Abstract
Aqueous infusions of the leaves of the shrub Albertisia delagoensis (Menispermaceae) are used in South Africa in traditional Zulu medicine to alleviate a variety of symptoms, including fever, and intestinal problems. We report the analysis of such an aqueous extract using the HPLC-NMR technique. A number of polar compounds were identified, including proto-quercitol, nicotinic acid, allantoic acid, 3,4-dihydroxy-benzoic acid, phthalic acid and the aporphine alkaloid derivative roemrefidine. Allantoic acid and roemrefidine have been fully characterised by 1H- and 13C-NMR and mass spectrometry. Earlier reports of antiplasmodial activity of roemrefidine and of A. delagoensis extracts are correlated with this study and with the antipyretic properties of neutral aqueous extracts.
1. Introduction
Albertisia delagoensis N.E. Br. Forman [=Epinetrum delagoensis] is a suffrutescent of the Menispermaceae family found in Mozambique and north-eastern KwaZulu-Natal, South Africa. A number of Albertisia species are used medicinally throughout tropical and sub-tropical Africa [1], however A. delagoensis is the only species found in southern Africa, and the leaves are reportedly used as an antipyretic [2], antidiarrhoeal and antiemetic [3,4] and the roots are used for menstrual pain, sexual performance in men, chest pain and back pain, among other uses [3,4,5]. An early phytochemical report on A. delagoensis [6] described the isolation of three alkaloids from the acidified ethanolic root bark extracts, and reported by mass spectrometry m/z (M+) values and molecular formulae for the three compounds. On the basis of the mass spectrometry fragmentation patterns it was suggested that all three belonged to the bis-benzylisoquinoline class of alkaloids, but chemical structures were not suggested. The alkaloids were tested [6,7] for cytotoxicity on continuous cell lines (VERO cells) and found to be highly active. More recently the isolation of three alkaloids, cocsoline, cocsuline and O-methylcocsoline from dilute sulphuric acid extracts of the rhizomes of A. delagoensis, and an additional two alkaloids, cycleanine and dicentrine, from similar extracts of the leaves was reported [3]. Subsequently De Wet et al. [4] reported that methanol extracts of the dried, milled rhizomes and leaves exhibited a high level of antiparasitic activity on the chloroquine-resistant Gambian FCR-3 strain of Plasmodium falciparum, but the same extracts showed low level cytotoxicity against Graham cells (transformed human kidney epithelium cells). Crude alkaloidal extracts of A. Delagoensis leaves showed a high level of cytotoxicity against selected breast, melanoma and renal cancer cell lines [8]. However the above studies, identifying antiplasmodial activity and cytotoxicity used compounds extracted into acidified aqueous or alcoholic solution, whereas in traditional medicine in southern Africa it is the neutral aqueous extracts of roots or leaves that are drunk. In the present study we sought to analyse leaf extracts obtained in this traditional manner, and did not seek to target only alkaloids as had been the case in previous studies.
The crude extract mixture was inspected by the hyphenated HPLC-DAD-NMR method, in an on-flow experiment where the 1H-NMR spectrometer acted as a detector identifying components of the mixture which were present in sufficient quantity to merit more detailed structural analysis by 2-D NMR methods. Those selected components were then separated and accumulated in multiple HPLC-DAD runs with the selected eluent collected in loops (the loop storage method). The HPLC-DAD-NMR and HPLC-DAD-loop storage are among an array of powerful hyphenated analytical methods employing NMR for molecular structure determination, and have been recently and extensively reviewed [9,10,11].
2. Results and Discussion
The chromatograms (HPLC conditions as for the loop storage method) of the extract showed eight peaks which eluted within the first 25 min, were well resolved and symmetric. In addition there was a broad peak (with tailing) with retention time 28.5 min.
The on-flow HPLC-NMR experiment on the extract showed five components of the mixture gave sufficiently strong 1H-NMR spectra that it was considered useful to isolate these components in sufficient quantity to enable 2-D NMR experiments for structural elucidation. These five peaks in the chromatogram corresponded to four of the eight well-resolved peaks (retention times 2.0, 7.0, 13.2 and 16.8 min) and the broad (tailing) peak (retention time 28.5 min) identified in the shorter (40 min) run (HPLC conditions for loop storage). The collection and accumulation of these five fractions in capillary loops is described in Section 3.4 below.
