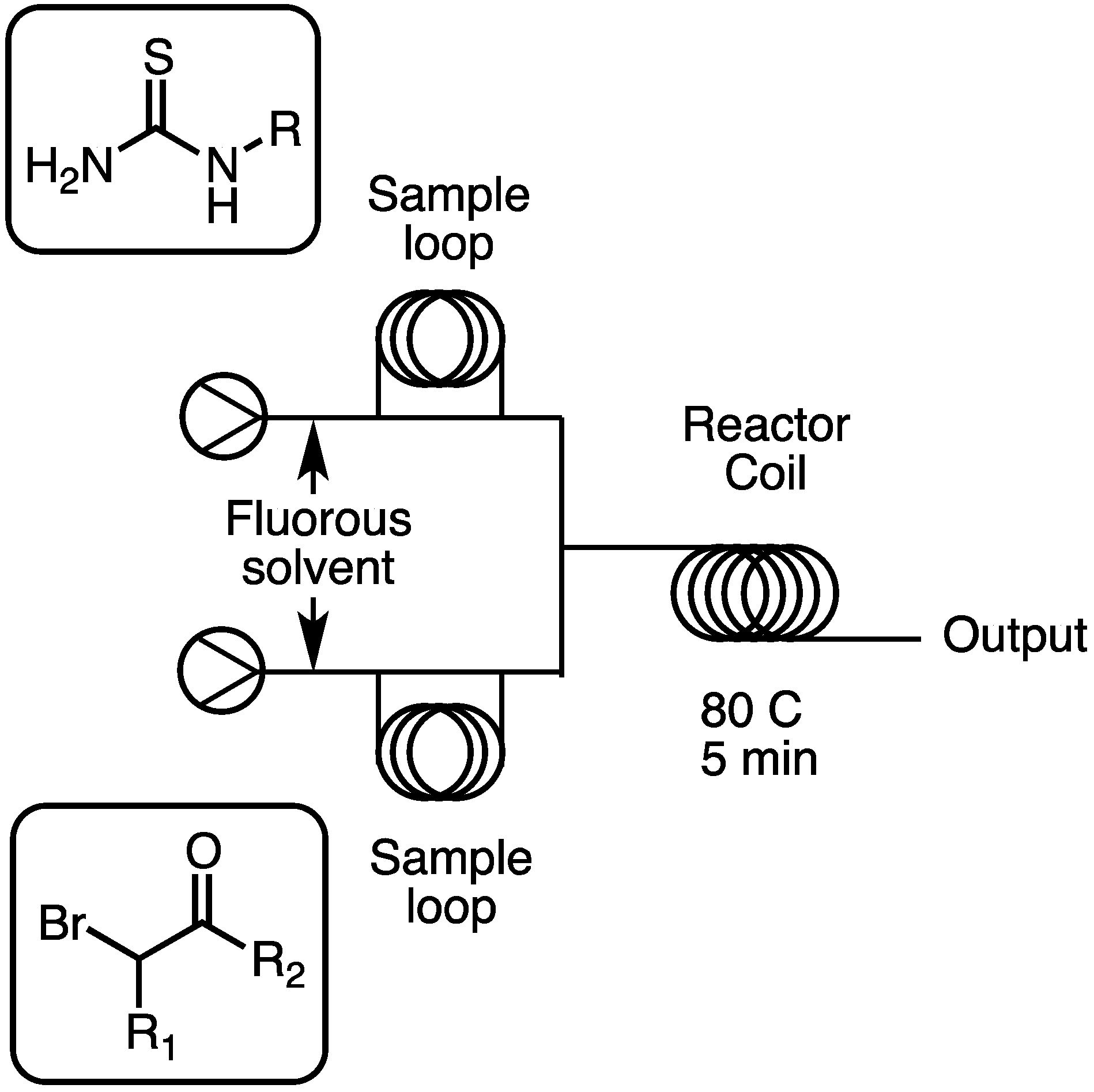
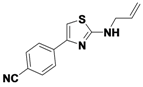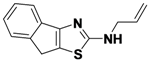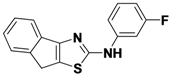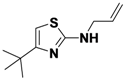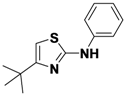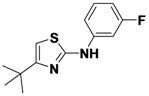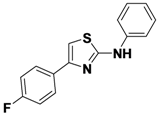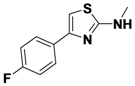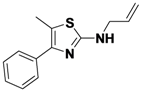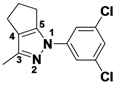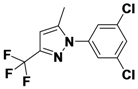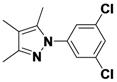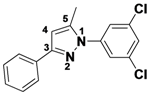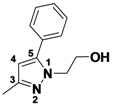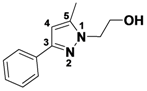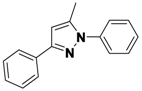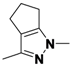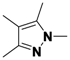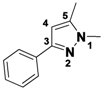3.2. Standard Protocol for Library Synthesis
Stock solutions of each desired starting material were first created by adding 2 mmol of a given starting material to a 20 mL scintillation vial. To each of these vials was then added 2 mL of dry DMF, and the vials were then sonicated to form clear solutions. For each library member, 100 μL of stock solution from each of the required building blocks was loaded into a metering loop of known volume on a Uniqsis flow system. The machine then injected both metering loops to form a single cohesive droplet that was then flowed at 1.3 mL/min through an 80 °C heated coil, giving a final reaction time of 5 min. One minute after the library member was injected, a blank plug of 200 mL of DMF was added to the machine, and both the library droplet and the wash droplet were collected into tubes. The DMF layer was then removed, the DMF was evaporated under warm nitrogen, and the resulting residue was purified by preparative HPLC on a Waters Symmetry C8 column (25 mm × 100 mm, 7 μm particle size) using a gradient of 10% to 100% acetonitrile: 0.1% aqueous TFA over 8 min (10 min run time) at a flow rate of 40 mL/min to give the desired compound. The pyrrazole library was repeated in triplicate, and gave a 5.1% standard deviation in yields.
