Communic Acids: Occurrence, Properties and Use as Chirons for the Synthesis of Bioactive Compounds
Abstract
:1. Introduction
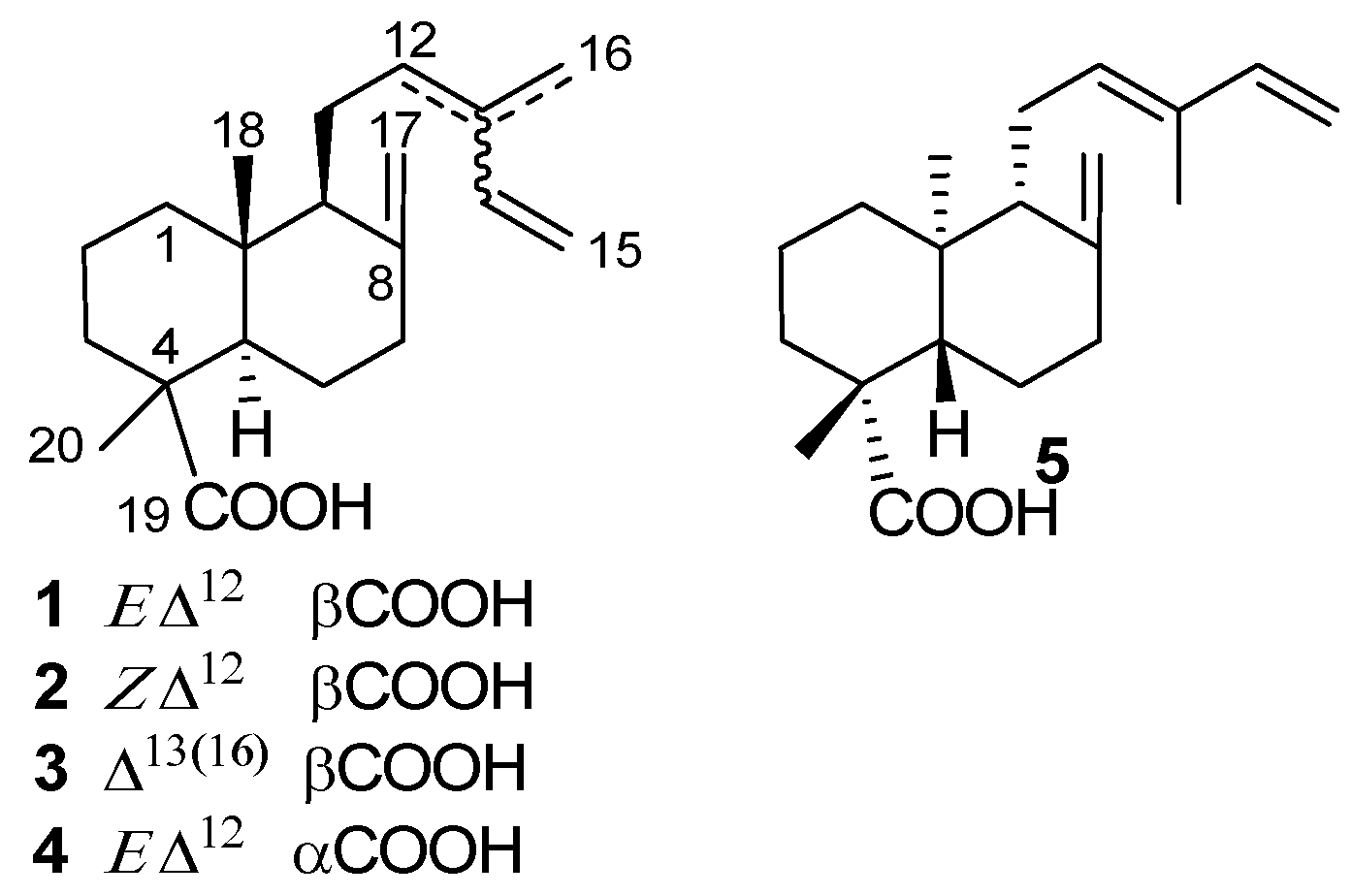
2. Sources