The 1H 1-D NMR spectra of the 2.0 min fraction indicated the presence of a major and two minor components. The 1-D 13C spectrum showed six signals, five to higher frequency (71.8, 72.2, 74.5, 75.7 and 78.0 δ), and one at 36.8 δ. The COSY, TOCSY and HSQC spectra are all consistent with this major component being a pentahydroxycyclohexane, and the 1H- and 13C-NMR data are in agreement with those reported [12] for proto-quercitol (2, see Figure 1). The two minor components were identified as nicotinic acid [13] and allantoic acid (1, see Figure 1).
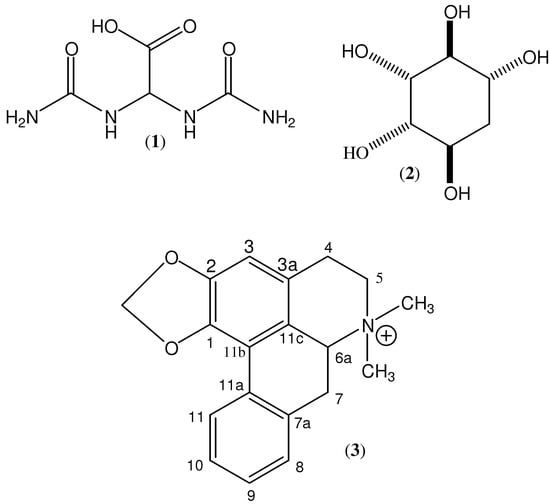
Figure 1.
Chemical structures of allantoic acid (1), proto-quercitol (2), and roemrefidine (3).
The 1H- and 13C-NMR spectra of the 7.0 and 13.2 min fractions showed these to be 3,4-dihydroxy-benzoic acid and phthalic acid, respectively, and these were confirmed by comparison with the spectra of authentic samples (see also [14]).
The 1H, and 13C-NMR spectra of the 16.8 min fraction showed this to be a mixture of two components, both aromatic in nature. The major component gave 1H-NMR signals at δ 6.96 (d, J = 8.3 Hz), δ 7.5 (d,d J = 1.9, 8.3 Hz) and δ 7.73 (d, J = 1.9 Hz). A combination of HSQC and HMBC spectra gave 13C-H signals at δ 121.1, 126.6 and 117.4 and non-protonated 13C signals at δ 134.5, 148.9, 153.7 and 174.8. Similarly the minor component gave 1H signals at δ 6.65 (d, J = 8.3 Hz), δ 7.02 (d, J = 1.8 Hz) and δ 7.22 (d,d J = 1.8, 8.3 Hz), with corresponding 13C shifts measured from the HSQC spectrum at δ 117.7, 117.3 and 125.8. There was insufficient signal/noise in the HMBC spectrum to give information on the non-protonated 13C shifts. However these data were not sufficient to assign structures.
The 1H- and 13C-NMR spectra of the 28.5 min fraction indicated a structure more complex than any of the above. An accurate m/z value of 294.1503 was measured; the molecular formula C19H20NO2 has calculated mass 294.1489, i.e., Δ = 4.8 ppm. Consideration of all available 1-D and 2-D NMR data led to the candidate structure 3 (see Figure 1). This was identified as roemrefidine, which has been reported previously [15], and is the N-methyl derivative of the alkaloid roemerine.
The 1H and 13C chemical shifts determined here for roemrefidine are given in Table 1. The 3-bond (vicinal) 1H-1H coupling constants between the protons at positions 4 and 5, and positions 6 and 7 fall into two ranges; 3.9 to 5.8 Hz, and 13.0 to 14.0 Hz. The smaller coupling range is typical for C-H bond dihedral angles ca. 60° or ca. 120°, whereas the larger coupling constants are typical for dihedral angles nearer 180°.

Table 1.
1H- and 13C-NMR data for roemrefidine (3) a.
