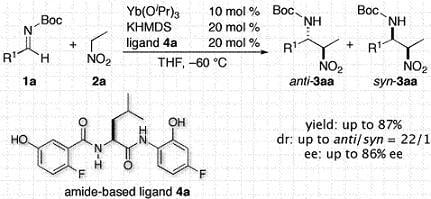
Catalytic Asymmetric Nitro-Mannich Reactions with a Yb/K Heterobimetallic Catalyst
Abstract
:1. Introduction
2. Results and Discussion
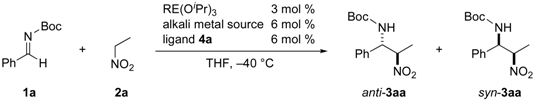
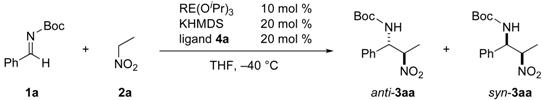
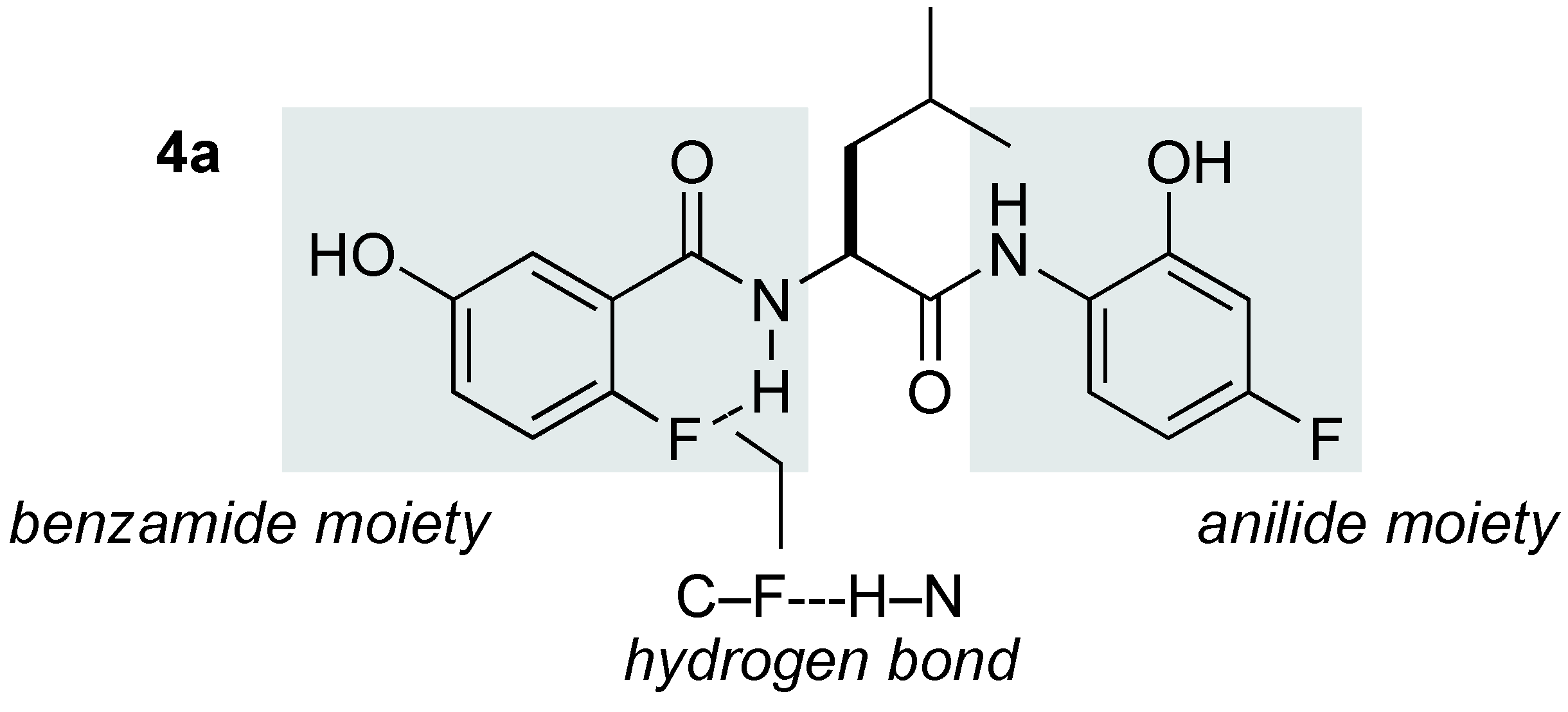
2.1. Identification of a Suitable Heterobimetallic Catalyst for Asymmetric Nitro-Mannich Reaction
 | |||||||
| Entry | RE(O i Pr)3 | Alkali metal source d | Time (h) | Yield e (%) | dr (anti/syn) | ee (anti) (%) | |
| 1 b | Nd5O(O iPr)13c | NaHMDS | 21 | 87 | 3.7/1 | 0 | |
| 2 | Nd5O(O iPr)13c | LHMDS | 21 | trace | ND | ND | |
| 3 | Nd5O(O iPr)13c | KHMDS | 21 | trace | ND | ND | |
| 4 | La(O iPr)3 | NaHMDS | 19 | 72 | 7.2/1 | 7 | |
| 5 | Sm(O iPr)3 | NaHMDS | 19 | 89 | 3.5/1 | 11 | |
| 6 | Gd(O iPr)3 | NaHMDS | 19 | 80 | 4.4/1 | 8 | |
| 7 | Er(O iPr)3 | NaHMDS | 19 | 83 | 5/1 | 3 | |
| 8 | Yb(O iPr)3 | NaHMDS | 19 | 92 | 5.2/1 | 6 |
 | |||||||
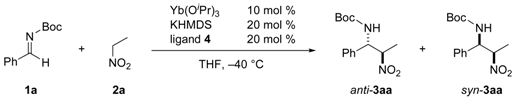
| Entry | RE(OiPr)3 | Time (h) | Yieldb (%) | dr (anti/syn) | ee (anti) (%) | ||
| 1 | La(OiPr)3 | 27 | 0 | ND | ND | ||