3. Biological Activity
4. Chemical Reactivity
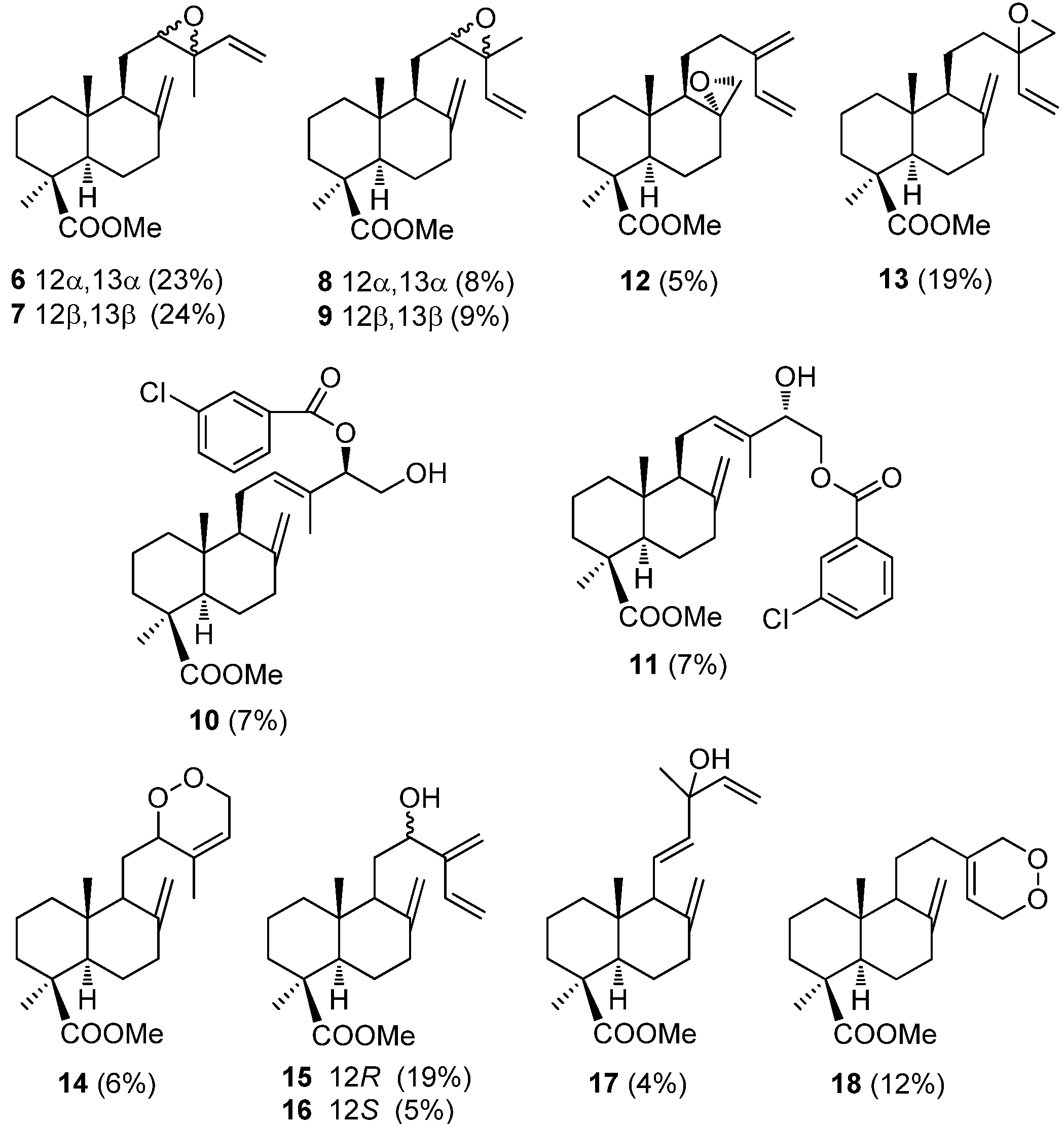



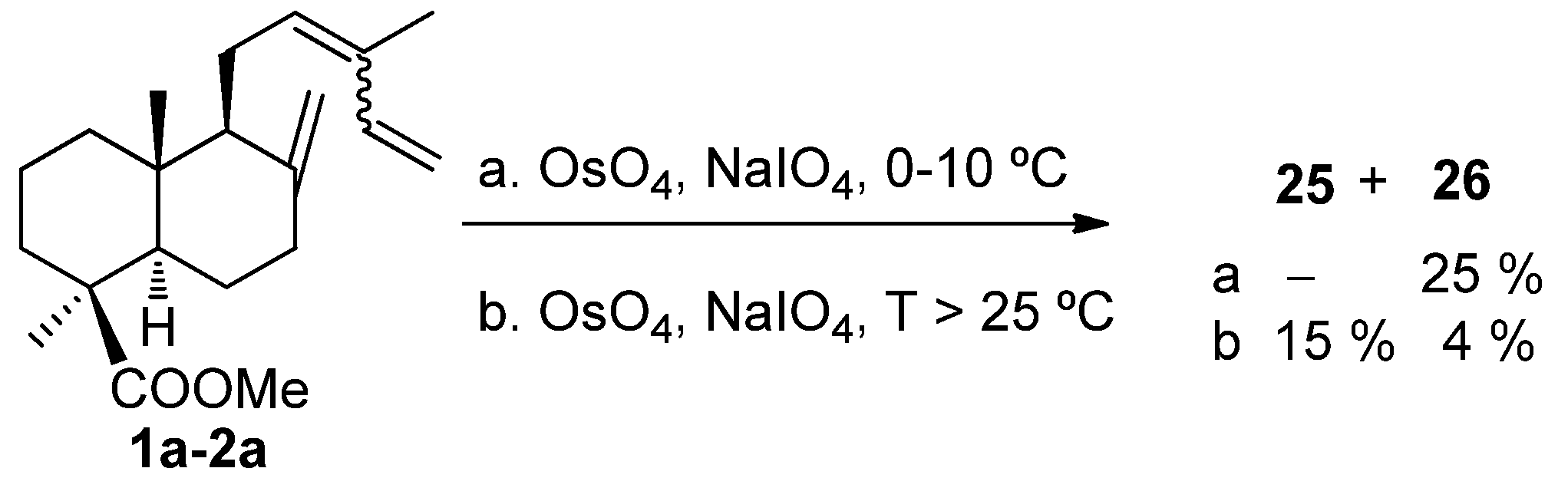
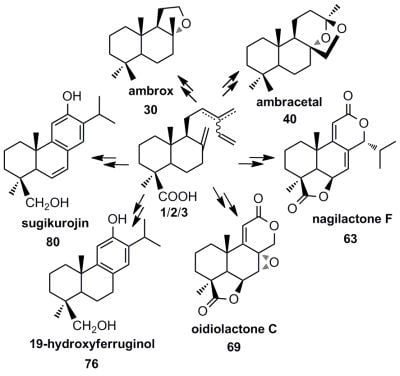