| Position | δ 1H | δ 13C b |
|---|---|---|
| 1 | 143.3 c | |
| 2 | 147.6 c | |
| 3 | 6.82 (s) | 106.8 |
| 3a | 122.6 | |
| 4 (Ψe) | 3.05 (d,d J = 4.5, 18.0 Hz) | 23.1 |
| 4 (Ψa) | 3.32 (d,d,d J = 5.8, 13.0, 18.0 Hz) | |
| 5 (Ψa) | 3.67 (d,t J = 4.5, 13.0 Hz) | 61.2 |
| 5 (Ψe) | 3.76 (d,d J = 5.8, 13.0 Hz) | |
| 6 (Ψa) | 4.64 (d,d J = 3.9, 14.0 Hz) | 68.2 |
| 7 (Ψa) | 3.09 (t J = 14.0 Hz) | 28.1 |
| 7 (Ψe) | 3.44 (d,d J = 3.9, 14.0 Hz) | |
| 7a | 130.5 | |
| 8 | 7.43 (d J = 7.4 Hz) | 128.0 |
| 9 | 7.39 (t J = 7.4 Hz) | 128.4 |
| 10 | 7.43 (t J = 7.4 Hz) | 128.0 |
| 11 | 8.12 (d J = 7.4 Hz) | 126.2 |
| 11a | 130.4 | |
| 11b | 115.3 | |
| 11c | 118.5 | |
| OCH2O | 6.07 (d J = 0.8 Hz) | 101.3 |
| 6.20 (d J = 0.8 Hz) | ||
| CH3 (Ψa) | 3.05 (s) | 42.4 |
| (Ψe) | 3.38 (s) | 53.0 |
a Solution in CD3CN-D2O (1:1); b Measured from HSQC and HMBC spectra, and values are ± 0.3 δ; c These two assignments may be interchanged.
Accordingly an energy minimized structure for roemrefidine was generated (see section 3.8) and this is shown in Figure 2. Relative to the average plane of the molecule it is clear that the protons at positions 4,5,6 and 7 can be assigned as pseudo-axial (Ψa) or pseudo-equatorial (Ψe), and on this basis the smaller vicinal coupling constants are assigned to Ψe/Ψe interactions or to Ψa/Ψe interactions, and the larger vicinal coupling constants to Ψa/Ψa interactions.
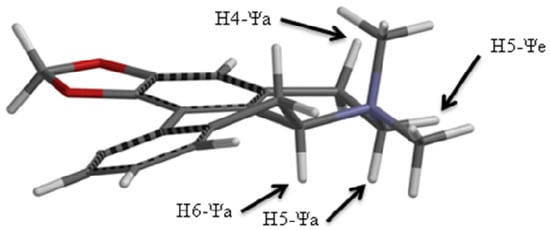
Figure 2.
Spartan energy minimized structure of roemrefidine.
The methyl signals were assigned as CH3 (Ψa) and CH3 (Ψe) since the NOESY spectrum showed a strong correlation between the CH3 signal at 3.38 δ and 4.64 δ (H6 Ψa), but only a very weak correlation between 3.05 δ and 4.64 δ. This indicates the methyl group giving the signal at 3.38 δ is closer in space to the H6 (Ψa) proton, which is the case for the Ψe methyl group (see Figure 2).
3. Experimental
3.1. Plant Material
Samples of the leaves of Albertisia delagoensis were collected in Tembe Elephant Park, Sihangwane [2732 AB], South Africa, in June 2005 and a voucher specimen [De Wet and SJ Siebert 100 (ZULU)] was deposited in the herbarium of the University of Zululand for verification purposes.
3.2. Extraction
The leaves were air-dried and powdered in a mill. Powdered leaf sample (10 g) was added to distilled water (100 mL), heated under reflux for one hour, cooled, filtered and lyophilised, yielding a brown powder (876 mg).
3.3. HPLC-DAD-on-flow-NMR
The hyphenated HPLC-DAD-NMR system consisted of an Agilent 1100 HPLC equipped with a column oven, a Bruker u.v. diode array detector (DAD) in the range 200 to 700 nm, a Bruker 36 place capillary loop storage device (BPSU36), and a Bruker DRX 600 NMR spectrometer with 3 mm cryogenic flow probe. The system was controlled with the Bruker HyStar2.1 software.
HPLC conditions: A Thermo Hypersil (DBS) C18 column (5 μm, 150 × 4.6 mm) with an oven temperature 305 K. The gradient elution used D2O and CD3CN both containing 0.05% DCOOD, started with 98:2 (v/v) D2O-CD3CN 98:2, and linearly changed to 46:54 over 267 min, then was held at 46:54 for 33 min. Flow rate: 0.15 mL/min; Injection volume 100 μL; Sample concentration: a sample of the leaf extract (63 mg) was dissolved in D2O-CD3CN-DCOOD 98:2:0.05 (1 mL). The solution was filtered (0.45 μm filter with centrifugation at 10,000 g for 10 min), and the clear supernatant collected. DAD conditions: full range 200 to 700 nm. Alternative conditions used the same solvent composition but with the linear variation over 800 min, then held for 100 min. Flow rate: 0.05 mL/min.
