Efficacy Evaluation of Plant Products in the Treatment of Erectile Dysfunction Related to Diabetes
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Supplies
4.3. Establishment of the Diabetic Rat Model
4.4. Study Design
4.5. Glucose Measurement
4.6. Efficacy Evaluation
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, J.; Dagur, G.; Warren, K.; Smith, N.L.; Sheynkin, Y.R.; Zumbo, A.; Khan, S.A. The Role of Diabetes Mellitus in Sexual and Reproductive Health: An Overview of Pathogenesis, Evaluation and Management. Curr. Diabetes Rev. 2017, 13, 573–581. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabet. Med. 2017, 34, 1185–1192. [Google Scholar] [CrossRef]
- Binmoammar, T.A.; Hassounah, S.; Alsaad, S.; Rawaf, S.; Majeed, A. The impact of poor glycaemic control on the prevalence of erectile dysfunction in men with type 2 diabetes mellitus: A systematic review. JRSM Open 2016, 7, 2054270415622602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenz de Tejada, I.; Goldstein, I.; Azadzoi, K.; Krane, R.J.; Cohen, R.A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N. Engl. J. Med. 1989, 320, 1025–1030. [Google Scholar] [CrossRef]
- Cartledge, J.J.; Eardley, I.; Morrison, J.F. Advanced glycation end-products are responsible for the impairment of corpus cavernosal smooth muscle relaxation seen in diabetes. BJU Int. 2001, 87, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kawamura, N.; Dyck, P.J.; Dyck, P.J.B.; Kihara, M.; Low, P.A. Spectrum of diabetic neuropathies. Diabetol. Int. 2020, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Dandona, P. Hypogonadotropic Hypogonadism in Men with Diabesity. Diabetes Care 2018, 41, 1516–1525. [Google Scholar] [CrossRef] [Green Version]
- Andersen, I.; Heitmann, B.L.; Wagner, G. Obesity and sexual dysfunction in younger Danish men. J. Sex. Med. 2008, 5, 2053–2060. [Google Scholar] [CrossRef]
- Masuku, N.P.; Unuofin, J.O.; Lebelo, S.L. Promising role of medicinal plants in the regulation and management of male erectile dysfunction. Biomed. Pharmacother. 2020, 130, 110555. [Google Scholar] [CrossRef]
- Kirby, M. The Circle of Lifestyle and Erectile Dysfunction. Sex. Med. Rev. 2015, 3, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.S.; Geethakumari, A.M.; Biswas, K.H. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. 2021, 134, 111128. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Comeglio, P.; Filippi, S.; Sarchielli, E.; Vignozzi, L.; Maneschi, E.; Cellai, I.; Gacci, M.; Lenzi, A.; Vannelli, G.B.; et al. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: An experimental study in the rabbit. Prostate 2013, 73, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Vignozzi, L.; Gacci, M.; Cellai, I.; Morelli, A.; Maneschi, E.; Comeglio, P.; Santi, R.; Filippi, S.; Sebastianelli, A.; Nesi, G.; et al. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013, 73, 1391–1402. [Google Scholar] [CrossRef]
- Vignozzi, L.; Filippi, S.; Comeglio, P.; Cellai, I.; Morelli, A.; Maneschi, E.; Sarchielli, E.; Gacci, M.; Carini, M.; Vannelli, G.B.; et al. Tadalafil effect on metabolic syndrome-associated bladder alterations: An experimental study in a rabbit model. J. Sex. Med. 2014, 11, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Hanchanale, V.; Eardley, I. Alprostadil for the treatment of impotence. Expert Opin. Pharmacother. 2014, 15, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Burri, A.; Serra, E.; Gianfrilli, D.; Mannucci, E.; Jannini, E.A.; Maggi, M. First-generation phosphodiesterase type 5 inhibitors dropout: A comprehensive review and meta-analysis. Andrology 2016, 4, 1002–1009. [Google Scholar] [CrossRef]
- Porst, H. Transurethral alprostadil with MUSE (medicated urethral system for erection) vs intracavernous alprostadil—A comparative study in 103 patients with erectile dysfunction. Int. J. Impot. Res. 1997, 9, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Srivatsav, A.; Balasubramanian, A.; Pathak, U.I.; Rivera-Mirabal, J.; Thirumavalavan, N.; Hotaling, J.M.; Lipshultz, L.I.; Pastuszak, A.W. Efficacy and Safety of Common Ingredients in Aphrodisiacs Used for Erectile Dysfunction: A Review. Sex. Med. Rev. 2020, 8, 431–442. [Google Scholar] [CrossRef]