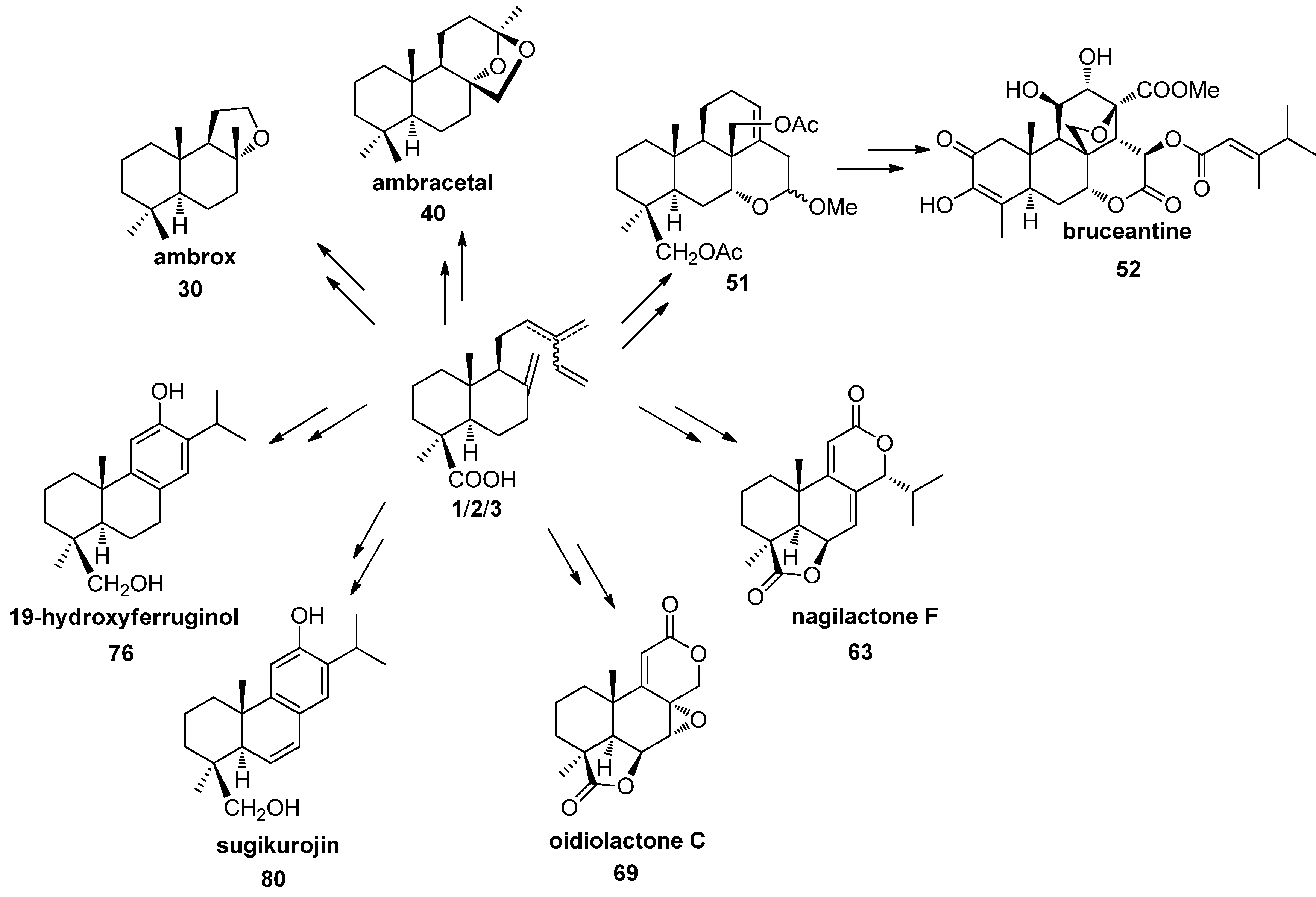
5. Use of Communic Acids as Starting Materials for the Synthesis of Compounds of High Added Value
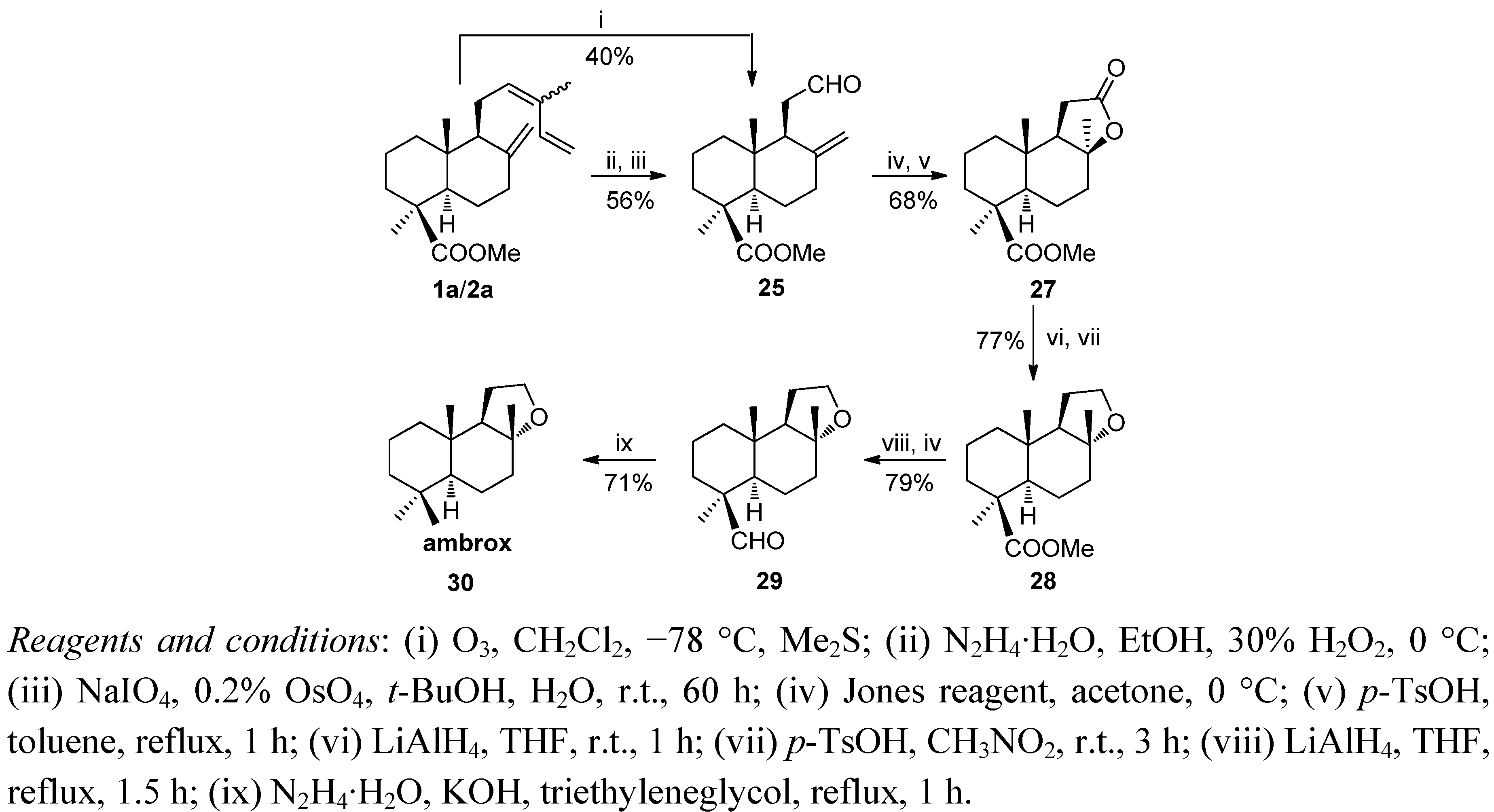