4-(2-(Allylamino)thiazol-4-yl)benzonitrile (
1-1).
1H-NMR (500 MHz, DMSO) δ 8.00 (d,
J = 8.4, 3H), 7.83 (d,
J = 8.3, 2H), 7.41 (s, 1H), 5.94 (ddd,
J = 22.5, 10.5, 5.4, 1H), 5.28 (d,
J = 17.2, 1H), 5.14 (d,
J = 10.2, 1H), 3.93 (d,
J = 19.5, 2H).
13C-NMR (101 MHz, DMSO) δ 168.36, 147.98, 138.82, 134.76, 132.41, 126.17, 119.04, 115.97, 109.33, 105.09, 46.61.MS (DCI+)
m/z 242.1 [M+H]
+. 42% yield.
4-(2-(Phenylamino)thiazol-4-yl)benzonitrile (
1-2).
1H-NMR (500 MHz, DMSO) δ 10.36 (s, 1H), 8.11 (d,
J = 8.4, 2H), 7.90 (d,
J = 8.4, 2H), 7.72 (d,
J = 7.9, 2H), 7.66 (s, 1H), 7.36 (t,
J = 7.9, 2H), 6.99 (t,
J = 7.3, 1H).
13C-NMR (126 MHz, DMSO) δ 163.37, 148.33, 140.95, 138.54, 132.72, 129.03, 126.23, 121.43, 119.01, 116.91, 109.58, 106.87. MS (DCI+)
m/z 278.3 [M+H]
+. 48% yield.
4-(2-(3-Fluorophenylamino)thiazol-4-yl)benzonitrile (
1-3).
1H-NMR (500 MHz, DMSO) δ 10.61 (s, 1H), 8.10 (d,
J = 8.3, 2H), 7.92 (d,
J = 8.3, 2H), 7.78 (d,
J = 11.8, 1H), 7.72 (s, 1H), 7.44–7.27 (m, 3H), 6.80 (m, 1H).
13C-NMR (126 MHz, DMSO) δ 162.96, 162.54 (d,
J = 241), 148.36, 142.55 (d,
J = 11), 138.39, 132.78, 130.58 (d,
J = 10), 126.20, 118.98, 112.83 (d,
J = 2), 109.71, 107.65 (d,
J = 21), 107.51, 103.58 (d,
J = 27). MS (DCI+)
m/z 296.3 [M+H]
+. 49% yield.
4-(2-(Methylamino)thiazol-4-yl)benzonitrile (
1-4).
1H-NMR (500 MHz, DMSO) δ 8.01 (d,
J = 8.2, 2H), 7.90 (d,
J = 8.2, 2H), 7.39 (s, 1H), 3.15 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 169.48, 147.79, 138.66, 132.53, 126.22, 119.02, 109.36, 104.88, 31.01. MS (DCI+)
m/z 216.3 [M+H]
+. 48% yield.