- Shamsa, A.; Hosseinzadeh, H.; Molaei, M.; Shakeri, M.T.; Rajabi, O. Evaluation of Crocus sativus L. (saffron) on male erectile dysfunction: A pilot study. Phytomedicine 2009, 16, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Maisto, E.; Romis, L.; Celentano, G. Use of a natural compound made of Ecklonia bicyclis seaweed.; Tribulus terrestris and water-soluble chitosan oligosaccharide.; in male sexual asthenia with mild or mild-moderate erectile dysfunction and serum testosterone levels at the lower limit of normal. Health 2016, 8, 1668–1678. [Google Scholar]
- Choi, Y.D.; Park, C.W.; Jang, J.; Kim, S.H.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Chung, W.S. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: A multicenter.; placebo-controlled.; double-blind clinical study. Int. J. Impot. Res. 2013, 25, 45–50. [Google Scholar] [CrossRef]
- De Andrade, E.; De Mesquita, A.A.; Claro, J.D.A.; De Andrade, P.M.; Ortiz, V. Study of the efficacy of Korean red ginseng in the treatment of erectile dysfunction. Asian J. Androl. 2007, 9, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Mirone, V.; Napolitano, L.; D’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Sorrentino, R.; Vanelli, A.; Vanacore, D.; Turnaturi, C.; La Rocca, R.; Celentano, G.; et al. A new original nutraceutical formulation ameliorates the effect of Tadalafil on clinical score and cGMP accumulation. Arch. Ital. Urol. Androl. 2021, 93, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile Dysfunction: AUA Guideline. J. Urol. 2018, 200, 633–641. [Google Scholar] [CrossRef]
- Rendell, M.S.; Rajfer, J.; Wicker, P.A.; Smith, M.D. Sildenafil for treatment of erectile dysfunction in men with diabetes: A randomized controlled trial. Sildenafil Diabetes Study Group. JAMA 1999, 281, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, I.; Young, J.M.; Fischer, J.; Bangerter, K.; Segerson, T.; Taylor, T.; Vardenafil Diabetes Study Group. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: A multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care 2003, 26, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.G.; Feldman, R.A.; Auerbach, S.M.; Deriesthal, H.; Wilson, N.; Multicenter Study Group. Long-term safety and effectiveness of sildenafil citrate in men with erectile dysfunction. Ther. Clin. Risk Manag. 2007, 3, 975–981. [Google Scholar]
- Corona, G.; Maggi, M.; Jannini, E.A. EDEUS.; a Real-Life Study on the Users of Phosphodiesterase Type 5 Inhibitors: Prevalence, Perceptions and Health Care-Seeking Behavior among European Men with a Focus on 2nd-Generation Avanafil. Sex. Med. 2018, 6, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Yafi, F.A.; Sharlip, I.D.; Becher, E.F. Update on the Safety of Phosphodiesterase Type 5 Inhibitors for the Treatment of Erectile Dysfunction. Sex. Med. Rev. 2018, 6, 242–252. [Google Scholar] [CrossRef] [PubMed]
- The European Alprostadil Study Group. The long-term safety of alprostadil (prostaglandin-E1) in patients with erectile dysfunction. Br. J. Urol. 1998, 82, 538–543. [Google Scholar]
- Heaton, J.P.; Lording, D.; Liu, S.N.; Litonjua, A.D.; Guangwei, L.; Kim, S.C.; Kim, J.J.; Zhi-Zhou, S.; Israr, D.; Niazi, D.; et al. Intracavernosal alprostadil is effective for the treatment of erectile dysfunction in diabetic men. Int. J. Impot. Res. 2001, 13, 317–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.K.; Lee, T.; Kim, D.J.; Park, I.S.; Yoon, S.M.; Lee, H.S.; Song, S.U.; Suh, J.K. Free radical-scavenging activity of Korean red ginseng for erectile dysfunction in non-insulin-dependent diabetes mellitus rats. Urology 2005, 65, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, Y.F.; Jia, C.; Yang, B.H.; Tao, X.; Li, J.; Fang, X. Ginsenoside Rg3 improves erectile function in streptozotocin-induced diabetic rats. J. Sex. Med. 2015, 12, 611–620. [Google Scholar] [CrossRef]
