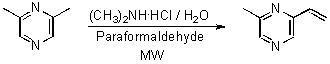
Extraction with SPME and Synthesis of 2-Methyl-6-vinylpyrazine by a ‘One Pot’ Reaction Using Microwaves
Abstract
:Introduction
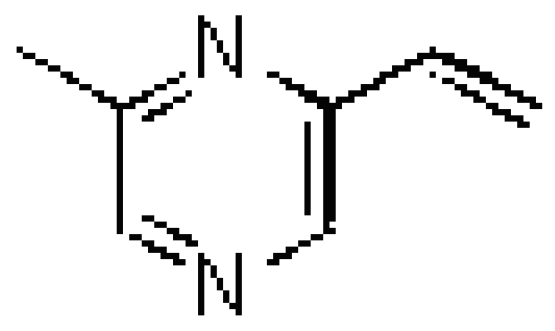
Results and Discussion
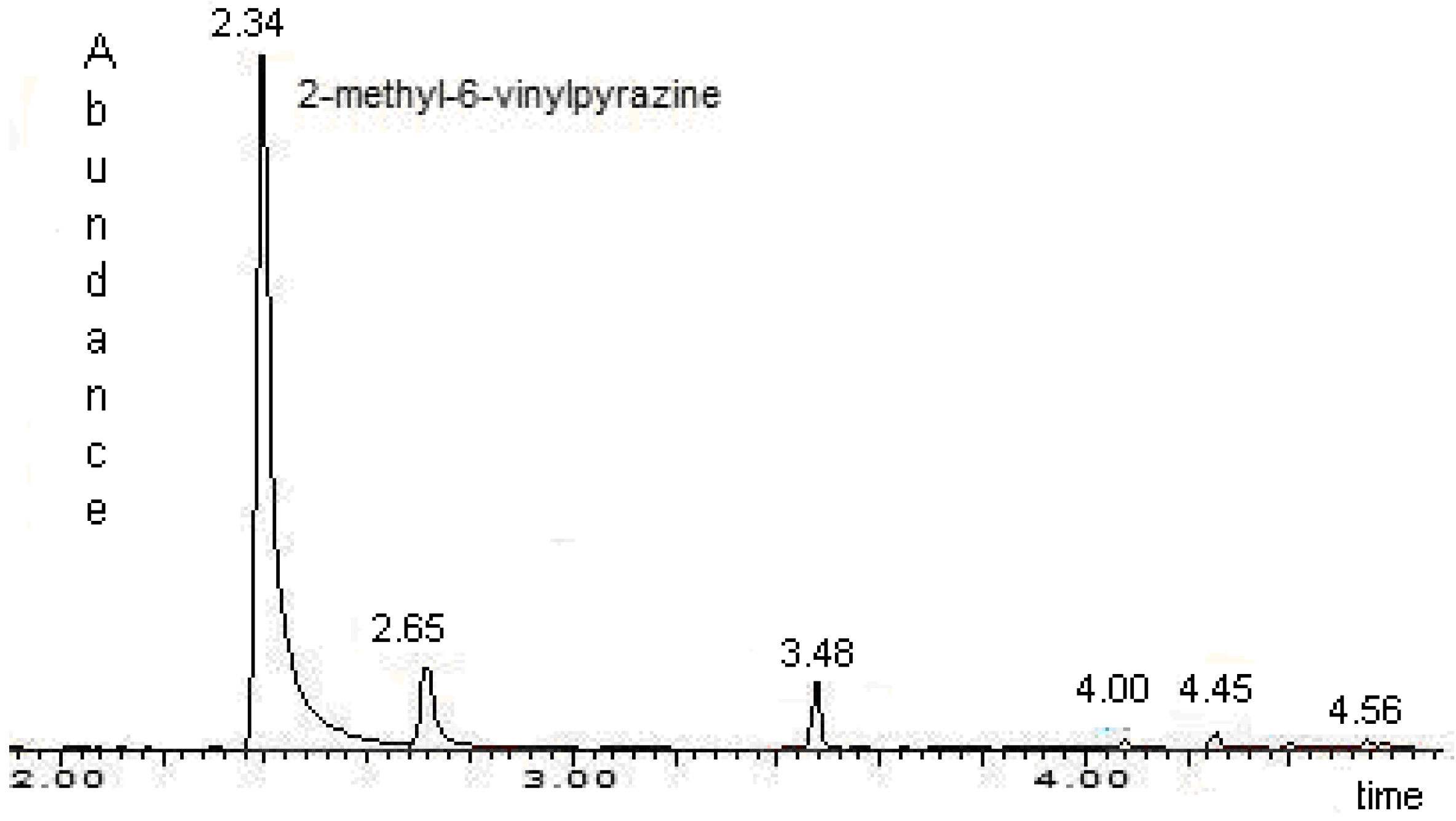
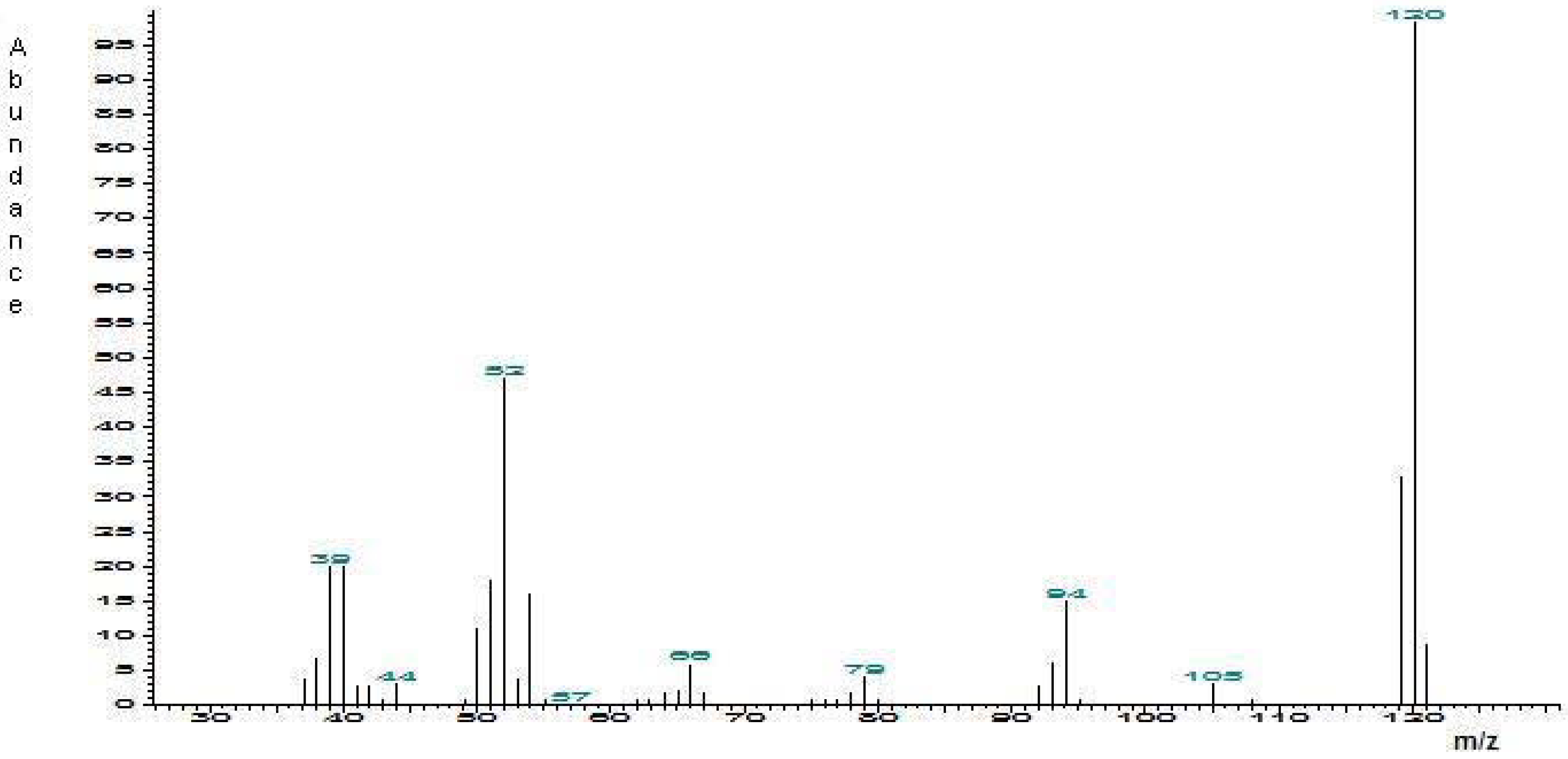
Identification of 2-methyl-6-vinylpyrazine in male emissions
Synthesis of 2-methyl-6-vinylpyrazine
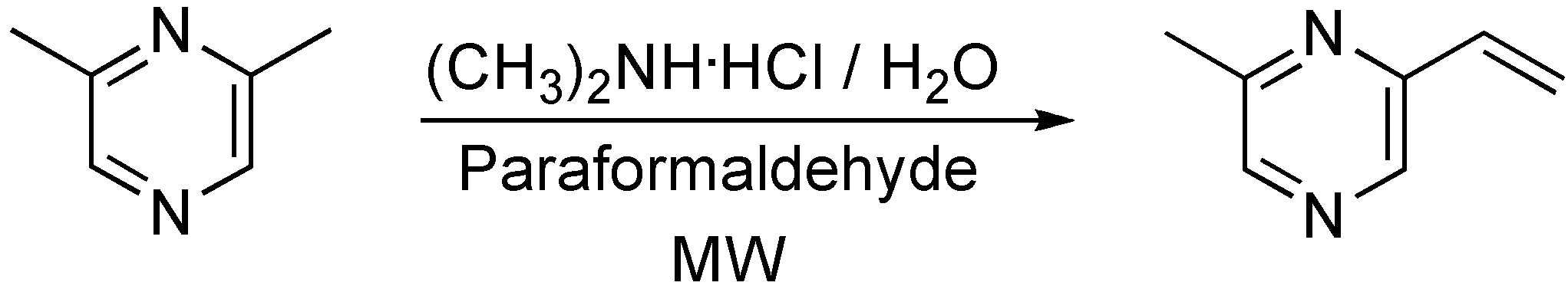
Conclusions
Experimental
General
Insects
Extraction and identification of volatile compounds
Quantitative Analysis
Acknowledgements
References and Notes
- Landolt, P.J. Chemical Ecology of the Papaya Fruit flies. Fruit flies. Biology and Management; Aluja, M., Liedo, P., Eds.; Springer Verlag Inc.: New York, NY, USA, 1993; pp. 207–210. [Google Scholar]
- Chuman, T.; Landolt, P.J.; Heath, R.R.; Tumilson, J.H. Isolation, identification and synthesis of male-produced sex pheromone of papaya fruit fly, Toxotrypana curvicauda Gerstaecker (Diptera: Tephritidae). J. Chem. Ecol. 1987, 13, 1979–1992. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Zhang, Y. Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J. Agric. Food Chem. 2007, 55, 9956–9962. [Google Scholar] [CrossRef]