N-Allyl-8H-indeno[1,2-d]thiazol-2-amine (
1-5).
1H-NMR (500 MHz, DMSO) δ 7.47 (dd,
J = 13.3, 7.4, 2H), 7.31 (t,
J = 7.5, 1H), 7.18 (t,
J = 7.4, 1H), 5.95 (ddt,
J = 17.0, 10.4, 5.3, 1H), 5.41–5.22 (m, 1H), 5.17 (dd,
J = 10.2, 1.4, 1H), 4.02 (s, 1H).
13C-NMR (126 MHz, DMSO) δ 173.04, 145.42, 136.79, 134.33, 126.65, 124.71, 124.34, 122.51, 117.68, 116.17, 46.72, 32.48. MS (DCI+)
m/z 229.1 [M+H]
+. 12% yield.
N-Phenyl-8H-indeno[1,2-d]thiazol-2-amine (
1-6).
1H-NMR (500 MHz, DMSO) δ 10.33 (s, 1H), 7.73 (d,
J = 7.9, 2H), 7.54 (dd,
J = 17.7, 7.4, 2H), 7.37–7.31 (m,
J = 7.5, 3.9, 3H), 7.21 (t,
J = 9.5, 5.4, 1H), 6.97 (t,
J = 7.3, 1H), 3.81 (s, 2H).
13C-NMR (126 MHz, DMSO) δ 167.47, 156.16, 145.40, 141.05, 137.37, 129.00, 126.71, 124.78, 124.40, 121.26, 117.76, 116.91, 32.29. MS (DCI+)
m/z 265.1 [M+H]
+. 34% yield.
N-(3-fluorophenyl)-8H-indeno[1,2-d]thiazol-2-amine (
1-7).
1H-NMR (500 MHz, DMSO) δ 7.81 (d,
J = 7.3, 1H), 7.58 (d,
J = 7.5, 1H), 7.53 (d,
J = 7.4, 1H), 7.38–7.32 (m, 3H), 7.22 (t,
J = 7.5, 1H), 6.81–6.75 (m, 1H), 3.83 (s, 2H).
13C-NMR (101 MHz, DMSO) δ 166.95, 162.60 (d,
J = 241), 156.14, 145.38, 142.67 (d,
J = 11), 137.21, 130.51 (d,
J = 10), 126.76, 125.18, 124.83, 124.53, 117.84, 112.78 (d,
J = 2), 107.43 (d,
J = 21), 103.57 (d,
J = 27), 32.32. MS (DCI+)
m/z 283.3 [M+H]
+. 24% yield.
N-methyl-8H-indeno[1,2-d]thiazol-2-amine (
1-8).
1H-NMR (500 MHz, DMSO) δ 7.51–7.44 (m, 2H), 7.32 (t,
J = 7.5, 1H), 7.19 (t,
J = 7.5, 1H), 3.72 (s,
J = 18.8, 2H), 2.96 (s,
J = 5.4, 3H).
13C-NMR (101 MHz, DMSO) δ 173.89, 145.39, 136.38, 126.67, 124.73, 124.47, 122.08, 117.72, 32.61, 31.19. MS (DCI+)
m/z 203.3 [M+H]
+. 53% yield.
N-Allyl-4-tert-butylthiazol-2-amine (
1-9).
1H-NMR (500 MHz, DMSO) δ 6.46 (s, 1H), 5.88 (m, 1H), 5.31 (d,
J = 17.2, 1H), 5.23 (d,
J = 10.3, 1H), 3.96 (s,
J = 4.1, 2H), 1.24 (s, 9H).
13C-NMR (101 MHz, DMSO) δ 169.78, 149.34, 131.76, 117.94, 100.05, 48.11, 33.25, 28.39. MS (DCI+)
m/z 197.1 [M+H]
+. 50% yield.
4-tert-Butyl-N-phenylthiazol-2-amine (
1-10).
1H-NMR (500 MHz, DMSO) δ 10.08 (s, 1H), 7.68–7.54 (m, 2H), 7.30 (t,
J = 7.7, 2H), 6.92 (t,
J = 7.3, 1H), 6.41 (s, 1H), 1.31 (s, 9H).
13C-NMR (101 MHz, DMSO) δ 162.93, 160.86, 141.29, 128.96, 121.15, 116.87, 99.21, 34.31, 29.54. MS (DCI+)
m/z 233.2 [M+H]
+. 40% yield.
4-tert-Butyl-N-(3-fluorophenyl)thiazol-2-amine (
1-11).