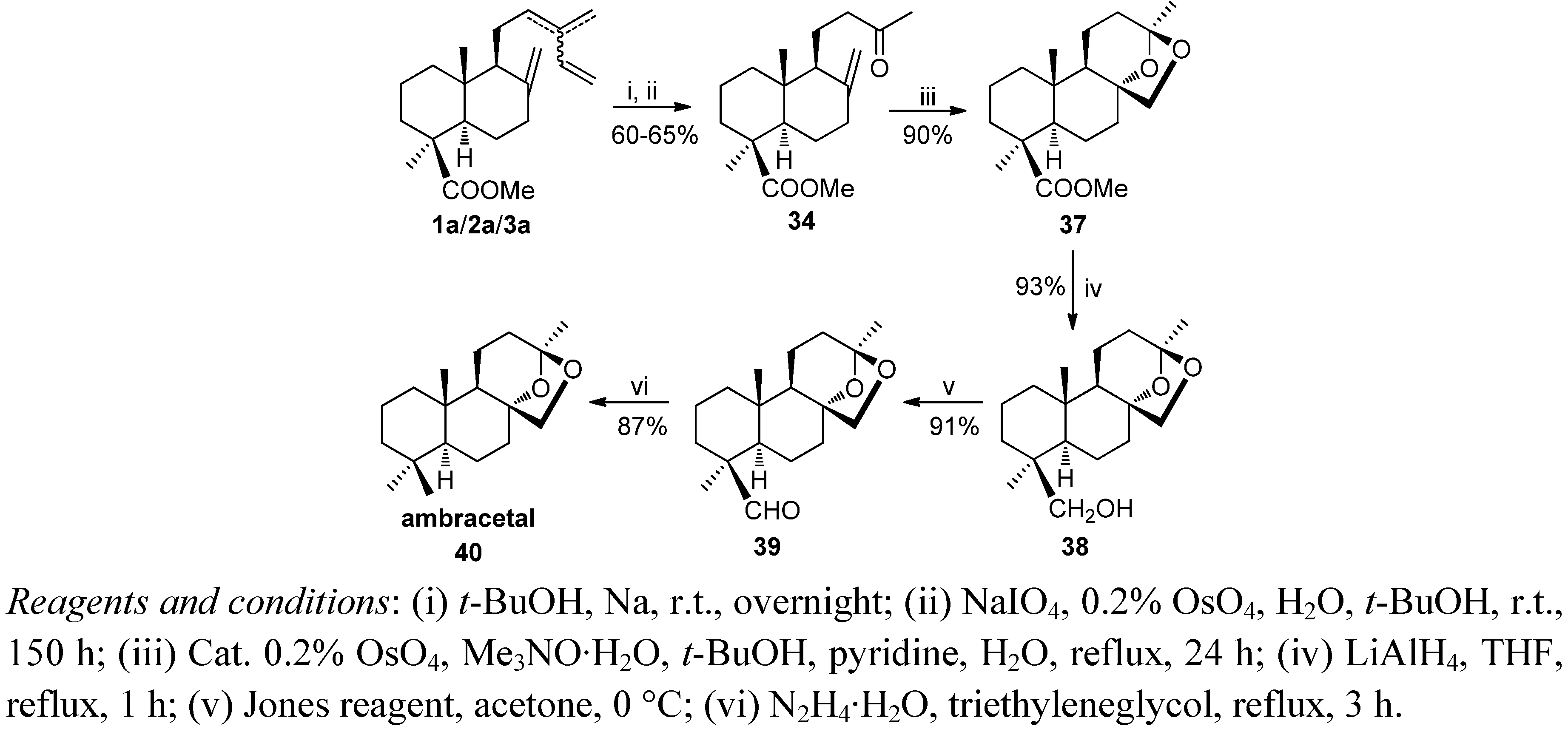
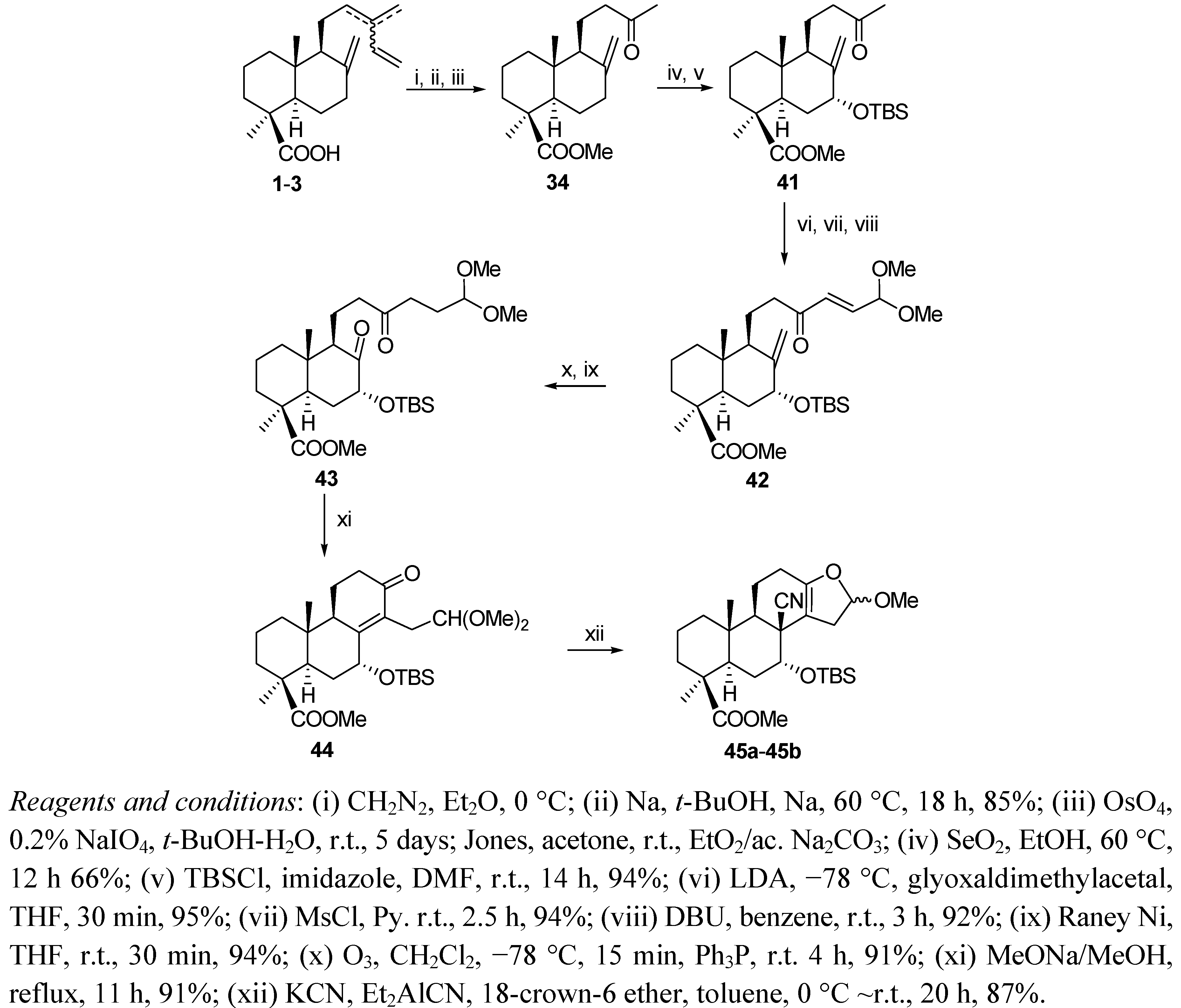
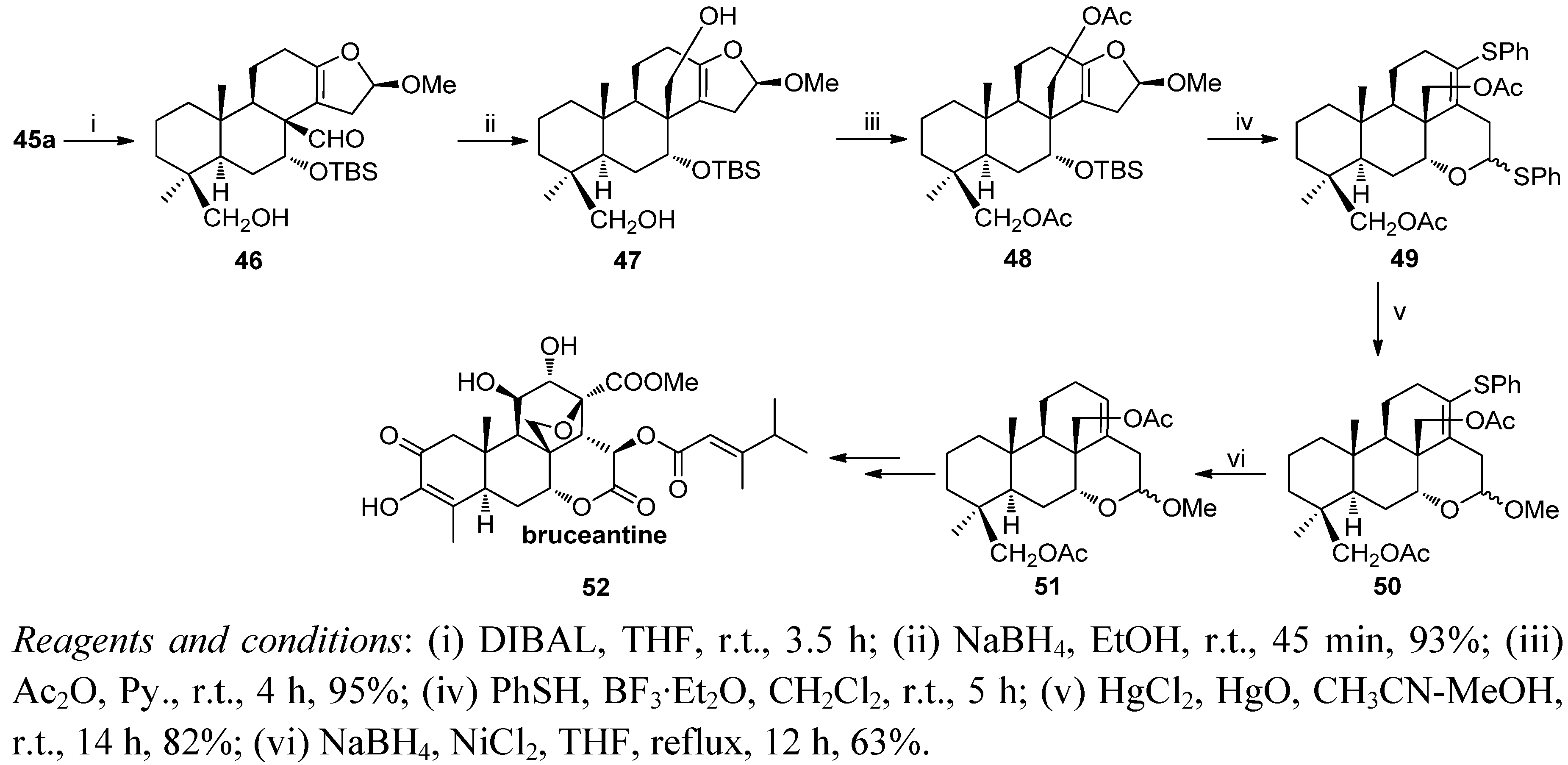

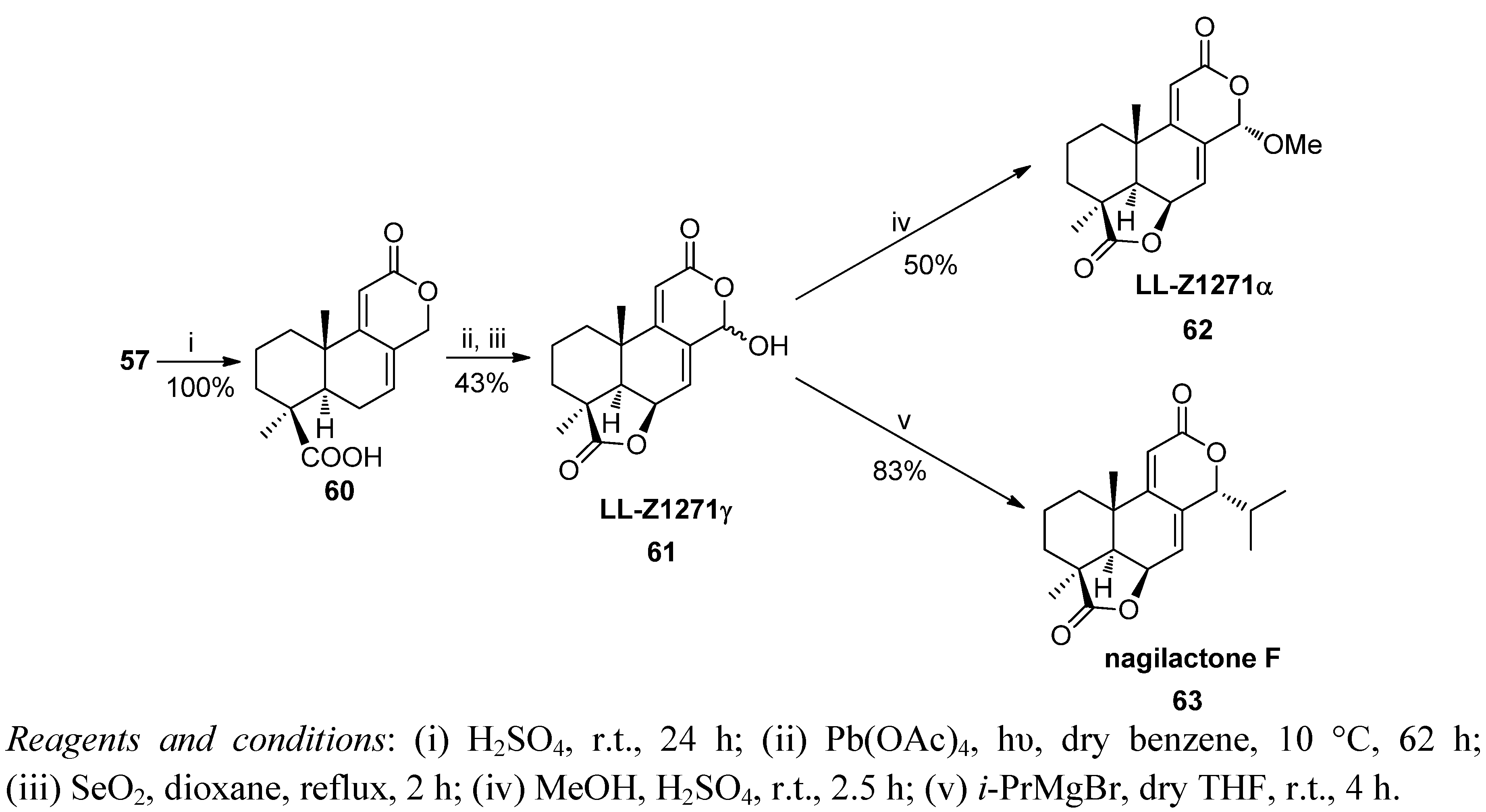
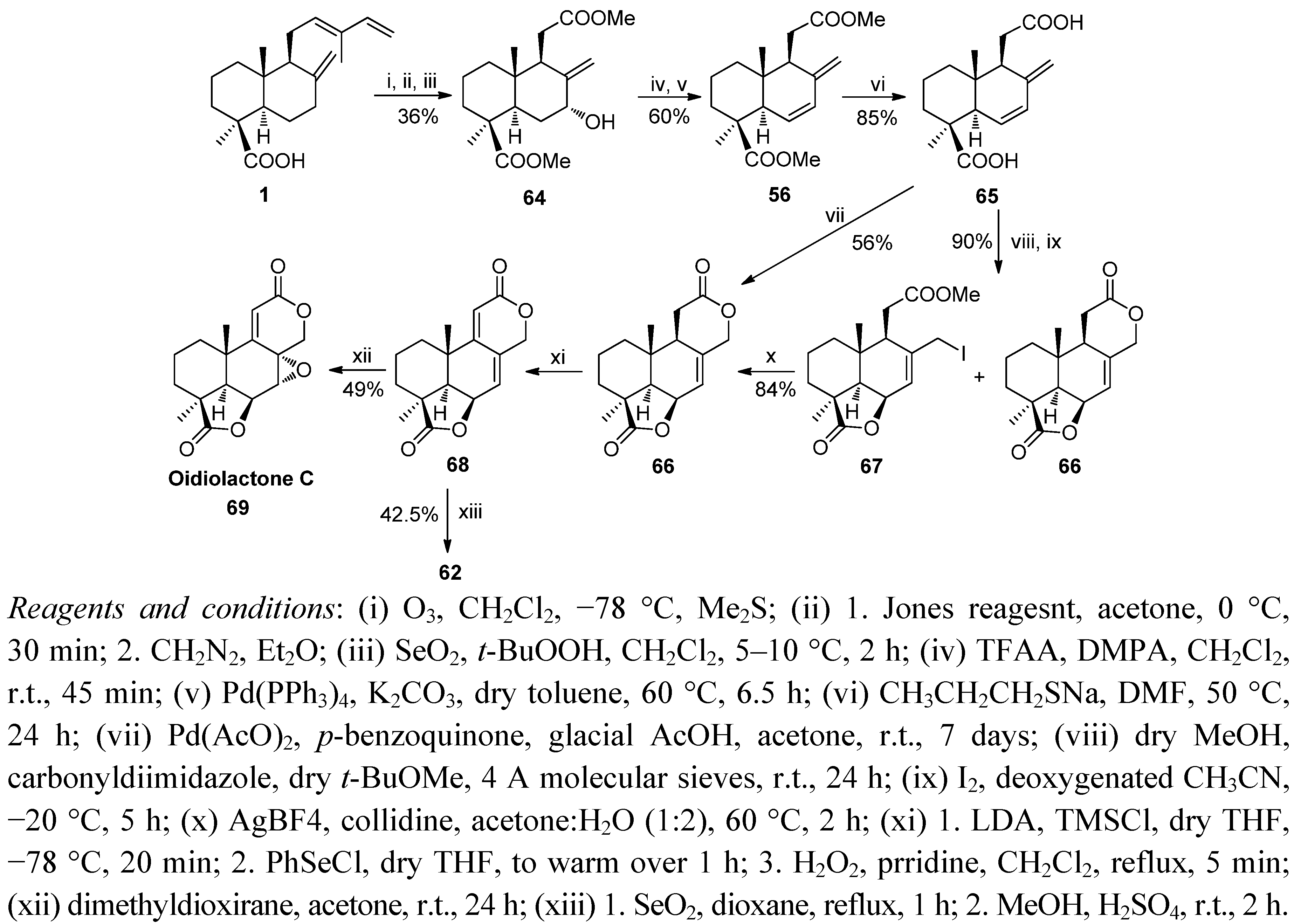