- Sanz, C.; Ansorena, D.; Bello, J.; Cid, C. Optimizing headspace temperature and time sampling for identification of volatile compounds in Ground Roasted Arabica coffee. J. Agric. Food Chem. 2001, 49, 1364–1369. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Elmore, J.S.; Mottram, D.S. Comparison of the Aroma Characteristics of Acid-Hydrolyzed and Enzyme-Hydrolyzed Vegetable Proteins Produced from Soy. J. Agric. Food Chem. 1998, 46, 5225–5231. [Google Scholar] [CrossRef]
- Bredie, W.L.P.; Mottram, D.S.; Guy, R.C.E. Aroma Volatiles Generated during Extrusion Cooking of Maize Flour. J. Agric. Food Chem. 1998, 46, 1479–1487. [Google Scholar] [CrossRef]
- Bredie, W.L.P.; Mottram, D.S.; Guy, R.C.E. Effect of Temperature and pH on the Generation of Flavor Volatiles in Extrusion Cooking of Wheat Flour. J. Agric. Food Chem. 2002, 50, 1118–1125. [Google Scholar] [CrossRef]
- Boulton, A.J.; McKillop, A.; Rowbottom, P.M. The Synthesis of (1,2-Dihydroxyethyl)pyrazines, and their Identification in Caramels. J. Chem. Res. Syn. 1989, 59. [Google Scholar]
- Yang, X.; Peppard, T. Solid- Phase Microextraction for Flavor Analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar]
- Matloubi, M.F.; Rezanejade, B.G.; Ismaili, H. Microwave-Assisted One-Pot Synthesis of Symmetrical 4H- Pyran-4-ones. J. Braz. Chem. Soc. 2007, 18, 1024–1027. [Google Scholar] [CrossRef]