1H-NMR (500 MHz, DMSO) δ 10.33 (s, 1H), 7.82–7.65 (m, 1H), 7.41–7.16 (m, 2H), 6.71–6.65 (m, 1H), 6.57–6.38 (m, 1H), 1.27 (s, 9H).
13C-NMR (126 MHz, DMSO) δ 162.56 (d,
J = 241), 162.04, 161.53, 143.12 (d,
J = 12), 130.36 (d,
J = 10), 112.44, 106.95 (d,
J = 21), 103.24 (d,
J = 27), 99.98, 34.39, 29.59. MS (DCI+)
m/z 251.1 [M+H]
+. 49% yield.
4-tert-Butyl-N-methylthiazol-2-amine (
1-12).
1H-NMR (500 MHz, DMSO) δ 6.50 (s, 1H), 2.97 (s, 3H), 1.32 (s, 9H).
13C-NMR (101 MHz, DMSO) δ 170.27, 162.27, 148.86, 100.12, 33.19, 28.34. MS (DCI+)
m/z 171.3 [M+H]
+. 36% yield.
N-Allyl-4-(4-fluorophenyl)thiazol-2-amine (
1-13).
1H-NMR (500 MHz, DMSO) δ 7.90–7.74 (m, 2H), 7.21 (t,
J = 8.8, 2H), 7.06 (s, 1H), 5.93–5.85 (m, 1H), 5.28 (d,
J = 8.8, 2H), 5.15 (d,
J = 10.3, 2H), 3.95 (d,
J = 5.2, 1H).
13C-NMR (101 MHz, DMSO) δ 168.42, 161.53 (d,
J = 244), 147.87, 134.65, 130.91 (d,
J = 3), 127.65 (d,
J = 8), 116.05, 115.29 (d,
J = 21), 100.96, 46.77. MS (DCI+)
m/z 235.2 [M+H]
+. 63% yield.
4-(4-Fluorophenyl)-N-phenylthiazol-2-amine (
1-14).
1H-NMR (500 MHz, DMSO) δ 10.27 (s, 1H), 8.03–7.89 (m, 2H), 7.72 (d,
J = 8.3, 2H), 7.42–7.30 (m, 2H), 7.24–7.3 (t,
J = 7.25, 2H), 6.98 (t,
J = 6.98, 1H).
13C-NMR (101 MHz, DMSO) δ 163.17, 161.60 (d,
J = 244), 149.01, 141.14, 131.13 (d,
J = 3), 128.99, 127.62 (d,
J = 8), 121.22, 116.80, 115.46 (d,
J = 21), 102.63. MS (DCI+)
m/z 271.3 [M+H]
+. 31% yield.
N-(3-Fluorophenyl)-4-(4-fluorophenyl)thiazol-2-amine (
1-15).
1H-NMR (500 MHz, DMSO) δ 10.52 (s, 1H), 7.95 (dd,
J = 8.4, 5.7, 2H), 7.79 (d,
J = 11.0, 1H), 7.42–7.32 (m, 3H), 7.29 (t,
J = 8.8, 2H), 6.78 (m, 1H).
13C-NMR (126 MHz,z DMSO) δ 162.73, 162.55 (d,
J = 241), 161.65 (d,
J = 244), 149.04, 142.73 (d,
J = 11), 130.98 (d,
J = 3), 130.52 (d,
J = 10), 127.59 (d,
J = 8), 115.55 (d,
J = 21), 112.70 (d,
J = 2), 107.41 (d,
J = 21), 103.45 (d,
J = 27), 103.34. MS (DCI+)
m/z 289.4 [M+H]
+. 46% yield.
4-(4-Fluorophenyl)-N-methylthiazol-2-amine (
1-16)
1H-NMR (500 MHz, DMSO) δ 7.90–7.80 (m, 2H), 7.28–7.18 (m, 2H), 7.23 (s, 1H), 2.91 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 169.45, 161.52 (d,
J = 244), 148.07, 130.92, 127.65 (d,
J = 8), 115.28 (d,
J = 22), 100.72. MS (DCI+)
m/z 209.2 [M+H]
+. 55% yield.
N-Allyl-5-methyl-4-phenylthiazol-2-amine (
1-17).
1H-NMR (500 MHz, DMSO) δ 7.55 (d,
J = 8.1, 2H), 7.46 (t,
J = 7.6, 2H), 7.38 (t,
J = 7.3, 1H), 5.91 (dtd,
J = 15.8, 10.5, 5.3, 1H), 5.30 (d,
J = 10.2, 1H), 5.18 (d,
J = 10.3, 1H), 3.95 (s, 2H), 2.31 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 166.55, 136.86, 132.60, 129.95, 128.89, 128.66, 117.39, 114.58, 114.49, 47.48, 11.66. MS (DCI+)
m/z 231.2 [M+H]
+. 41% yield.