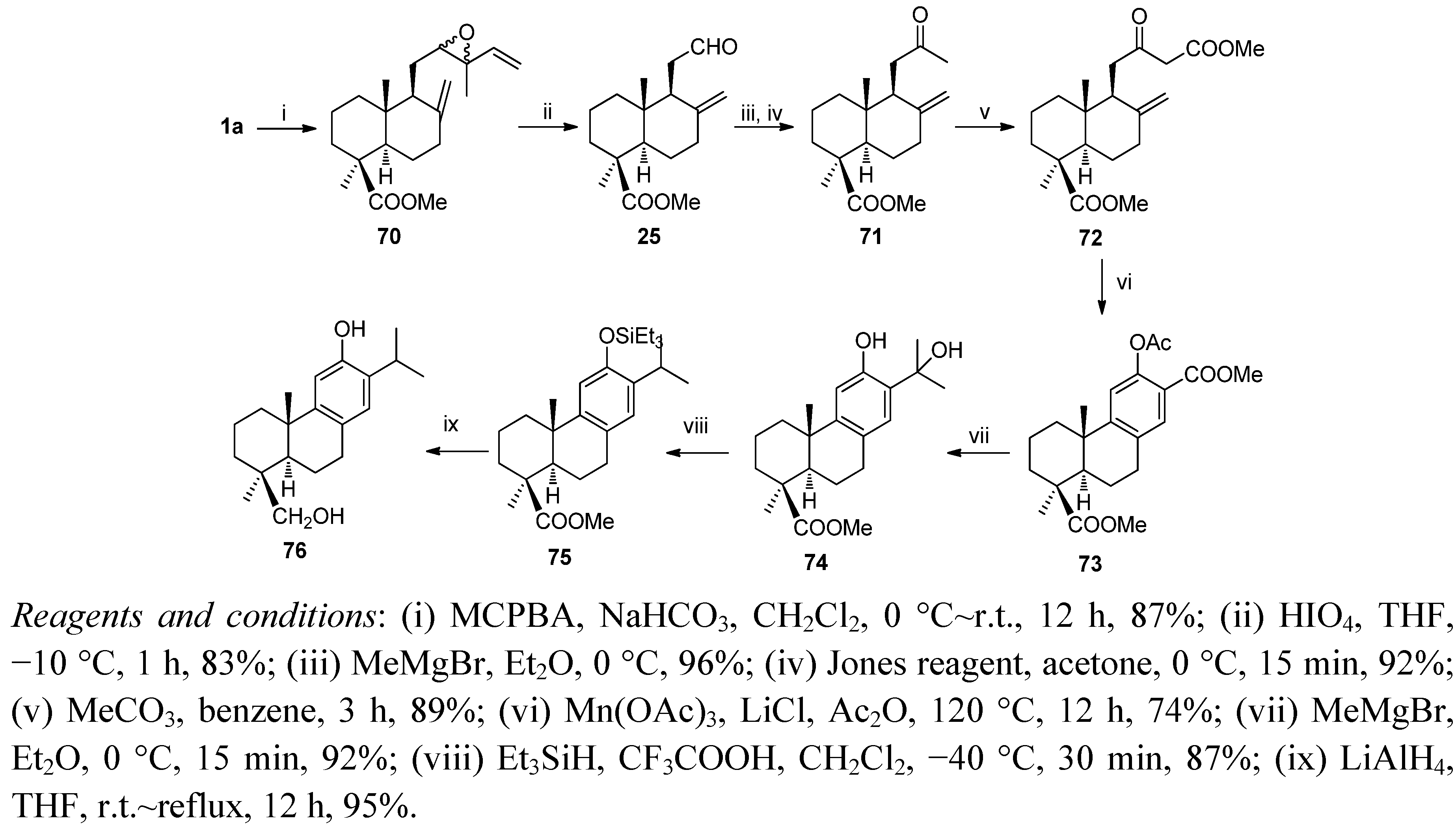

6. Conclusions
References
- Dictionary of Terpenoids; Connolly, J.D.; Hill, R.A. (Eds.) Chapman & Hall: London, UK, 1991; Volume 2.