| 2 | Pr(OiPr)3 | 27 | 66 | 5.3/1 | 6 | ||
| 3 | Sm(OiPr)3 | 27 | 73 | 5.8/1 | 18 | ||
| 4 | Gd(OiPr)3 | 27 | 66 | 7.7/1 | 0 | ||
| 5 | Dy(OiPr)3 | 17 | 61 | 6.7/1 | 20 | ||
| 6 | Er(OiPr)3 | 17 | 68 | 9.0/1 | 51 | ||
| 7 | Yb(OiPr)3 | 27 | 72 | 11/1 | 68 | ||
| 8 | Yb(OiPr)3 | 17 | 78 | 6.6/1 | 55 | ||
 | ||||||
| Entry | Amide-based ligand 4 | Time (h) | Yield c (%) | dr (anti/syn) | ee (anti) (%) | |
| 1 |  | 4a | 27 | 72 | 11/1 | 68 |
| 2 | 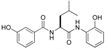 | 4b | 23 | 88 | 3.1/1 | 17 |
| 3 | 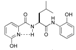 | 4c | 17 | 91 | 2.9/1 | 2 |
| 4 | 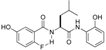 | 4d | 23 | 68 | 4.5/1 | 19 |
| 5 | 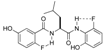 | 4e | 23 | 66 | 4.5/1 | 31 b |
 | |||||||||
| Entry | x | y | z | Solvent | Temp (°C) | Time (h) | Yield b (%) | dr (anti/syn) | ee (anti) (%) |
| 1 | 10 | 20 | 20 | THF | –40 | 27 | 72 | 11/1 | 68 |
| 2 | 10 | 10 | 10 | THF | –40 | 13 | 23 | 3.8/1 | 17 |
| 3 | 10 | 20 | 10 | THF | –40 | 13 | 72 | 2.3/1 | 4 |
| 4 | 10 | 10 | 20 | THF | –40 | 13 | 62 | 7.6/1 | 52 |
| 5 | 10 | 20 | 20 | CH2Cl2 | –40 | 12 | 26 | 4.4/1 | 4 |