5-Methyl-N,4-diphenylthiazol-2-amine (
1-18).
1H-NMR (500 MHz, DMSO) δ 10.05 (s, 1H), 7.66 (dd,
J = 12.6, 8.3, 4H), 7.46 (t,
J = 7.6, 2H), 7.37–7.26 (m, 3H), 6.92 (td,
J = 7.4, 0.9, 1H), 2.43 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 159.59, 144.82, 141.21, 134.86, 129.00, 128.36, 128.05, 127.21, 121.21, 116.96, 116.30, 11.93. MS (DCI+)
m/z 267.2 [M+H]
+. 40% yield.
N-(3-Fluorophenyl)-5-methyl-4-phenylthiazol-2-amine (
1-19).
1H-NMR (500 MHz, DMSO) δ 10.30 (s, 1H), 7.74 (d,
J = 12.2, 1H), 7.67 (d,
J = 8.0, 2H), 7.47 (t,
J = 7.6, 2H), 7.40–7.24 (m, 3H), 6.82–6.65 (m, 1H), 2.44 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 162.54 (d,
J = 241), 158.71, 145.30, 142.90 (d,
J = 12 Hz), 134.98, 130.40 (d,
J = 10), 128.36, 127.92, 127.17, 117.04, 112.51 (d,
J = 2), 107.02 (d,
J = 21), 103.25 (d,
J = 27), 11.91.MS (DCI+)
m/z 285.4 [M+H]
+. 31% yield.
N,5-Dimethyl-4-phenylthiazol-2-amine (
1-20).
1H-NMR (500 MHz, DMSO) δ 7.55 (d,
J = 7.5, 2H), 7.49 (t,
J = 7.6, 2H), 7.43 (t,
J = 7.2, 1H), 2.96 (s, 3H), 2.27 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 166.86, 134.06, 129.42, 128.98, 128.72, 128.28, 114.50, 31.96, 11.61. MS (DCI+)
m/z 205.2 [M+H]
+. 61% yield.
2-(3,5-Dichlorophenyl)-3-methyl-2,4,5,6-tetrahydrocyclopenta[c]pyrazole (
2-1a).
1H-NMR (400 MHz, DMSO) δ 7.59 (m, 3H), 2.63 (t,
J = 7.4, 2H), 2.56 (t,
J = 7.1, 2H), 2.39–2.33 (m, 2H), 2.33 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 149.31, 144.74, 141.60, 134.89, 129.22, 124.16, 116.20, 30.39, 26.13, 21.65, 12.50 (s). MS (DCI+)
m/z 267.1 [M+H]
+. 8% yield. In the ROESY spectrum an NOE correlation was observed between the methyl protons and resonances for the aryl ringverifying the regioisomer.
1-(3,5-Dichlorophenyl)-3-methyl-1,4,5,6-tetrahydrocyclopenta[c]pyrazole (
2-1b).
1H-NMR (400 MHz, DMSO) δ 7.57 (d,
J = 1.8, 2H), 7.45 (t,
J = 1.8, 1H), 3.05 (m, 2H), 2.59–2.49 (m, 4H), 2.16 (s, 3H). MS (DCI+)
m/z 267.1 [M+H]
+. 1% yield. In the ROESY spectrum NOE correlations were observed between methylene protons of the cyclopentyl ring and aryl protons verifying the regioisomer.
1-(3,5-Dichlorophenyl)-5-methyl-3-(trifluoromethyl)-1H-pyrazole (
2-2).
1H-NMR (400 MHz, DMSO) δ 7.84 (dt,
J = 16.5, 1.8, 1H), 7.76 (d,
J = 1.8, 2H), 6.81 (s, 1H), 2.40 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 151.34, 144.72, 133.82, 123.92 (q,
J = 287), 119.10, 114.40, 92.23 (q,
J = 32), 47.70, 15.31. MS (DCI+)
m/z 294.1 [M+H]
+. 15% yield. In the ROESY spectrum an NOE correlation was observed between the methyl protons and aryl protons, verifying the regioisomer.
1-(3,5-Dichlorophenyl)-3,4,5-trimethyl-1H-pyrazole (
2-3).