- Hayes, B. L. Microwave Synthesis. Chemistry at the Speed of Light; CEM Publishing: Mattehews, NC, USA, 2002; pp. 11–26. [Google Scholar]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem., Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Neuschl, M.; Bogdal, D.; Potacek, M. Microwave-Assisted Synthesis of Substituted Hexahydropyrrolo [3,2-c] quinolines. Molecules 2007, 12, 49–59. [Google Scholar] [CrossRef]
- Escalante, J.; Carrillo-Morales, M.; Linzaga, I. Michael Additions of Amines to Methyl Acrylates Promoted by Microwave Irradiation. Molecules 2008, 13, 340–347. [Google Scholar] [CrossRef]
- Laroche, M.F.; Belotti, D.; Cossy, J. A One-Pot Reformatsky/Cyclopropanation Sequence Induced by Diethylzinc. Org Lett. 2005, 7, 171–173. [Google Scholar] [CrossRef]
- Unciti-Broceta, A.; Pineda de las Infantas, M. J.; Díaz Mochón, J.J.; Romagnoli, R.; Baraldi, P.G.; Gallo, M.A.; Espinosa, A. Regioselective One-Pot Synthesis of 9-Alkyl-6-chloropyrido [3,2-e][1,2,4]triazolo[4,3-a]pyrazines. Reactivity of Aliphatic and Aromatic Hydrazides. J. Org. Chem. 2005, 70, 2878–2880. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, Z.; Guo, H.; Geng, M.; Xia, R. Microwave-promoted Facile and Efficient Preparation of N-(alkoxycarbonylmethyl) Nucleobases − Building Blocks for Peptide Nucleic Acids. Molecules 2007, 12, 543–551. [Google Scholar] [CrossRef]
- Sample Availability: Small samples of 2-methyl-6-vinylpyrazine (a few milligrams) are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Robledo, N.; Escalante, J.; Arzuffi, R. Extraction with SPME and Synthesis of 2-Methyl-6-vinylpyrazine by a ‘One Pot’ Reaction Using Microwaves. Molecules 2009, 14, 2160-2166. https://doi.org/10.3390/molecules14062160
Robledo N, Escalante J, Arzuffi R. Extraction with SPME and Synthesis of 2-Methyl-6-vinylpyrazine by a ‘One Pot’ Reaction Using Microwaves. Molecules. 2009; 14(6):2160-2166. https://doi.org/10.3390/molecules14062160
Chicago/Turabian StyleRobledo, Norma, Jaime Escalante, and René Arzuffi. 2009. "Extraction with SPME and Synthesis of 2-Methyl-6-vinylpyrazine by a ‘One Pot’ Reaction Using Microwaves" Molecules 14, no. 6: 2160-2166. https://doi.org/10.3390/molecules14062160
APA StyleRobledo, N., Escalante, J., & Arzuffi, R. (2009). Extraction with SPME and Synthesis of 2-Methyl-6-vinylpyrazine by a ‘One Pot’ Reaction Using Microwaves. Molecules, 14(6), 2160-2166. https://doi.org/10.3390/molecules14062160