| 6 | 10 | 20 | 20 | toluene | –40 | 12 | 46 | 2.2/1 | 4 |
| 7 | 10 | 20 | 20 | EtOAc | –40 | 12 | 79 | 3.1 | 20 |
| 8 | 10 | 20 | 20 | tBuOMe | –40 | 12 | 70 | 7.6 | 52 |
| 9 | 10 | 20 | 20 | THF | –60 | 24 | 77 | 18/1 | 73 |
2.2. Scope of the Catalytic Asymmetric Nitro-Mannich Reaction with Yb/K/4a Heterobimetallic Catalyst
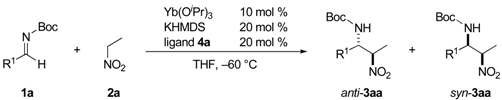 | ||||||||
| Entry | R1 | Product | Time (h) | Yield b (%) | dr (anti/syn) | ee (anti) (%) | ||
| 1 | Ph | 1a | 3aa | 22 | 80 | 18/1 | 73 | |
| 2 | 2-naph | 1b | 3ba | 22 | 71 | 17/1 | 72 | |
| 3 | 4-Me | 1c | 3ca | 44 | 87 | 22/1 | 86 | |
| 4 | 3-Me | 1d | 3da | 20 | 76 | 13/1 | 75 | |
| 5 | 4-OMe | 1e | 3ea | 44 | 79 | 19/1 | 82 | |
| 6 | 4-Cl | 1f | 3fa | 20 | 74 | 6.6/1 | 50 | |
| 7 | 4-CF3 | 1g | 3ga | 20 | 72 | 2.4/1 | 14 |
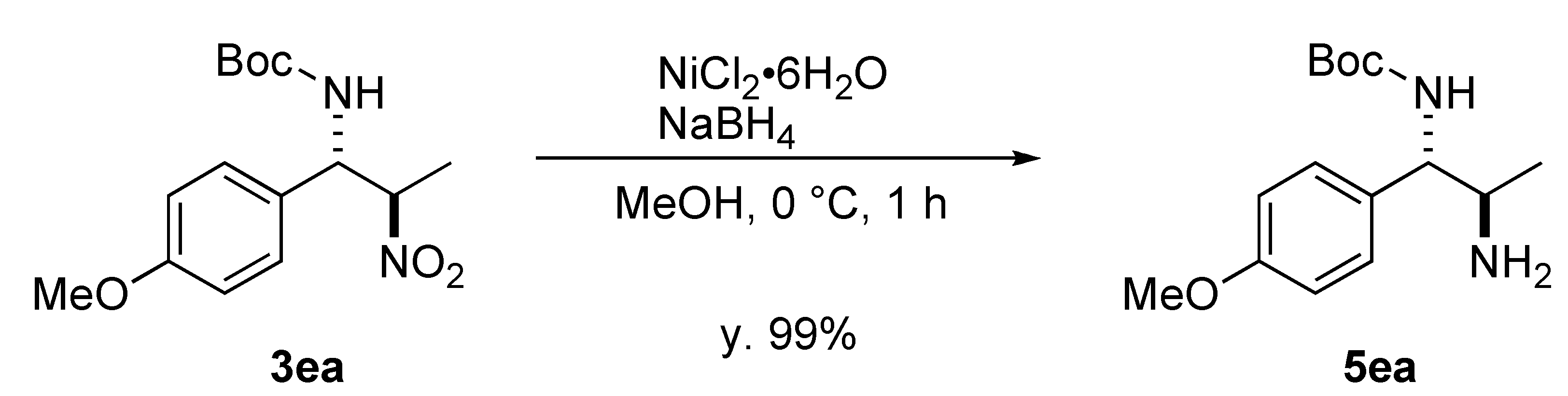
3. Experimental
3.1. General
3.2. General Procedure for Catalytic Asymmetric Nitro-Mannich Reaction with Yb/K/4a Heterobimetallic Catalyst (Table 5, Entry 1)
3.3. Reduction of Nitro Group of 3ea
4. Conclusions
Acknowledgements
- Samples Availability: Amide-based ligand 4a is available from the authors.