1H-NMR (400 MHz, DMSO) δ 7.67–7.43 (m, 3H), 2.27 (s, 3H), 2.12 (s, 3H), 1.92 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 148.64, 141.80, 136.25, 134.31, 125.69, 121.70, 114.16, 11.66, 10.83, 7.82. MS (DCI+)
m/z 255.1 [M+H]
+. 26% yield.
1-(3,5-Dichlorophenyl)-5-methyl-3-phenyl-1H-pyrazole (
2-4a).
1H-NMR (400 MHz, DMSO) δ 7.85 (dt,
J = 8.2, 1.7, 2H), 7.75 (d,
J = 1.8, 2H), 7.70 (t,
J = 1.8, 1H), 7.38–7.32 (m, 2H), 6.81 (d,
J = 0.8, 1H), 2.45 (d,
J = 0.5, 3H).
13C-NMR (126 MHz, DMSO) δ 149.74, 143.53, 141.46, 134.02, 129.64, 128.74, 128.52, 126.39, 122.90, 109.03, 13.22. MS (DCI+)
m/z 303.2 [M+H]
+. 14% yield. In the ROESY spectrum an NOE correlation was observed between the methyl protons and aryl protons of the chlorinated ring, verifying the regioisomer.
1-(3,5-Dichlorophenyl)-3-methyl-5-phenyl-1H-pyrazole (
2-4b).
1H-NMR (400 MHz, DMSO) δ 7.57 (t,
J = 1.9, 1H), 7.47–7.36 (m, 3H), 7.32–7.25 (m, 3H), 7.26 (d,
J = 0.6, 1H), 6.48 (s, 1H), 2.24 (d,
J = 34.1, 3H) MS (DCI+)
m/z 303.2 [M+H]
+. 9% yield. In the ROESY spectrum NOE correlations were observed between the aryl resonances for the chlorinated ring and the phenyl ring, verifying the regioisomer.
2-(3-Methyl-5,6-dihydrocyclopenta[c]pyrazol-1(4H)-yl)ethanol (
2-5).
1H-NMR (400 MHz, DMSO) δ 3.94 (t,
J = 5.6, 2H), 3.63 (t,
J = 5.6, 2H), 2.70–2.65 (m, 2H), 2.48–2.40 (m, 4H), 2.07 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 153.97, 139.13, 124.94, 76.27, 59.47, 52.43, 29.83, 23.75, 22.20, 11.08. MS (DCI+)
m/z 167.2 [M+H]
+. 50% yield. In the ROESY spectrum NOE correlations were observed between methylene protons in the cyclopentyl ring and the methylene protons for the alcohol substituent, verifying the regioisomer.
2-(5-Methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)ethanol (
2-6).
1H-NMR (400 MHz, DMSO) δ 6.45 (s, 1H), 4.92 (t,
J = 5.4, 1H), 4.21–4.08 (m, 2H), 3.71 (q,
J = 5.5, 2H), 2.30 (dd,
J = 17.1, 10.9, 3H).
13C-NMR (101 MHz, DMSO) δ 141.76, 139.76 (q,
J = 37), 121.99 (q,
J = 268), 103.30, 60.33, 51.90, 10.78. MS (DCI+)
m/z 195.1 [M+H]
+. 17% yield. In the ROESY spectrum NOE correlations were observed between methyl protons and methylene protons for the alcohol substituent, verifying the regioisomer.
2-(3,5-Dimethyl-1H-pyrazol-1-yl)ethanol (
2-7).
1H-NMR (400 MHz, DMSO) δ 4.06 (t,
J = 5.6, 2H), 3.64 (t,
J = 5.6, 2H), 2.18 (s, 3H), 2.11 (s, 3H), 1.90–1.82 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 143.46, 139.36, 111.58, 59.87, 50.65, 10.55, 9.19, 7.44. MS (DCI+)
m/z 155.1 [M+H]
+. 37% yield.
2-(3-Methyl-5-phenyl-1H-pyrazol-1-yl)ethanol (
2-8a).
1H-NMR (400 MHz, DMSO) δ 7.64–7.27 (m, 5H), 6.15 (s, 1H), 4.03 (t,
J = 5.9, 2H), 3.75 (t,
J = 5.9, 2H), 2.20 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 146.50, 144.43, 130.42, 128.81, 128.70, 128.38, 105.35, 59.98, 50.84, 13.28. MS (DCI+)
m/z 203.1 [M+H]
+. 57% yield. In the ROESY spectrum NOE correlations were observed between resonances for the phenyl ring and methylene protons of the alcohol substituent, verifying the regioisomer.