- Hanson, J.R. Diterpenoids of terrestrial origen. Nat. Prod. Rep. 2011, 28, 1755–1772. [Google Scholar] [CrossRef]
- Peters, R.J. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2004, 21, 312–320. [Google Scholar] [CrossRef]
- Sakar, M.K.; Er, N.; Dilek, E.; Del Olmo, E.; San Feliciano, A. (–)-Desoxypodophyllotoxin and diterpenoids from Juniperus nana Willd. berries. Acta Pharm. Turcica 2002, 44, 213–219. [Google Scholar]
- De Pascual Teresa, J.; San Feliciano, A.; Barrero, A.F. Composition of Juniperus communis (common juniper) fruit. I. An. Quim. 1973, 69, 1065–1067. [Google Scholar]
- De Pascual Teresa, J.; San Feliciano, A.; Miguel del Corral, J.M. Components of Juniperus oxycedrus fruits. An. Quim. 1974, 70, 1015–1019. [Google Scholar]
- Fang, J.-M.; Chen, Y.-C.; Wang, B.-W.; Cheng, Y.-S. Terpenes from heartwood of Juniperus chinensis. Phytochemistry 1996, 41, 1361–1365. [Google Scholar] [CrossRef]
- Fang, J.M.; Sou, Y.C.; Chiu, Y.H.; Cheng, Y.S. Diterpenes from the bark of Juniperus chinensis. Phytochemistry 1993, 34, 1581–1584. [Google Scholar] [CrossRef]
- Chang, C.-I.; Chen, W.-C.; Shao, Y.-Y.; Yeh, G.-R.; Yang, N.-S.; Chiang, W.; Kuo, Y.-H. A new labdane-type diterpene from the bark of Juniperus chinensis Linn. Nat. Prod. Res. 2008, 22, 1158–1162. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quilez del Moral, J.F.; Herrador, M.M.; Akssira, M.; Bennamara, A.; Akkad, S.; Aitigri, M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry 2004, 65, 2507–2515. [Google Scholar] [CrossRef]
- Sakar, M.K.; San Feliciano, A. Diterpenoids of Juniperus foetidissima unripe berries. Fitoterapia 1994, 65, 304–306. [Google Scholar]
- Barrero, A.F.; Sanchez, J.F.; Altarejos, J. Resin acids in the woods of Juniperus sabina L. and Juniperus oxycedrus L. Ars Pharm. 1987, 28, 449–457. [Google Scholar]
- Su, W.-C.; Fang, J.-M.; Cheng, Y.-S. Diterpenoids from leaves of Cryptomeria japonica. Phytochemistry 1996, 41, 255–261. [Google Scholar]
- Wang, Y.-Z.; Tang, C.-P.; Ke, C.-Q.; Weiss, H.-C.; Gesing, E.-R.; Ye, Y. Diterpenoids from the pericarp of Plactycladus orientalis. Phytochemistry 2008, 69, 518–526. [Google Scholar] [CrossRef]
- Fang, S.; Gu, Y.; Yu, H.; Musadillin, S. The chemical nature of antitumor compounds from Sabina vulgaris Ant. Zhiwu Xuebao 1989, 31, 382–388. [Google Scholar]
- Gu, Y.; Xu, Y.; Fang, S.; He, Q. The chemical constituents from Podocarpus imbricatus. Zhiwu Xuebao 1990, 32, 631–636. [Google Scholar]
- Smith, R.M.; Marty, R.A.; Peters, C.F. The diterpene acids in the bled resins of three Pacific kauri, Agathis vitiensis, A. lanceolata, and A. macrophylla. Phytochemistry 1981, 20, 2205–2207. [Google Scholar] [CrossRef]
- Hafez, S.S. New labdane-type diterpenoids from the stem bark of Thuja occidentalis L. J. Pharm. Sci. 2004, 20, 34–47. [Google Scholar]
- Bohlmann, F.; Zdero, C. Naturally occurring terpene derivatives. XXXIII. Constituents of Hermas villosa. Chem. Ber. 1974, 107, 1416–1419. [Google Scholar] [CrossRef]
- Topcu, G.; Erenler, R.; Cakmak, O.; Johansson, C.B.; Celik, C.; Chai, H.-B.; Pezzuto, J.M. Diterpenes from the berries of Juniperus excelsa. Phytochemistry 1999, 50, 1195–1199. [Google Scholar] [CrossRef]
- Tchinda, A.T.; Fuendjiep, V.; Mekonnen, Y.; Ngo, B.B.; Dagne, E. A bioactive diterpene from Entada abyssinica. Nat. Prod. Commun. 2007, 2, 9–12. [Google Scholar]
- Takahashi, K.; Nagahama, S.; Nakashima, T.; Suenaga, H. Chemotaxonomy on the leaf constituents of Thujopsis dolabrata Sieb. et Zucc.-analysis of acidic extracts. Biochem. Syst. Ecol. 2003, 31, 723–738. [Google Scholar] [CrossRef]
- Hasegawa, S.; Hirose, Y. Terpenoids from the seed of Thujopsis dolabrata. Phytochemistry 1981, 20, 508–510. [Google Scholar] [CrossRef]
- Nagahama, S.; Tajima, M.; Nishimura, K. Chemotaxonomy of ate (Thujopsis dolabrata) of Noto, Ishikawa Prefecture. Mokuzai Gakkaishi 1996, 42, 698–702. [Google Scholar]
- Minami, T.; Wada, S.; Tokuda, H.; Tanabe, G.; Muraoka, O.; Tanaka, R. Potential antitumor-promoting diterpenes from the cones of Pinus luchuensis. J. Nat. Prod. 2002, 65, 1921–1923. [Google Scholar] [CrossRef]
- Fukushima, J.; Yatagai, M.; Ohira, T. Abietane-type and labdane-type diterpenoids from the cones of Chamaecyparis obtusa. J. Wood Sci. 2002, 48, 326–330. [Google Scholar] [CrossRef]
- Hanari, N.; Yamamoto, H.; Kuroda, K. Comparison of terpenes in extracts from the resin and the bark of the resinous stem canker of Chamaecyparis obtusa and Thujopsis dolabrata var. hondae. J. Wood Sci. 2002, 48, 56–63. [Google Scholar] [CrossRef]
- Yamamoto, H.; Asano, N.; Sawano, C.; Sone, T.; Gasha, T.; Ono, Y. Diterpenes Isolated from the Resin of the Resinous Stem Canker of Japanese Cypress, Chamaecyparis obtusa. Mokuzai Gakkaishi 1997, 43, 558–565. [Google Scholar]
- Minami, T.; Iwamoto, M.; Ohtsu, H.; Ohishi, H.; Tanaka, R.; Yoshitake, A. Aromatase inhibitory activities of standishinal and the diterpenoids from the bark of Thuja standishii. Planta Med. 2002, 68, 742–745. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ohtsu, H.; Tokuda, H.; Nishino, H.; Matsunaga, S.; Tanaka, R. Anti-tumor promoting diterpenes from the stem bark of Thuja standishii (Cupressaceae). Bioorg. Med. Chem. 2001, 9, 1911–1921. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Ferreira, A.J.S.; Sartorelli, P.; Lopes, N.P.; Floh, E.I.S.; Handro, W.; Kato, M.J. Phenylpropanoid derivatives and biflavones at different stages of differentiation and development of Araucaria angustifolia. Phytochemistry 2000, 55, 575–580. [Google Scholar]
- Lin, T.-C.; Fang, J.-M.; Cheng, Y.-S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar] [CrossRef]
- Bungert, M.; Gabler, J.; Adam, K.-P.; Zapp, J.; Becker, H. Pinguisane sesquiterpenes from the liverwort Porella navicularis. Phytochemistry 1998, 49, 1079–1083. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. Labdane diterpenes in Curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferatio. Food Chem. 2011, 124, 527–532. [Google Scholar] [CrossRef]
- Sakar, M.K. Diterpenes from ripe “fruits” of Juniperus drupaceae Labill. Acta Pharm. Turcica 1987, 29, 65–68. [Google Scholar]
- Hasegawa, S.; Hirose, Y. Diterpenes from the seed of Sciadopitys verticillata. Phytochemistry 1985, 24, 2041–2046. [Google Scholar] [CrossRef]
- Kitajima, J.; Komori, T.; Kawasaki, T. Studies on the constituents of the crude drug “Fritillariae Bulbus.” III. On the diterpenoid constituents of fresh bulbs of Fritillaria thunbergii Miq. Chem. Pharm. Bull. 1982, 30, 3912–3921. [Google Scholar] [CrossRef]
- Ding, T.; Liu, H.; Pu, Q. Studies on the resin constituents of Taxodiaceae. I. Sesquiterpene and diterpene components of the resin from Cunninghamia unicanaliculata var. pyramidalis Yunnan Zhiwu Yanjiu 1982, 4, 307–311. [Google Scholar]
- Bohlmann, F.; Fiedler, L. Naturally occurring terpene derivatives. Part 131. A new communic acid derivative from Chromolaena collina (DC) K. et R. J. Indian Chem. Soc. 1978, 55, 1161–1162. [Google Scholar]