- Li, H.; He, W.Y.; Lin, F.; Gou, X. Panax notoginseng saponins improve erectile function through attenuation of oxidative stress.; restoration of Akt activity and protection of endothelial and smooth muscle cells in diabetic rats with erectile dysfunction. Urol. Int. 2014, 93, 92–99. [Google Scholar] [CrossRef]
- Lin, F.; Gou, X. Panax notoginseng saponins improve the erectile dysfunction in diabetic rats by protecting the endothelial function of the penile corpus cavernosum. Int. J. Impot. Res. 2013, 25, 206–211. [Google Scholar] [CrossRef]
- Prabsattroo, T.; Wattanathorn, J.; Iamsaard, S.; Somsapt, P.; Sritragool, O.; Thukhummee, W.; Muchimapura, S. Moringa oleifera extract enhances sexual performance in stressed rats. J. Zhejiang Univ. Sci. B 2015, 16, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, S.K.; Inamdar, M.N.; Dethe, S.M.; Gururaj, G.M.; Jamwal, R.; Bhaskar, A.; Mundkinajeddu, D.; Agarwal, A. Erectogenic and Aphrodisiac Property of Moringa oleifera: Involvement of Soluble Epoxide Hydrolase Enzyme. Phytother. Res. 2016, 30, 1119–1127. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef] [Green Version]
- Oyeleye, S.I.; Ojo, O.R.; Oboh, G. Moringa oleifera leaf and seed inclusive diets influenced the restoration of biochemicals associated with erectile dysfunction in the penile tissue of STZ-induced diabetic male rats treated with/without Acarbose drug. J. Food Biochem. 2021, 45, e13323. [Google Scholar] [CrossRef] [PubMed]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves.; stem.; and root barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef]
- Oboh, G.; Adebayo, A.A.; Ademosun, A.O.; Boligon, A.A. In vitro inhibition of phosphodiesterase-5 and arginase activities from rat penile tissue by two Nigerian herbs (Hunteria umbellata and Anogeissus leiocarpus). J. Basic Clin. Physiol. Pharmacol. 2017, 28, 393–401. [Google Scholar] [CrossRef]
- Al-Roujeaie, A.S.; Abuohashish, H.M.; Ahmed, M.M.; Alkhamees, O.A. Effect of rutin on diabetic-induced erectile dysfunction: Possible involvement of testicular biomarkers in male rats. Andrologia 2017, 49, e12737. [Google Scholar] [CrossRef]
- Butchi Akondi, R.; Kumar, P.; Annapurna, A.; Pujari, M. Protective Effect of Rutin and Naringin on Sperm Quality in Streptozotocin (STZ) Induced Type 1 Diabetic Rats. Iran. J. Pharm. Res. 2011, 10, 585–596. [Google Scholar]
- Boydens, C.; Pauwels, B.; Vanden Daele, L.; Van de Voorde, J. Protective effect of resveratrol and quercetin on in vitro-induced diabetic mouse corpus cavernosum. Cardiovasc. Diabetol. 2016, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Zhang, Z.; Su, H.; Xu, P.; Qi, H.; Zhao, D.; Li, X. Panax ginseng clinical trials: Current status and future perspectives. Biomed. Pharmacother. 2020, 132, 110832. [Google Scholar] [CrossRef]
- Shergis, J.L.; Thien, F.; Worsnop, C.J.; Lin, L.; Zhang, A.L.; Wu, L.; Chen, Y.; Xu, Y.; Langton, D.; Da Costa, C.; et al. 12-month randomised controlled trial of ginseng extract for moderate COPD. Thorax 2019, 74, 39–545. [Google Scholar] [CrossRef] [PubMed]
- Gambo, A.; Moodley, I.; Babashani, M.; Babalola, T.K. Impact of Moringa Oleifera leaves supplementation on quality of life of people living with HIV: A double-blind randomized controlled trial. Qual. Life Res. 2021, 30, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Tshingani, K.; Donnen, P.; Mukumbi, H.; Duez, P.; Dramaix-Wilmet, M. Impact of Moringa oleifera lam. Leaf powder supplementation versus nutritional counseling on the body mass index and immune response of HIV patients on antiretroviral therapy: A single-blind randomized control trial. BMC Complement Altern. Med. 2017, 17, 420. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Impact of Rutin and Vitamin C Combination on Oxidative Stress; Insulin Sensitivity and Lipid Profile in Type 2 Diabetic Patients—NCT03437902. Available online: www.clinicaltrials.gov (accessed on 10 December 2021).
- Evaluation of The Effect of Rutin and Vitamin C on the Oxidative Stress in Hemodialysis Patients—NCT04955145. Available online: www.clinicaltrials.gov (accessed on 10 December 2021).
