Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria
Abstract
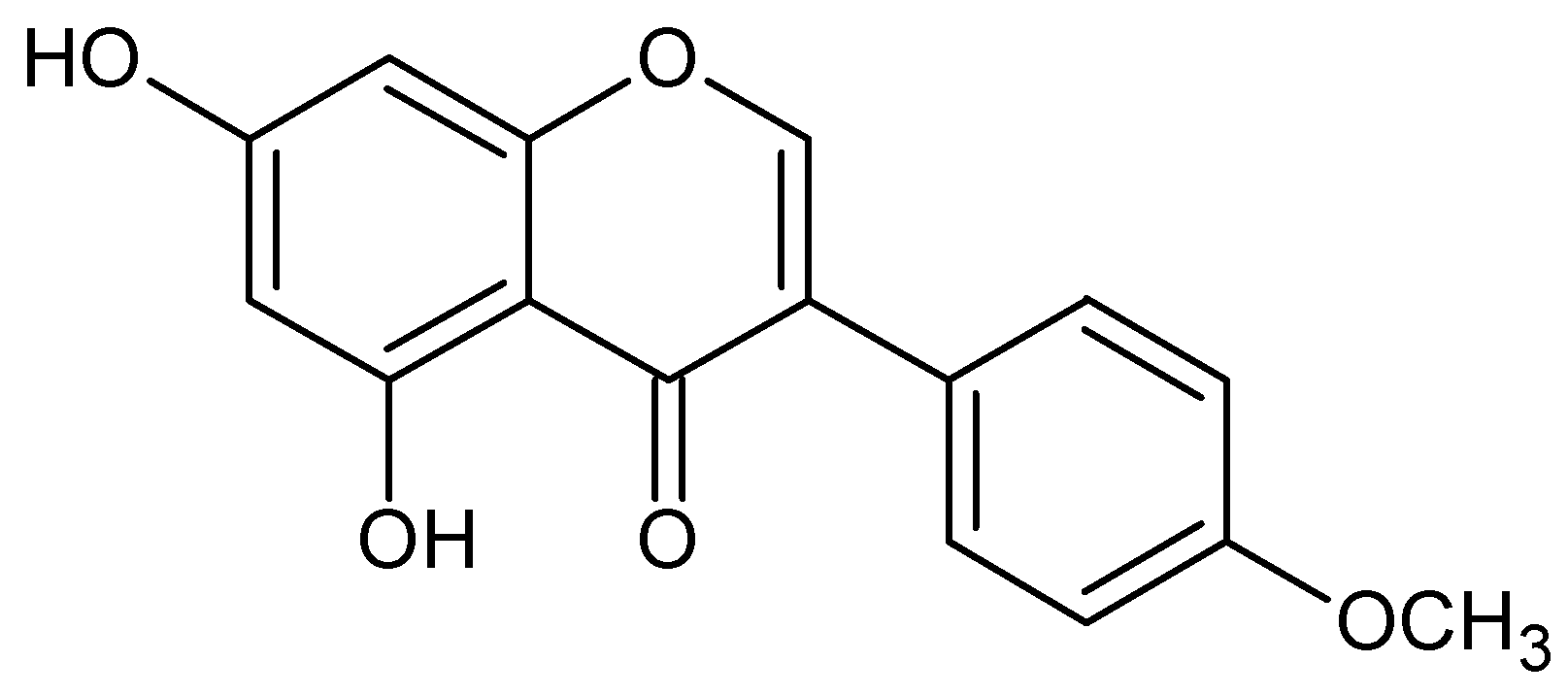
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Microbial cultures and media
3.2. Antimicrobial assay
4. Conclusions
Acknowledgements
References and Notes
- Viswanathan, V.K.; Hodges, K.; Hecht, G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhea. Nat. Rev. Microbiol. 2009, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Nord, C.E. The place of probiotics in human intestinal infections. Int. J. Antimicrob. Agents 2002, 20, 313–319. [Google Scholar] [CrossRef]
- Kuijper, E.J.; Coignard, B.; Tull, P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 2006, 12, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Fooks, L.J.; Fuller, R.; Gibson, G.R. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 1999, 9, 53–61. [Google Scholar] [CrossRef]
- Bartlett, J.G. Narrative review: The new epidemic of clostridium difficile-associated enteric disease. Ann. Intern Med. 2006, 145, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.E.; Price, A.B.; Honour, P.; Borriello, S.P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet 1978, 311, 1063–1066. [Google Scholar] [CrossRef]
- Lavigne, J.-P.; Bouziges, N.; Sotto, A.; Leroux, J.-L.; Michaux-Charachon, S. Spondylodiscitis Due to Clostridium ramosum Infection in an Immunocompetent Elderly Patient. J. Clin. Microbiol. 2003, 41, 2223–2226. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, O.F.; Solaroglu, I.; Tun, K.; Beskonakli, E.; Taskin, Y. Secondary infection of intracranial hydatid cyst with Clostridium ramosum. Child’s Nerv. Syst. 2005, 21, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Borregagarcia, P.; Jimenezmejias, M.E.; Chinchon, I.; Cuellocontreras, J.A. Paravertebral abscess and meningitis by Clostridium clostridioforme. Med. Clin.-Barcelona 1994, 102, 279. [Google Scholar]
- Spitzer, R.D.; Ratzan, K.R. Chronic osteomyelitis due to Clostridium clostridiiforme. South. Med. J. 1991, 84, 671–672. [Google Scholar] [PubMed]
- Marrie, T.J.; Costerton, J.W. Mode of growth of bacterial pathogens in chronic polymicrobial human osteomyelitis. J. Clin. Microbiol. 1985, 22, 924–933. [Google Scholar] [PubMed]
- Drudy, D.; Harnedy, N.; Fanning, S.; Hannan, M.; Kyne, L. Emergence and control of fluoroquinolone-resistant, toxin a-negative, toxin B-Positive Clostridium difficile. Infect. Control Hosp. Epidemiol. 2007, 28, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Hookman, P.; Barkin, J.S. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroentero. 2009, 15, 1554–1580. [Google Scholar] [CrossRef]