3.4. HPLC-DAD-loop Storage
The HPLC-DAD, column and temperature, and DAD conditions were the same as used in the on-flow NMR experiments (see above).
HPLC conditions: Gradient elution used H2O and CH3CN both containing 0.1% HCOOH and the same solvent compositions were used as for the on-flow experiments. The linear variation of composition was over 40 min, then held for 5 min. Flow rate: 1 mL/min; Injection volume 20 μL; Sample concentration: as prepared for SPE (see above), but using normal (non-deuterated) solvents with 0.1% HCOOH.
The fractions for the specified peaks in the chromatogram were stored in separate loops. The experiment was repeated 6 times, and the corresponding fractions were combined. Each combined fraction was lyophilised, redissolved in D2O-CD3CN 1:1 (600 μL) containing 0.05% TSP (see below) and transferred to a 5 mm o.d. NMR tube for NMR measurements.
3.5. NMR Spectroscopy
Off-line NMR measurements used a Bruker DRX 600 spectrometer with a standard 5 mm 1H/13C inverse mode probe (1H-NMR at 600 MHz, 13C-NMR at 151 MHz). The residual 1H solvent signals from deuterated water and acetonitrile were suppressed using the WET method [16] with 13C decoupling. All spectra were measured at 292 K. The 2-D NMR experiments used were homonuclear 1H COSY, NOESY and TOCSY, and heteronuclear 1H-13C HSQC and HMBC, and these techniques have been summarised by Braun et al. [17].
3.6. Molecular Modelling
The software used for molecular modelling was Spartan ’10 from Wavefunction Inc. (Irvine, CA, USA).
3.7. Mass Spectrometery
The accurate m/z value was measured by the King’s College, University of London, Mass Spectrometry service using a Bruker Apex III system with electrospray ionisation from methanol.
3.8. Allantoic Acid
An authentic sample of allantoic acid (1) was prepared by alkaline hydrolysis of allantoin, as described by Behrend and Schultz [18]. 1H-NMR (DMSO-d6) δ: 5.20 (1H, t, J = 7.9 Hz, CH), 5.77 (4H, br s, NH2), 6.78 (2H, d, J = 7.9 Hz, NH). 13C-NMR (DMSO-d6) δ: 57.8 (CH), 157.9 (CONH2), 171.3 (CO2H).
4. Conclusions
HPLC-NMR analysis of the neutral aqueous extract of the leaves of Albertisia delagoensis identified the compounds present including proto-quercitol, nicotinic acid, allantoic acid, 3,4-dihydroxybenzoic acid, phthalic acid, and the aporphine alkaloid derivative roemrefidine. The 1H- and 13C-NMR spectroscopic data for allantoic acid and roemrefidine are reported for the first time. In addition molecular modelling of the roemrefidine structure allowed stereospecific assignment of the 1H resonances by comparison with vicinal coupling constants measured from the 1H spectrum.
Munoz et al. [19] showed that roemrefidine, isolated from the stem bark of Sparattanthelium amazonum Martius (Hernandiaceae), is active against the malaria parasite Plasmodium falciparumin vitro and against P. berghei in mice, but showed no cytotoxic activity against the cell lines KB, HEp-2, and HeLa. It is aqueous extracts of the leaves of A. delagoensis that are reported [2] to be used in traditional medicine to treat fever. Since roemrefidine is a salt (quaternary nitrogen) it is more likely to be extracted into neutral aqueous solution than the less polar MOalkaloids [3,4,5,6,7,8], and therefore roemrefidine must be considered a candidate for the febrifugal activity of the traditional preparations. In addition we have recently demonstrated [20] the antimicrobial activity of roemrefidine towards Bacillus cereus, Escherichia coli and Staphylococcus aureus, which may be relevant to the use of A. delagoensis for the treatment of intestinal disorders.
Acknowledgments
The authors acknowledge technical assistance from Bhavin Dalal, Heather Matta and S.M. Goldup at Queen Mary, and from William Nel at UZULU. We acknowledge financial support from Royal Society/ National Research Foundation South Africa – UK Science Networks programme.