- Alvarez-Manzaneda Roldan, E.; Chahboun, R. Span. Procedure for isolation of trans-communic acid and derivatives from Cupressus sempervirens. Patent ES 2284341 A1 20071101, 2284. [Google Scholar]
- Rawat, P.; Khan, M.F.; Kumar, M.; Tamarkar, A.K.; Srivastava, A.K.; Arya, K.R.; Maurya, R. Constituents from fruits of Cupressus sempervirens. Fitoterapia 2010, 81, 162–166. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; Barrero, A.F.; Muriel, L.; San Feliciano, A.; Grande, M. New natural diterpene acids from Juniperus communis. Phytochemistry 1980, 19, 1153–1156. [Google Scholar]
- Xiao, Z.-Y.; Wang, X.-C.; Zhang, G.-P.; Huang, Z.-L.; Hu, L.-H. Terpenoids from roots of Chloranthus spicatus. Helv. Chim. Acta 2010, 93, 803–810. [Google Scholar] [CrossRef]
- Wang, W.; Ba, H.; Hajia, A.; Liao, L.; Duo, L. Study on chemical constituents of Sabina vulgaris Antoine. Tianran Chanwu Yanjiu Yu Kaifa 2005, 17, 588–591. [Google Scholar]
- Chen, R.; Zhang, Y.; Fang, S. Inhibitors of human DNA polymerase β isolated from Jack Rorreya (Torreya jackii). Zhongcaoyao 1997, 28, 707–710. [Google Scholar]
- Xu, Y.; Fang, S.; He, Q. The chemical constituents in Dacrydium pierrei. Zhiwu Xuebao 1991, 33, 646–648. [Google Scholar]
- Dawidar, A.M.; Ezmirly, S.T.; Abdel-Mogib, M. Sesquiterpenes and diterpenes from Juniperus phoenicea L. Pharmazie 1991, 46, 472–473. [Google Scholar]
- Lee, G.H.; Lin, C.C.; Cheng, Y.S.; Peng, S.M. Structure of methyl trans-communate. Acta Crystallogr. C Cryst. Str. 1987, C43, 1382–1384. [Google Scholar]
- Bohlmann, F.; Dhar, A.K.; Jakupovic, J.; King, R.M.; Robinson, H. A caryophyllene derivative from Fleischmannia pycnocephaloides. Phytochemistry 1981, 20, 1425–1426. [Google Scholar]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Litaudon, M.; Bousserouel, H.; Martin, M.-T.; Thoison, O.; Leonce, S.; Dumontet, V.; Sevenet, T.; Gueritte, F. Sesquiterpenoids and Cytotoxic Lignans from the Bark of Libocedrus chevalieri. J. Nat. Prod. 2007, 70, 1368–1370. [Google Scholar] [CrossRef]
- Sultan, M.Z.; Jeon, Y.-M.; Moon, S.-S. Labdane-type diterpenes active against acne from pine cones (Pinus densiflora). Planta Med. 2008, 74, 449–452. [Google Scholar] [CrossRef]
- Mendes, C.C.; Cruz, F.G.; Guedes, M.L.S.; Roque, N.F. Terpenes from Mikania aff. jeffreyi (Asteraceae). Zeitschrift Fuer Naturforschung B Chem. Sci. 2005, 60, 875–879. [Google Scholar]
- Smith, E.C.J.; Williamson, E.M.; Wareham, N.; Kaatz, G.W.; Gibbons, S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry 2007, 68, 210–217. [Google Scholar]
- Shmidt, E.N.; Pentegova, V.A. Chemical composition of Larix dahurica soft resin. Khimiya Prirodnykh Soedinenii 1974, 675–676. [Google Scholar]
- Hsieh, Y.-L.; Fang, J.-M.; Cheng, Y.-S. Terpenoids and flavonoids from Pseudotsuga wilsoniana. Phytochemistry 1998, 47, 845–850. [Google Scholar]
- Yoshikawa, K.; Kokudo, N.; Tanaka, M.; Nakano, T.; Shibata, H.; Aragaki, N.; Higuchi, T.; Hashimoto, T. Novel abietane diterpenoids and aromatic compounds from Cladonia rangiferina and their antimicrobial activity against antibiotics resistant bacteria. Chem. Pharm. Bull. 2008, 56, 89–92. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; San Feliciano, A.; Miguel del Corral, M.J. Components of Juniperus oxycedrus berries. I. An. Quim. 1972, 68, 1061–1062. [Google Scholar]
- Zhou, X.; Cheng, C.; Roshchin, V.I. Study on the chemical composition of substances extracted from the green crown of Cunninghamia lanceolata Hook. Linchan Huaxue Yu Gongye 1997, 17, 55–60. [Google Scholar]
- Carman, R.M.; Cowley, D.E. Diterpenoids. XII. Dundatholic acid. Aust. J. Chem. 1967, 20, 193–196. [Google Scholar] [CrossRef]
- Carman, R.M.; Cowley, D.E.; Marty, R.A. Diterpenoids. XXV. Dundathic acid and polycommunic acid. Aust. J. Chem. 1970, 23, 1655–1665. [Google Scholar] [CrossRef]
- Scalarone, D.; van der Horst, J.; Boon, J.J.; Chiantore, O. Direct-temperature mass spectrometric detection of volatile terpenoids and natural terpenoid polymers in fresh and artificially aged resins. J. Mass Spectrom. 2003, 38, 607–617. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Sabatini, A.G. A new type of European propolis, containing bioactive labdanes. Rivista Italiana EPPOS 2003, 36, 3–7. [Google Scholar]
- Hafez, S.S. New labdane-type diterpenoids from the stem bark of Thuja occidentalis L. J. Pharm. Sci. 2004, 20, 34–47. [Google Scholar]
- Muhammad, I.; Mossa, J.S.; Al-Yahya, M.A.; Ramadan, A.F.; El-Feraly, F.S. Further antibacterial diterpenes from the bark and leaves of Juniperus procera Hochst. ex Endl. Phytother. Res. 1995, 9, 584–588. [Google Scholar] [CrossRef]
- Samoylenko, V.; Dunbar, D.C.; Gafur, M.A.; Khan, S.I.; Ross, S.A.; Mossa, Jaber S.; El-Feraly, F.S.; Tekwani, B.L.; Bosselaers, J.; Muhammad, I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother. Res. 2008, 22, 1570–1576. [Google Scholar] [CrossRef]
- Hirayama, T.; Someya, K.; Kuroyanagi, M.; Nakane, T. Natural antibacterial agents containing labdatrienoic acid and oral hygiene compositions containing them. Jpn. Kokai Tokkyo Koho 2009, JP 2009023920 A 20090205. [Google Scholar]
- Jeon, Y.M.; Moon, S.S.; Lee, T.H.; Cho, S.C.; Yim, B.K. Extract of pine cone having antibacterial and antifungal activity against particularly bacteria causing acne or athlete's foot, and cosmetic composition for improving skin disease without side effects on skin comprising the same or compound isolated from the same. Repub. Korean Kongkae Taeho Kongbo 2006, KR 2006104161 A 20061009. [Google Scholar]
- Xue, J.-J.; Fan, C.-Q.; Dong, L.; Yang, S.-P.; Yue, J.-M. Novel antibacterial diterpenoids from Larix chinensis beissn. Chem. Biodiver. 2004, 1, 1702–1707. [Google Scholar] [CrossRef]
- Perry, N.B.; Foster, L.M. Antitumor lignans and cytotoxic resin acids from a New Zealand gymnosperm, Libocedrus plumosa. Phytomedicine 1994, 1, 233–237. [Google Scholar] [CrossRef]
- Gordien, A.Y.; Gray, A.I.; Franzblau, S.G.; Seidel, V. Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae). J. Ethnopharmacol. 2009, 126, 500–505. [Google Scholar] [CrossRef]
- Tanaka, R.; Ohtsu, H.; Iwamoto, M.; Minami, T.; Tokuda, H.; Nishino, H.; Matsunaga, S.; Yoshitake, A. Cancer chemopreventive agents, labdane diterpenoids from the stem bark of Thuja standishii (Gord.) Carr. Cancer Lett. 2000, 161, 165–170. [Google Scholar] [CrossRef]
- Ribeiro, L.A.A.; Tavares, J.F.; de Andrade, N.C.; da Silva, M.S.; da Silva, B.A. The (8)17,12E,14-labdatrien-18-oic acid (labdane 302), a labdane-type diterpene isolated from Xylopia langsdorffiana St. Hil. & Tul. (Annonaceae), relaxes the guinea pig trachea. Revista Brasileira de Farmacognosia 2007, 17, 197–203. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, W.-K. Hypolipidemic effects of Korean softwood components. Han'guk Sikp'um Yongyang Kwahak Hoechi 2001, 30, 1204–1214. [Google Scholar]