- Bartlett, J.G. The case for vancomycin as the preferred drug for treatment of Clostridium difficile infection. Clin. Infect. Dis. 2008, 46, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.; Safdar, N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int. J. Infect Dis. 2009, 13, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Weintraub, A.; Fang, H.; Nord, C.E. Antimicrobial resistance in Clostridium difficile. Int. J. Antimicrob. Agents 2009, 34, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yamamoto, K.; Tamura, Y.; Takahashi, T. Tetracycline-resistance genes of Clostridium perfringens, Clostridium septicum and Clostridium sordellii isolated from cattle affected with malignant edema. Vet. Microbiol. 2001, 83, 61–69. [Google Scholar] [CrossRef]
- Fekety, R.; Shah, A.B. Diagnosis and treatment of Clostridium difficile colitis. JAMA 1993, 269, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Fekety, R.; Silva, J.; Kauffman, C.; Buggy, B.; Deery, H.G. Treatment of antibiotic-associated clostridium difficile colitis with oral vancomycin - comparasion of 2 dosage regimens. Am. J. Med. 1989, 86, 15–19. [Google Scholar] [CrossRef]
- Walters, B.A.J.; Roberts, R.; Stafford, R.; Seneviratne, E. Relapse on antibiotic associated colitis - endogenous persistence of Clostridium-difficile during vancomycin therapy. Gut 1983, 24, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The crisis in antibiotic-resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. A randomized placebo-controlled trial of Saccharomyces-boulardii in combination with standard antibodies for Clostridium difficile disease. JAMA 1994, 272, 518. [Google Scholar]
- Tannock, G.W. Identification of Lactobacilli and Bifidobacteria. Curr. Issues Mol. Biol. 1999, 1, 53–64. [Google Scholar] [PubMed]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Trejo, F.M.; Minnaard, J.; Perez, P.F.; De Antoni, G.L. Inhibition of Clostridium difficile growth and adhesion to enterocytes by Bifidobacterium supernatants. Anaerobe 2006, 12, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yu, W.K.; Heo, T.R. Identification and screening for antimicrobial activity against Clostridium difficile of Bifidobacterium and Lactobacillus species isolated from healthy infant faeces. Int. J. Antimicrob. Agents 2003, 21, 340–346. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Phillips, D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Johnson, G.; Maag, D.D.; Johnson, D.K.; Thomas, R.D. Possible role of phytoalexins in resistance of sugarbeet (Beta vulgaris) to Cercospora beticola. Physiol. Plant Pathol. 1976, 8, 225–230. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Manna, A.; Kumar, K.A.; Mazumdar, K.; Dutta, N.K.; Chakrabarty, A.N.; Motohashi, N.; Shirataki, Y. Studies on the antibacterial potentiality of isoflavones. Int. J. Antimicrob. Agents 2004, 23, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Gnanamanickam, S.S.; Smith, D.A. Selective toxicity of isoflavonoid phytoalexins to Gram-positive bacteria. Phytopathology 1980, 70, 894–896. [Google Scholar] [CrossRef]