References and Notes
- Westermann, B. Asymmetric catalytic aza-Henry reactions leading to 1,2-diamines and 1,2-diaminocarboxylic acids. Angew. Chem., Int. Ed. 2003, 42, 151–153. [Google Scholar]
- Marqués-López, E.; Merino, P.; Tejero, T.; Herrera, R.P. Catalytic enantioselective aza-Henry reactions. Eur. J. Org. Chem. 2009, 2401–2420. [Google Scholar]
- Ono, N., Ed. The Nitro Group in Organic Synthesis; John Wiley & Sons: New York, USA, 2001. [Google Scholar]
- Ballini, R.; Petrini, M. Recent synthetic developments in the nitro to carbonyl conversion (Nef reaction). Tetrahedron 2004, 60, 1017–1047. [Google Scholar] [CrossRef]
- Czekelius, C.; Carreira, E.M. Convenient transformation of optically active nitroalkanes into chiral aldoximes and nitriles. Angew. Chem., Int. Ed. 2005, 44, 612–615. [Google Scholar]
- Yamada, K.I.; Harwood, S.J.; Gröger, H.; Shibasaki, M. The first catalytic asymmetric nitro-Mannich-type reaction promoted by a new heterobimetallic complex. Angew. Chem., Int. Ed. 1999, 38, 3504–3506. [Google Scholar]
- Yamada, K.-I.; Moll, G.; Shibasaki, M. The first enantioselective and diastereoselective catalytic nitro-Mannich reaction: a new entry to chiral vicinal diamines. Synlett 2001, 980–982. [Google Scholar]
- Nishiwaki, N.; Knudsen, K.R.; Gothelf, K.V.; Jørgensen, K.A. Catalytic enantioselective addition of nitro compounds to imines - a simple approach for the synthesis of optically active α-nitro-β-Amino Esters. Angew. Chem. Int. Ed. 2001, 40, 2992–2995. [Google Scholar]
- Knudsen, K.R.; Risgaard, T.; Nishiwaki, N.; Gothelf, K.V.; Jørgensen, K.A. The first catalytic asymmetric aza-Henry reaction of nitronates with imines: a novel approach to optically active β-nitro-α-amino acid- and α,β-diamino acid derivatives. J. Am. Chem. Soc. 2001, 123, 5843–5844. [Google Scholar]
- Lee, A.; Kim, W.; Lee, J.; Hyeon, T.; Kim, B.M. Heterogeneous asymmetric nitro-Mannich reaction using a bis(oxazoline) ligand grafted on mesoporous silica. Tetrahedron: Asymmetry 2004, 15, 2595–2598. [Google Scholar]
- Anderson, J.C.; Howell, G.P.; Lawrence, R.M.; Wilson, C.S. An asymmetric nitro-Mannich reaction applicable to alkyl, aryl, and heterocyclic imines. J. Org. Chem. 2005, 70, 5665–5670. [Google Scholar] [CrossRef]
- Trost, B.M.; Lupton, D.W. Dinuclear zinc-catalyzed enantioselective aza-Henry reaction. Org. Lett. 2007, 9, 2023–2026. [Google Scholar] [CrossRef]
- Handa, S.; Gnanadesikan, V.; Matsunaga, S.; Shibasaki, M. syn-Selective catalytic asymmetric nitro-Mannich reactions using a heterobimetallic Cu−Sm−Schiff base complex. J. Am. Chem. Soc. 2007, 129, 4900–4901. [Google Scholar]
- Yoon, T.P.; Jacobsen, E.N. Highly enantioselective thiourea-catalyzed nitro-Mannich reactions. Angew. Chem., Int. Ed. 2005, 44, 466–468. [Google Scholar]
- Xu, X.; Furukawa, T.; Okino, T.; Miyabe, H.; Takemoto, Y. Bifunctional-thiourea-catalyzed diastereo- and enantioselective aza-Henry reaction. Chem. Eur. J. 2006, 12, 466–476. [Google Scholar] [CrossRef]
- Bode, C.M.; Ting, A.; Schaus, S.E. A general organic catalyst for asymmetric addition of stabilized nucleophiles to acyl imines. Tetrahedron 2006, 62, 11499–11505. [Google Scholar] [CrossRef]