2-(5-Methyl-3-phenyl-1H-pyrazol-1-yl)ethanol (
2-8b).
1H-NMR (400 MHz, DMSO) δ 7.73 (dd,
J = 8.3, 1.2, 2H), 7.44–7.31 (m, 2H), 7.25 (ddd,
J = 8.6, 2.6, 1.3, 1H), 6.44 (s, 1H), 4.09 (t,
J = 5.8, 2H), 3.72 (t,
J = 5.8, 2H), 2.30 (s, 3H). MS (DCI+)
m/z 203.1 [M+H]
+. 22% yield. In the ROESY spectrum NOE correlations were observed between methyl protons and methylene protons of the alcohol substituent, verifying the regioisomer.
3-Methyl-1-phenyl-1,4,5,6-tetrahydrocyclopenta[c]pyrazole (
2-9).
1H-NMR (400 MHz, DMSO) δ 7.59 (d,
J = 8.8, 2H), 7.51–7.34 (m, 2H), 7.31–7.14 (m, 1H), 3.10–2.91 (m, 2H), 2.15 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 148.68, 143.03, 139.94, 129.43, 127.94, 125.03, 118.03, 30.53, 26.25, 21.76, 12.53. MS (DCI+)
m/z 199.2 [M+H]
+. 32% yield. In the ROESY spectrum NOE correlations were observed between methylene protons for the cyclopentyl ring and resonances for the phenyl ring, verifying the regioisomer.
5-Methyl-1-phenyl-3-(trifluoromethyl)-1H-pyrazole (
2-10).
1H-NMR (400 MHz, DMSO) δ 7.55 (m, 5H), 6.76 (s, 1H), 2.36 (s, 3H).
13C-NMR (101 MHz, DMSO) δ 141.57, 140.99 (q,
J = 37), 138.42, 129.34, 128.75, 125.03, 121.56 (q,
J = 268), 104.90 (q,
J = 2), 11.84.MS (DCI+)
m/z 227.2 [M+H]
+. 38% yield. In the ROESY spectrum an NOE correlation was observed between methyl protons and resonances for the phenyl ring verifying the regioisomer.
3,4,5-Trimethyl-1-phenyl-1H-pyrazole (
2-11).
1H-NMR (400 MHz, DMSO) δ 7.53–7.40 (m, 4H), 7.40–7.26 (m, 1H), 2.20 (s, 3H), 2.13 (s, 3H), 1.93 (m, 3H).
13C-NMR (126 MHz, DMSO) δ 147.03, 139.75, 135.83, 129.05, 126.78, 124.01, 112.89, 11.65, 10.75, 7.90. MS (DCI+)
m/z 187.3 [M+H]
+. 33% yield.
5-Methyl-1,3-diphenyl-1H-pyrazole (
2-12)
1H-NMR (400 MHz, DMSO) δ 7.46–7.26 (m, 6H), 7.27–7.13 (m, 3H), 6.45 (s, 1H), 2.25 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 148.53, 143.11, 139.86, 130.29, 128.93, 128.54, 128.32, 128.19, 127.23, 124.96, 107.80, 13.27. MS (DCI+)
m/z 235.3 [M+H]
+. 29% yield. In the ROESY spectrum an NOE correlation was observed between the methyl protons and resonances for the N-bound phenyl ring, verifying the regioisomer.
1,3-Dimethyl-1,4,5,6-tetrahydrocyclopenta[c]pyrazole (
2-13).
1H-NMR (400 MHz, DMSO) δ 3.57 (s, 1H), 2.59 (m, 1H), 2.42 (m, 1H), 1.98 (m, 1H).
13C-NMR (126 MHz, DMSO) δ 150.13, 139.74, 123.91, 36.29, 30.69, 23.10, 22.51, 12.39. MS (DCI+)
m/z 137.1 [M+H]
+. 43% yield. In the ROESY spectrum NOE correlations were observed between methylene resonances for the cyclopentyl ring and the N-bound methyl group, verifying the regioisomer.
1,3,4,5-Tetramethyl-1H-pyrazole (
2-15).
1H-NMR (400 MHz, DMSO) δ 3.57 (s, 3H), 2.08 (s, 3H), 1.99 (s, 3H), 1.82 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 143.96, 135.34, 110.06, 35.45, 11.50, 9.05, 7.85. MS (DCI+)
m/z 125.2 [M+H]
+. 44% yield.
1,5-Dimethyl-3-phenyl-1H-pyrazole (
2-16a).