- Hong, H.K.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Fujiwara, S.; Hirata, M.; Yamaguchi, R.; Etoh, H.; Tokuda, C. Antibacterial property of isoflavonoids isolated from Erythrina variegata against cariogenic oral bacteria. Phytomedicine 2003, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Weidenborner, M.; Hindorf, H.; Jha, H.C.; Tsotsonos, P.; Egge, H. Antifungal activity of isoflavonoids in different reduced stages on Rhizoctonia solani and Sclerotium rolfsii. Phytochemistry 1990, 29, 801–803. [Google Scholar] [CrossRef]
- Kramer, R.P.; Hindorf, H.; Jha, H.C.; Kallage, J.; Zilliken, F. Antifungal activity of soybean and chickpea isoflavonec and their reduced derivatives. Phytochemistry 1984, 23, 2203–2205. [Google Scholar] [CrossRef]
- Rojas, R.; Bustamante, B.; Ventosilla, P.; Fernadez, I.; Caviedes, L.; Gilman, R.H.; Lock, O.; Hammond, G.B. Larvicidal, antimycobacterial and antifungal compounds from the bark of the Peruvian plant Swartzia polyphylla DC. Chem. Pharm. Bull. 2006, 54, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Sklenickova, O.; Flesar, J.; Kokoska, L. Selective antimicrobial activity of isoflavonoids. Ann. Nutr. Metab. 2009, 55, 391. [Google Scholar]
- Paredes, C.J.; Alsaker, K.V.; Papoutsakis, E.T. A Comparative Genomic View of Clostridial Sporulation and Physiology. Nat. Rev. Microbiol. 2005, 3, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Yuli, S.; Chengxu, L.; Sydney, F.M. Multiplex PCR for rapid differentiation of three species in the “Clostridium clostridioforme group”. FEMS Microbiol. Lett. 2005, 244, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Clostridial infection in children. J. Med. Microbiol. 1995, 42, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, J.; van Hoek, A.; Domig, K.J.; Saarela, M.; Florez, A.B.; Brockmann, E.; Amtmann, E.; Mayo, B.; Aarts, H.J.M.; Danielsen, M. Susceptibility of human and probiotic Bifidobacterium spp. to selected antibiotics as determined by the Etest method. Int. Dairy J. 2007, 17, 1123–1131. [Google Scholar] [CrossRef]
- Kiwaki, M.; Sato, T. Antimicrobial susceptibility of Bifidobacterium breve strains and genetic analysis of streptomycin resistance of probiotic B. breve strain Yakult. Int. J. Food Microbiol. 2009, 134, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Stermitz, F.R.; Tegos, G.; Lewis, K. Isoflavones as potentiators of antibacterial activity. Int. J. Food Microbiol. 2003, 51, 5677–5679. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Saviranta, N.M.; Anttonen, M.J.; von Wright, A.; Karjalainen, R.O. Red clover (Trifolium pratense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar]
- Batterha, T.J.; Shutt, D.A.; Hart, N.K.; Braden, A.W.H.; Tweeddal, H.J. Metabolism of intraruminally administered [4-C-14]formononetin and [4-C-14]biochanin A in sheep. Aust. J. Agric. Res. 1971, 22, 131–138. [Google Scholar] [CrossRef]
- Davies, F.T.; Calderon, C.M.; Human, Z.; Gomez, R. Influence of a flavonoid (formononetin) on mycorrhizal activity and potato crop productivity in the highlands of Peru. Sci. Hortic.-Amsterdam 2005, 106, 318–329. [Google Scholar] [CrossRef]