- Robak, M.T.; Trincado, M.; Ellman, J.A. Enantioselective aza-Henry reaction with an N-sulfinyl urea organocatalyst. J. Am. Chem. Soc. 2007, 129, 15110–15111. [Google Scholar] [CrossRef]
- Wang, C.J.; Dong, X.Q.; Zhang, Z.H.; Xue, Z.Y.; Teng, H.L. Highly anti-selective asymmetric nitro-Mannich reactions catalyzed by bifunctional amine-thiourea-bearing multiple hydrogen-bonding donors. J. Am. Chem. Soc. 2008, 130, 8606–8607. [Google Scholar] [CrossRef]
- Rampalakos, C.; Wulff, W.D. A novel bis-thiourea organocatalyst for the asymmetric aza-Henry reaction. Adv. Synth. Catal. 2008, 350, 1785–1790. [Google Scholar] [CrossRef]
- Takada, K.; Nagasawa, K. Enantioselective aza-Henry reaction with acyclic guanidine-thiourea bifunctional organocatalyst. Adv. Synth. Catal. 2009, 351, 345–347. [Google Scholar] [CrossRef]
- Nugent, B.M.; Yoder, R.A.; Johnston, J.N. Chiral proton catalysis: a catalytic enantioselective direct aza-Henry reaction. J. Am. Chem. Soc. 2004, 126, 3418–3419. [Google Scholar]
- Rueping, M.; Antonchick, A.P. Brønsted-acid-catalyzed activation of nitroalkanes: a direct enantioselective aza-Henry reaction. Org. Lett. 2007, 10, 1731–1734. [Google Scholar]
- Palomo, C.; Oiarbide, M.; Laso, A.; López, R. Catalytic enantioselective aza-Henry reaction with broad substrate scope. J. Am. Chem. Soc. 2005, 127, 17622–17623. [Google Scholar] [CrossRef]
- Gomez-Bengoa, E.; Linden, A.; López, R.; Múgica- Mendiola, I.; Oiarbide, M.; Palomo, C. Asymmetric aza-Henry reaction under phase transfer catalysis: an experimental and theoretical study. J. Am. Chem. Soc. 2008, 130, 7955–7966. [Google Scholar]
- Jiang, X.; Zhang, Y.; Wu, L.; Zhang, G.; Liu, X.; Zhang, H.; Fu, D.; Wang, R. Doubly stereocontrolled asymmetric aza-Henry reaction with in situ generation of N-Boc-imines catalyzed by novel rosin-derived amine thiourea catalysts. Adv. Synth. Catal. 2009, 351, 2096–2100. [Google Scholar] [CrossRef]
- Nitabaru, T.; Kumagai, N.; Shibasaki, M. A catalytic asymmetric anti-selective nitroaldol reaction with a neodymium sodium heterobimetallic complex. Tetrahedron Lett. 2008, 49, 272–276. [Google Scholar] [CrossRef]
- Nitabaru, T.; Nojiri, A.; Kobayashi, M.; Kumagai, N.; Shibasaki, M. anti-Selective catalytic asymmetric nitroaldol reaction via a heterobimetallic heterogeneous catalyst. J. Am. Chem. Soc. 2009, 131, 13860–13869. [Google Scholar]
- Yamamoto, H.; Futatsugi, K. “Designer acids”: combined acid catalysis for asymmetric synthesis. Angew. Chem.Int. Ed. 2005, 44, 1924–1942. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem.Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yang, J.W.; Hoffmann, S.; List, B. Asymmetric enamine catalysis. Chem. Rev. 2007, 107, 5471–5569. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shibasaki, M. Multimetallic bifunctional asymmetric catalysis based on proximity effect control. Bull. Chem. Soc. Jpn. 2008, 81, 60–75. [Google Scholar] [CrossRef]
- Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Recent progress in asymmetric bifunctional catalysis using multimetallic systems. Acc. Chem. Res. 2009, 42, 1117–1127. [Google Scholar] [CrossRef]
- Mashiko, T.; Hara, K.; Tanaka, D.; Fujiwara, Y.; Kumagai, N.; Shibasaki, M. En route to an efficient asymmetric synthesis of AS-3201. J. Am. Chem. Soc. 2007, 129, 11342–11343. [Google Scholar] [CrossRef]