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- Oliver-Bever, B. Medicinal Plants in Tropical West Africa; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Jansen, P.M.C.; Mendes, O. Plantas Medicinais: Seu uso Tradicional em Moçambique 1; Instituto Nacional do Livroe do Disco: Maputo, Mozambique, 1983. [Google Scholar]
- De Wet, H. An Ethnobotanical and Chemotaxonomic Study of South African Menispermaceae. PhD Thesis, University of Johannesburg, Johannesburg, South Africa, 2006. [Google Scholar]
- De Wet, H.; van Heerden, F.R.; van Wyk, B.E.; van Zyl, R.L. Antiplasmodial activity and cytotoxicity of Albertisia delagoensis. Fitoterapia 2007, 78, 420–422. [Google Scholar] [CrossRef]
- De Wet, H.; van Wyk, B.E. An ethnobotanical survey of southern African Menispermaceae. S. Afr. J. Bot. 2008, 74, 2–9. [Google Scholar] [CrossRef]
- Rondanelli, R.; Guaglio, R.; Sartirana, E.; Zizzi, E.; Olliaro, P.; Benzi Cipelli, R. Evaluation of the cytotoxic activity of some alkaloids from Epinetrum delagoense Diels I. Il Farmaco 1986, 41, 185–189. [Google Scholar]
- Rondanelli, R.; Guaglio, R.; Guarnone, E.; Brè, E.; Olliaro, P.; Benzi Cipelli, R. Cytotoxic activity of some alkaloids from Epinetrum delagoense Diels: Electron microscopy studies II. Il Farmaco 1986, 41, 190–196. [Google Scholar]
- De Wet, H.; Fouche, G.; van Heerden, F.R. In vitro cytotoxicity of crude alkaloidal extracts of South African Menispermaceae against three cancer cell lines. Afr. J. Biotechnol. 2009, 8, 3332–3335. [Google Scholar]
- Corcoran, O.; Spraul, M. LC-NMR-MS in drug discovery. Drug Discov. Today 2003, 8, 624–631. [Google Scholar] [CrossRef]
- Jaroszewski, J.W. Hyphenated NMR methods in natural products research, Part 1: Direct hyphenation. Planta Med. 2005, 71, 691–700. [Google Scholar] [CrossRef]
- Joroszewski, J.W. Hyphenated NMR methods in natural products research, Part 2: HPLC-SPE-NMR and other new trends in NMR hyphenation. Planta Med. 2005, 71, 795–802. [Google Scholar] [CrossRef]
- Smallcombe, S.H.; Patt, S.L.; Keifer, P.A. WET solvent suppression and its application to LC NMR and high resolution NMR spectroscopy. J. Magn. Reson. Ser. A 1995, 117, 295–303. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.; Berger, S. 150 and More Basic NMR Experiments, 2nd ed; Wiley-VCH: Weinheim, Germany, 1998; pp. 477-489, 501, 504. [Google Scholar]
- Behrend, R.; Schultz, R. Ueber die Oxydation der Harnsäure in alkalischer Lösung. Justus Liebig’s Ann. Chem. 1909, 365, 21–37. [Google Scholar] [CrossRef]
- Salamci, E.; Secen, H.; Sütbeyaz, Y.; Balci, M. A concise and convenient synthesis of DL-proto-quercitol and DL-gala-quercitol via ene reaction of singlet oxygen combined with [2+4] cycloaddition to cyclohexadiene. J. Org. Chem. 1997, 62, 2453–2457. [Google Scholar] [CrossRef]
- Fan, T.W.-M. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Progr. Nucl. Magn. Reson. Spec. 1996, 28, 161–219. [Google Scholar]
- The Aldrich Library of 13C and 1H FT NMR Spectra; Pouchert, C.J.; Behnke, J. (Eds.) Aldrich Chemical Company Inc: St. Louis, MI, USA, 1993; Volume 2, p. 1116B, 1088A.
- Slavík, J.; Picka, K.; Slavíková, L.; Táborská, E.; Vĕžník, F. Quaternary alkaloids of some species of the Papaveraceae family. Coll. Czech. Chem. Comm. 1980, 45, 914–920. [Google Scholar]
- Muñoz, V.; Sauvin, M.; Mollinedo, P.; Callapa, J.; Rojas, I.; Gimenez, A.; Valentin, A.; Mallié, M. Antimalarial activity and cytotoxicity of (-)-roemrefidine isolated from the stem bark of Sparattanthelium amazonum. Planta Med. 1999, 65, 448–449. [Google Scholar] [CrossRef]
- Monteil, D.; Russell, C.; Beckonert, O.; Cutler, R.; Hawkes, G.E.; Li, J.V. Unpublished results.
- Sample Availability: Samples of allantoic acid are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).