- Occhiuto, F.; Zangla, G.; Samperi, S.; Palumbo, D.R.; Pino, A.; De Pasquale, R.; Circosta, C. The phytoestrogenic isoflavones from Trifolium pratense L. (Red clover) protects human cortical neurons from glutamate toxicity. Phytomedicine 2008, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Karita, S.; Kimura, T.; Sakka, K.; Ohmiya, K. Characterization of Clostridium paraputrificum Chitanase A from a Recombinant Escherichia coli. J. Biosci. Bioeng. 2001, 92, 466–468. [Google Scholar] [CrossRef]
- Evvyernie, D.; Yamazaki, S.; Morimoto, K.; Karita, S.; Kimura, T.; Sakka, K.; Ohmiya, K. Identification and Characterization of Clostridium paraputrificum M-21, a Chitinolytic, Mesophilic and Hydrogen-Producing Bacterium. J. Biosci. Bioeng. 2000, 89, 596–601. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Antimicrobial Susceptibility Testing: General Considerations. In Manual Of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Al., E., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 1469–1473. [Google Scholar]
Sample Availability: Samples of biochanin A are commercially available. |
| Bacterial strains | MIC1 (μg/mL) | |
|---|---|---|
| biochanin A | tetracycline | |
| Bifidobacterium spp. | ||
| B. animalis CCM2 4988 | >4096 | 1 |
| B. bifidum ATCC3 29 521 | >4096 | 1 |
| B. breve ATCC 15 700 | >4096 | 0.5 |
| B. catenulatum CCM 4989 | >4096 | 0.5 |
| B. infantis ATCC 17 930 | >4096 | 1 |
| B. longum ATCC 15 707 | >4096 | 1 |
| Average | >4096 | 0.83 |
| Clostridium spp. | ||
| Cl. butyricum DSM4 10702 | 512 | 0.0625 |
| Cl. clostridioforme DSM 933 | 64 | 0.025 |
| Cl. paraputrificum DSM 2630 | 256 | 8 |
| Cl. perfringens DSM 11778 | 1024 | 4 |
| Cl. ramosum DSM 1402 | 256 | 1 |
| Cl. tertium DSM 2485 | 512 | 0.0625 |
| Cl. acetobutylicum I51 | 512 | 0.0625 |
| Cl. butylicum I 2 | 512 | 0.0625 |
| Cl. clostridioforme I 3 | 64 | 0.0156 |
| Cl. difficile I 4 | 256 | 0.0625 |
| Cl. ramosum I 5 | 256 | 0.0625 |
| Average | 384 | 1.2196 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sklenickova, O.; Flesar, J.; Kokoska, L.; Vlkova, E.; Halamova, K.; Malik, J. Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria. Molecules 2010, 15, 1270-1279. https://doi.org/10.3390/molecules15031270
Sklenickova O, Flesar J, Kokoska L, Vlkova E, Halamova K, Malik J. Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria. Molecules. 2010; 15(3):1270-1279. https://doi.org/10.3390/molecules15031270
Chicago/Turabian StyleSklenickova, Olga, Jaroslav Flesar, Ladislav Kokoska, Eva Vlkova, Katerina Halamova, and Jan Malik. 2010. "Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria" Molecules 15, no. 3: 1270-1279. https://doi.org/10.3390/molecules15031270
APA StyleSklenickova, O., Flesar, J., Kokoska, L., Vlkova, E., Halamova, K., & Malik, J. (2010). Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria. Molecules, 15(3), 1270-1279. https://doi.org/10.3390/molecules15031270