- Ko, J.H.; Hwang, E.I.; Lee, K.W.; Choi, K.H. Testosterone 5α-reductase inhibitor for preventing and treating diseases associated with testosterone 5α-reductase comprising extract of pine needle or compounds isolated therefrom. Repub. Korean Kongkae Taeho Kongbo 2006, KR 2006067255 A 20060619. [Google Scholar]
- Shimizu, M.; Tsuji, H.; Shogawa, H.; Fukumura, H.; Tanaami, S.; Hayashi, T.; Arisawa, M.; Morita, N. Anti-inflammatory constituents of topically applied crude drugs. II. Constituents and anti-inflammatory effect of Cryptomeria japonica D. Don. Chem. Pharm. Bull. 1988, 36, 3967–3973. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; San Feliciano, A.; Miguel del Corral, J.M.; Barrero, A.F. Transformaciones químicas en la cadena lateral de los ácidos comúnicos. 1. Epoxidaciones. Studia Chemica 1984, 9, 255–267. [Google Scholar]
- De Pascual Teresa, J.; Mateos, A.F.; Barrero, A.F.; Pollos, P. Adiciones de oxígeno singlete a mirceocomunato de metilo y trans-comunato de metilo. Síntesis de lambertianato de metilo. Stud. Chem. 1984, 9, 49–56. [Google Scholar]
- Schulte-Elte, K.H.; Muller, B.L.; Rautenstrauch, V. Preference for syn ene additions of 1O2 to trisubstituted, acyclic olefins. Helv. Chim. Acta 1978, 61, 2777–2783. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sánchez, J.F.; Altarejos, J. Biomimetic Cyclization of Communic Acids to Pimarane Skeleton Via Organomercurial Intermediates. Tetrahedron Lett. 1988, 29, 3713–3716. [Google Scholar]
- Barrero, A.F.; Ramírez, A.; Salido, S.; Altarejos, J. Oxymercuriation-demedrcuriation reactions of methyl myrceocommunate. An. Quim. 1990, 86, 786–790. [Google Scholar]
- Barrero, A.F.; Sánchez, J.F.; Altarejos, J.; Perales, A. Oxymercuriation-demercuriation of the methyl esters of communic acids. X-ray molecular structures of methyl (8R,12R)-8,12-epoxyisopimar-15-en-19-oate. J. Chem. Soc. Perkin Trans. 1 1991, 2513–2523. [Google Scholar]
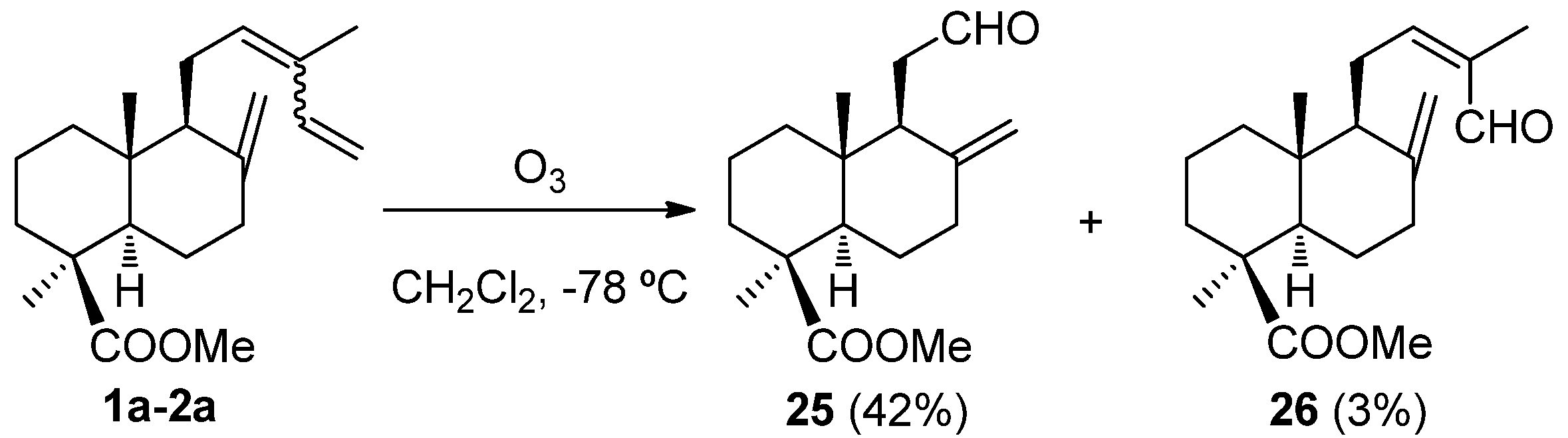
- Barrero, A.F.; Sánchez, J.F.; Altarejos, J. Selective ozonolysis of methyl trans-communate. Synthesis of drimanes. Tetrahedron Lett. 1989, 30, 5515–5518. [Google Scholar] [CrossRef]
- Barrero, A.F.; Alvarea-Manzaneda, E.J.; Altarejos, J.; Ramos, J.M.; Salido, S. Preferential oxidation reactions of the side chain of unsaturated labdanes. Bull. Soc. Chim. Fr. 1993, 130, 700–707. [Google Scholar]
- Barrero, A.F.; Altarejos, J.; Alvarea-Manzaneda, E.J.; Ramos, J.M.; Salido, S. Synthesis of ambrox from communic acids. Tetrahedron 1993, 49, 6251–6262. [Google Scholar]
- Barrero, A.F.; Altarejos, J.; Alvarea-Manzaneda, E.J.; Ramos, J.M.; Salido, S. Amber-type odorants from communic acids. Tetrahedron 1993, 49, 9525–9534. [Google Scholar] [CrossRef]
- Barrero, A.F.; Alvarez-Manzaneda, E.J.; Alvarez-Manzaneda, R.; Chahboun, R.; Meneses, R.; Cuerva, J.M.; Aparicio, M.; Romera, J.L. Approach to the synthesis of antitumor quassinoids from labdane diterpenes: An efficient synthesis of a picrasane-related intermediate. Org. Lett. 2001, 3, 647–650. [Google Scholar] [CrossRef]
- Dictionary of Terpenoids; Connolly, J.D.; Hill, R.A. (Eds.) Chapman-Hall: London, UK, 1991; Volume 2, pp. 853–857.
- Andersen, N.R.; Rasmunsen, P.R.; Falshaw, C.P.; King, T.J. The relative and absolute configuration of clerocidin and its cometabolites. Tetrahedron Lett. 1984, 25, 469–472. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Springer, J.P.; Cox, R.H.; Cutler, H.; Wicklow, D.T. Isolation and identification of two new biologically active norditerpene dilactones from Aspergillus wentii. Phytochemistry 1980, 19, 1157–1161. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H.; Kunstmann, M.P.; Lancaster, J.E.; Morton, G.O. Structure and chemistry of antibiotic LL-Z1271α, an antifungal carbon-17 terpene. J. Am. Chem. Soc. 1970, 92, 5483–5489. [Google Scholar] [CrossRef]
- Hosoe, T.; Nozawa, K.; Lumley, T.C.; Currah, R.S.; Fukushima, K.; Takizawa, K.; Miyaji, M.; Kawai, K. Tetranorditerpene lactones, potent antifungal antibiotics for human pathogenic yeasts, from a unique species of Oidiodendron. Chem. Pharm. Bull. 1999, 47, 1591–1597. [Google Scholar] [CrossRef]
- Ichikawa, K.; Ikunaka, M.; Kojima, N.; Nishida, H.; Yoshikawa, N. Terpenoid lactone compounds and their production process. 1999, EP 0 933 273 A1. [Google Scholar]
- Barrero, A.F.; Sánchez, J.F.; Elmerabet, J.; Jiménez-González, D.; Macías, F.A.; Simonet, A.M. Enantiospecific syntheses of the potent bioactives Nagilactone F and the mould metabolite LL-Z1271α. An evaluation of their allelopathic potential. Tetrahedron 1999, 55, 7289–7304. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quilez del Moral, J.F.; Cuerva, J.M.; Cabrera, E.; Jimenez-González, D. preparation of bioactive podolactones via a new Pd-catalysed bislactonisation reaction. Synthesis of oidiolactone C. Tetrahedron Lett. 2000, 41, 5203–5206. [Google Scholar] [CrossRef]
- Barrero, A.F.; Arseniyadis, S.; Quilez del Moral, J.F.; Herrador, M.M.; Valdivia, M.; Jimenez, D. First synthesis of the antifungal oidiolactone C from trans-communic acid: Cytotoxic and antimicrobial activity in podolactone-related compounds. J. Org. Chem. 2002, 67, 2501–2508. [Google Scholar] [CrossRef]
- Cambie, R.C.; Cox, R.E.; Sidwell, D. Phenolic diterpenoids of Podocarpus ferrugineus and other podocarps. Phytochemistry 1984, 23, 333–336. [Google Scholar] [CrossRef]
- Arihara, S.; Umeyama, A.; Bando, S.; Imoto, S.; Ono, M.; Tani, M.; Yoshikawa, K. A new abietane and two dimeric abietane diterpenes from the black heartwood of Cryptomeria japonica. Chem. Pharm. Bull. 2004, 52, 354–358. [Google Scholar] [CrossRef]
- Alvarez-Manzaneda, E.; Chahboun, R.; Cabrera, E.; Alvarez, E.; Alvarez-Manzaneda, R.; Lachkar, M.; Messouri, I. Synthesis of phenol abietane diterpenes based on the oxidative radical cyclization utilizing the Mn(OAc)3/Ac2O system. Synlett 2007, 2425–2429. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Arteaga, J.F.; Arteaga, A.F. Communic Acids: Occurrence, Properties and Use as Chirons for the Synthesis of Bioactive Compounds. Molecules 2012, 17, 1448-1467. https://doi.org/10.3390/molecules17021448
Barrero AF, Herrador MM, Arteaga P, Arteaga JF, Arteaga AF. Communic Acids: Occurrence, Properties and Use as Chirons for the Synthesis of Bioactive Compounds. Molecules. 2012; 17(2):1448-1467. https://doi.org/10.3390/molecules17021448
Chicago/Turabian StyleBarrero, Alejandro F., M. Mar Herrador, Pilar Arteaga, Jesús F. Arteaga, and Alejandro F. Arteaga. 2012. "Communic Acids: Occurrence, Properties and Use as Chirons for the Synthesis of Bioactive Compounds" Molecules 17, no. 2: 1448-1467. https://doi.org/10.3390/molecules17021448
APA StyleBarrero, A. F., Herrador, M. M., Arteaga, P., Arteaga, J. F., & Arteaga, A. F. (2012). Communic Acids: Occurrence, Properties and Use as Chirons for the Synthesis of Bioactive Compounds. Molecules, 17(2), 1448-1467. https://doi.org/10.3390/molecules17021448