1H-NMR (400 MHz, DMSO) δ 7.72 (dt,
J = 8.1, 1.6, 2H), 7.41–7.31 (m, 2H), 7.31–7.17 (m, 1H), 6.46 (s, 1H), 3.75 (s, 3H), 2.25 (s, 3H).
13C-NMR (126 MHz, DMSO) δ 146.04, 143.51, 130.31, 128.79, 128.33, 128.32, 105.28, 37.07, 13.14. MS (DCI+)
m/z 173.1 [M+H]
+. 15% yield. In the ROESY spectrum an NOE correlation was observed between the two methyl groups, verifying the regioisomer.
1,3-Dimethyl-5-phenyl-1H-pyrazole (
2-16b).
1H-NMR (400 MHz, DMSO) δ 7.61–7.27 (m, 5H), 6.18 (s, 1H), 3.76 (s, 3H), 2.20 (s, 3H). MS (DCI+)
m/z 173.1 [M+H]
+. 6% yield. In the ROESY spectrum an NOE correlation was observed between resonances for the phenyl ring and the N-bound methyl group, verifying the regioisomer.
1-Cyclohexyl-3-methyl-1,4,5,6-tetrahydrocyclopenta[c]pyrazole (
2-17).
1H-NMR (500 MHz, DMSO) δ 4.02-3.85 (m, 2H), 2.78–2.73 (m, 2H), 2.49–2.36 (m, 4H), 1.94 (s, 3H), 2.0–1.87 (m, 2H), 1.82–1.75 (m, 2H), 1.63 (qd,
J = 12.5, 3.5, 3H), 1.35 (qt,
J = 13.1, 3.4, 2H), 1.17 (qt,
J = 13.0, 3.6, 1H).
13C-NMR (101 MHz, DMSO) δ 151.43, 138.86, 125.16, 59.77, 31.89, 29.81, 24.80, 24.60, 24.41, 21.64, 11.14. MS (DCI+)
m/z 205.3 [M+H]
+. 40% yield. In the ROESY spectrum NOE correlations were observed between methylene resonances for the cyclopentyl ring and resonances for the cyclohexyl substituent, verifying the regioisomer.
1-Cyclohexyl-5-methyl-3-(trifluoromethyl)-1H-pyrazole (
2-18).
1H-NMR (400 MHz, DMSO) δ 6.43 (s, 1H), 4.18 (tt,
J = 11.5, 3.9, 1H), 2.32 (s, 3H), 1.85–1.60 (m, 8H), 1.48–1.34 (m, 2H), 1.28–1.14 (m, 1H).
13C-NMR (101 MHz, DMSO) δ 139.66, 138.99 (q,
J = 37), 121.81 (q,
J = 268), 103.08 (q,
J = 2), 57.00, 32.26, 24.81, 24.75, 10.34. MS (DCI+)
m/z 233.3 [M+H]
+. 23% yield. In the ROESY spectrum NOE correlations were observed between methyl protons and resonances for the cyclohexyl substituent, verifying the regioisomer.
1-Cyclohexyl-3,4,5-trimethyl-1H-pyrazole (
2-19).
1H-NMR (400 MHz, DMSO) δ 4.11–3.86 (m, 1H), 2.16 (s, 3H), 2.08 (s, 3H), 1.85 (s, 3H), 1.82–1.57 (m, 7H), 1.50–1.27 (m, 2H), 1.26–1.02 (m, 1H).
13C-NMR (101 MHz, DMSO) δ 143.58, 137.52, 111.25, 56.69, 32.05, 25.01, 24.81, 10.81, 9.05, 7.43. MS (DCI+)
m/z 193.2[M+H]
+. 34% yield.
1-Cyclohexyl-3-methyl-5-phenyl-1H-pyrazole (
2-20).
1H-NMR (400 MHz, DMSO) δ 7.57–7.27 (m, 5H), 6.06 (s, 1H), 3.98 (tt,
J = 11.2, 4.2, 1H), 2.19 (s, 3H), 2.00–1.68 (m, 6H), 1.52–1.50 (m, 1H), 1.36 –1.01 (m, 3H).
13C-NMR (101 MHz, DMSO) δ 146.10, 142.90, 130.66, 128.88, 128.66, 128.42, 105.20, 56.81, 33.04, 25.15, 24.88, 13.44. MS (DCI+)
m/z 241.2 [M+H]
+. 26% yield. In the ROESY spectrum an NOE correlation was observed between resonances for the phenyl ring and resonances for the cyclohexyl substituent, verifying the regioisomer.