925. [Google Scholar]
- Kim, J.H.; Kim, J.S.; Choi, H.; Lee, S.M.; Jun, B.H.; Yu, K.N.; Kuk, E.; Kim, Y.K.; Jeong, D.H.; Cho, M.H.; Lee, Y.S. Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal. Chem. 2006, 78, 6967–6973. [Google Scholar]
- Lutz, B.R.; Dentinger, C.E.; Nguyen, L.N.; Sun, L.; Zhang, J.W.; Allen, A.N.; Chan, S.; Knudsen, B.S. Spectral analysis of multiplex raman probe signatures. ACS Nano 2008, 2, 2306–2314. [Google Scholar]
- Gellner, M.; Kompe, K.; Schlucker, S. Multiplexing with SERS labels using mixed SAMs of Raman reporter molecules. Anal. Bioanal. Chem. 2009, 394, 1839–1844. [Google Scholar]
- Holmes, D.; Morgan, H.; Green, N.G. High throughput particle analysis: Combining dielectrophoretic particle focussing with confocal optical detection. Biosens. Bioelectron. 2006, 21, 1621–1630. [Google Scholar] [CrossRef]
- Watson, D.A.; Brown, L.O.; Gaskill, D.R.; Naivar, M.; Graves, S.W.; Doorn, S.K.; Nolan, J.P. A flow cytometer for the measurement of Raman spectra. Cytom. Part A 2008, 73A, 119–128. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jun, B.-H.; Kang, H.; Lee, Y.-S.; Jeong, D.H. Fluorescence-Based Multiplex Protein Detection Using Optically Encoded Microbeads. Molecules 2012, 17, 2474-2490. https://doi.org/10.3390/molecules17032474
Jun B-H, Kang H, Lee Y-S, Jeong DH. Fluorescence-Based Multiplex Protein Detection Using Optically Encoded Microbeads. Molecules. 2012; 17(3):2474-2490. https://doi.org/10.3390/molecules17032474
Chicago/Turabian StyleJun, Bong-Hyun, Homan Kang, Yoon-Sik Lee, and Dae Hong Jeong. 2012. "Fluorescence-Based Multiplex Protein Detection Using Optically Encoded Microbeads" Molecules 17, no. 3: 2474-2490. https://doi.org/10.3390/molecules17032474
APA StyleJun, B.-H., Kang, H., Lee, Y.-S., & Jeong, D. H. (2012). Fluorescence-Based Multiplex Protein Detection Using Optically Encoded Microbeads. Molecules, 17(3), 2474-2490. https://doi.org/10.3390/molecules17032474