- Mashiko, T.; Kumagai, N.; Shibasaki, M. An improved lanthanum catalytic system for asymmetric amination; toward a practical asymmetric synthesis of AS-3201 (ranirestat). Org. Lett. 2008, 10, 2725–2728. [Google Scholar] [CrossRef]
- Nojiri, A.; Kumagai, N.; Shibasaki, M. Asymmetric catalysis via dynamic substrate/ligand rare earth metal conglomerate. J. Am. Chem. Soc. 2008, 130, 5630–5631. [Google Scholar]
- Nojiri, A.; Kumagai, N.; Shibasaki, M. Linking structural dynamics and functional diversity in asymmetric catalysis. J. Am. Chem. Soc. 2009, 131, 3779–3784. [Google Scholar] [CrossRef]
- Mashiko, T.; Kumagai, N.; Shibasaki, M. Managing highly coordinative substrates in asymmetric catalysis: a catalytic asymmetric amination with a lanthanum-based ternary catalyst. J. Am. Chem. Soc. 2009, 131, 14990–14999. [Google Scholar]
- Boc. tert-butoxycarbonyl. N-Boc imines 1 were prepared by following the reported procedure; Kanazawa, A.M.; Denis, J.; Greene, A.E. Highly stereocontrolled and efficient preparation of the protected, esterification-ready docetaxel (taxotere) side chain. J. Org. Chem. 1994, 59, 1238. [CrossRef]
- Zhao, X.; Wang, X.Z.; Jiang, X.K.; Chen, Y.Q.; Li, Z.T.; Chen, G.J. Hydrazide-based quadruply hydrogen-bonded heterodimers. Structure, assembling selectivity, and supramolecular substitution. J. Am. Chem. Soc. 2003, 125, 15128–15139. [Google Scholar]
- Li, C.; Ren, S.F.; Hou, J.L.; Yi, H.P.; Zhu, S.Z.; Jiang, X.K.; Li, Z.T. F∙∙∙H–N hydrogen bonding driven foldamers: efficient receptors for dialkylammonium ions. Angew. Chem., Int. Ed. 2005, 44, 5725–5279. [Google Scholar]
- Solvents and Solvent Effects in Organic Chemistry; Reichardt, C. (Ed.) Wiley-VCH: Weinheim, Germany, 2003.
- The attempted reaction of 1a and 2a in the absence of ligand 4a under otherwise identical conditions (Yb(OiPr)3: 10 mol %, KHMDS: 20 mol %, THF, –60 °C, 22 h) afforded racemic 3aa in 53% yield (determined by 1H-NMR, anti/syn = 2.6/1), strongly suggested that unidentified achiral basic species promoted the background reaction to decrease the stereoselectivity.
- The identical reaction in Table 5, entry 3 gave the product 3ca after 20 h in 62% yield anti/syn = 18/1, 85% ee (anti), indicating the absence of retro-reaction and epimerization of the product during the reaction.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nitabaru, T.; Kumagai, N.; Shibasaki, M. Catalytic Asymmetric Nitro-Mannich Reactions with a Yb/K Heterobimetallic Catalyst. Molecules 2010, 15, 1280-1290. https://doi.org/10.3390/molecules15031280
Nitabaru T, Kumagai N, Shibasaki M. Catalytic Asymmetric Nitro-Mannich Reactions with a Yb/K Heterobimetallic Catalyst. Molecules. 2010; 15(3):1280-1290. https://doi.org/10.3390/molecules15031280
Chicago/Turabian StyleNitabaru, Tatsuya, Naoya Kumagai, and Masakatsu Shibasaki. 2010. "Catalytic Asymmetric Nitro-Mannich Reactions with a Yb/K Heterobimetallic Catalyst" Molecules 15, no. 3: 1280-1290. https://doi.org/10.3390/molecules15031280
APA StyleNitabaru, T., Kumagai, N., & Shibasaki, M. (2010). Catalytic Asymmetric Nitro-Mannich Reactions with a Yb/K Heterobimetallic Catalyst. Molecules, 15(3), 1280-1290. https://doi.org/10.3390/molecules15031280