- Adefegha, S.A.; Oyeleye, S.I.; Dada, F.A.; Olasehinde, T.A.; Oboh, G. Modulatory effect of quercetin and its glycosylated form on key enzymes and antioxidant status in rats penile tissue of paroxetine-induced erectile dysfunction. Biomed. Pharmacother. 2018, 107, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Qinna, N.; Taha, H.; Matalka, K.Z.; Badwan, A.A. A new herbal combination, Etana, for enhancing erectile function: An efficacy and safety study in animals. Int. J. Impot. Res. 2009, 21, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.D.; Kim, Y.J.; Huh, J.S.; Kim, S.W.; Sohn, D.W. Improvement of erectile function by Korean red ginseng (Panax ginseng) in a male rat model of metabolic syndrome. Asian J. Androl. 2013, 15, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Kopalli, S.R.; Hwang, S.Y.; Won, Y.J.; Kim, S.W.; Cha, K.M.; Han, C.K.; Hong, J.Y.; Kim, S.K. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp. Gerontol. 2015, 69, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
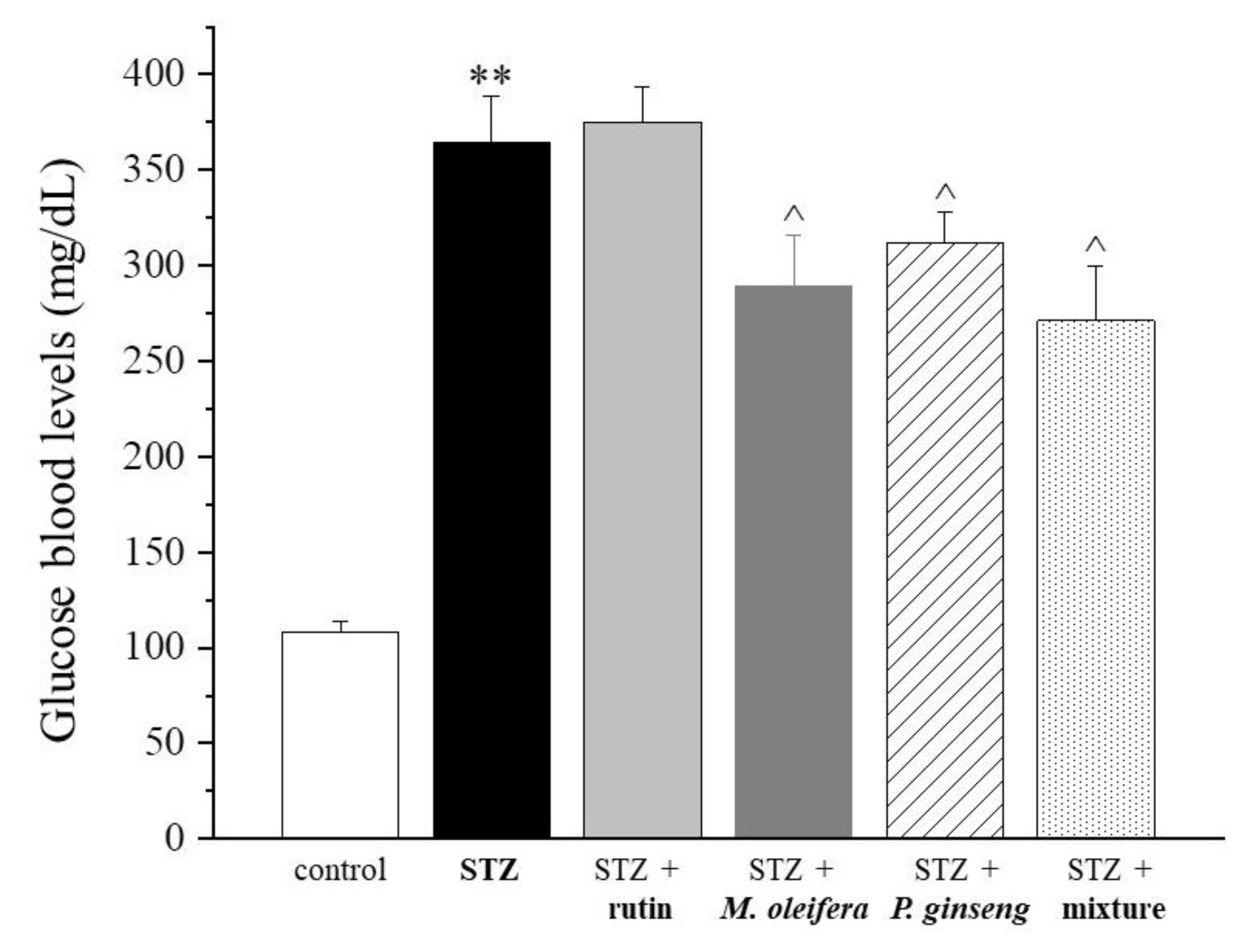
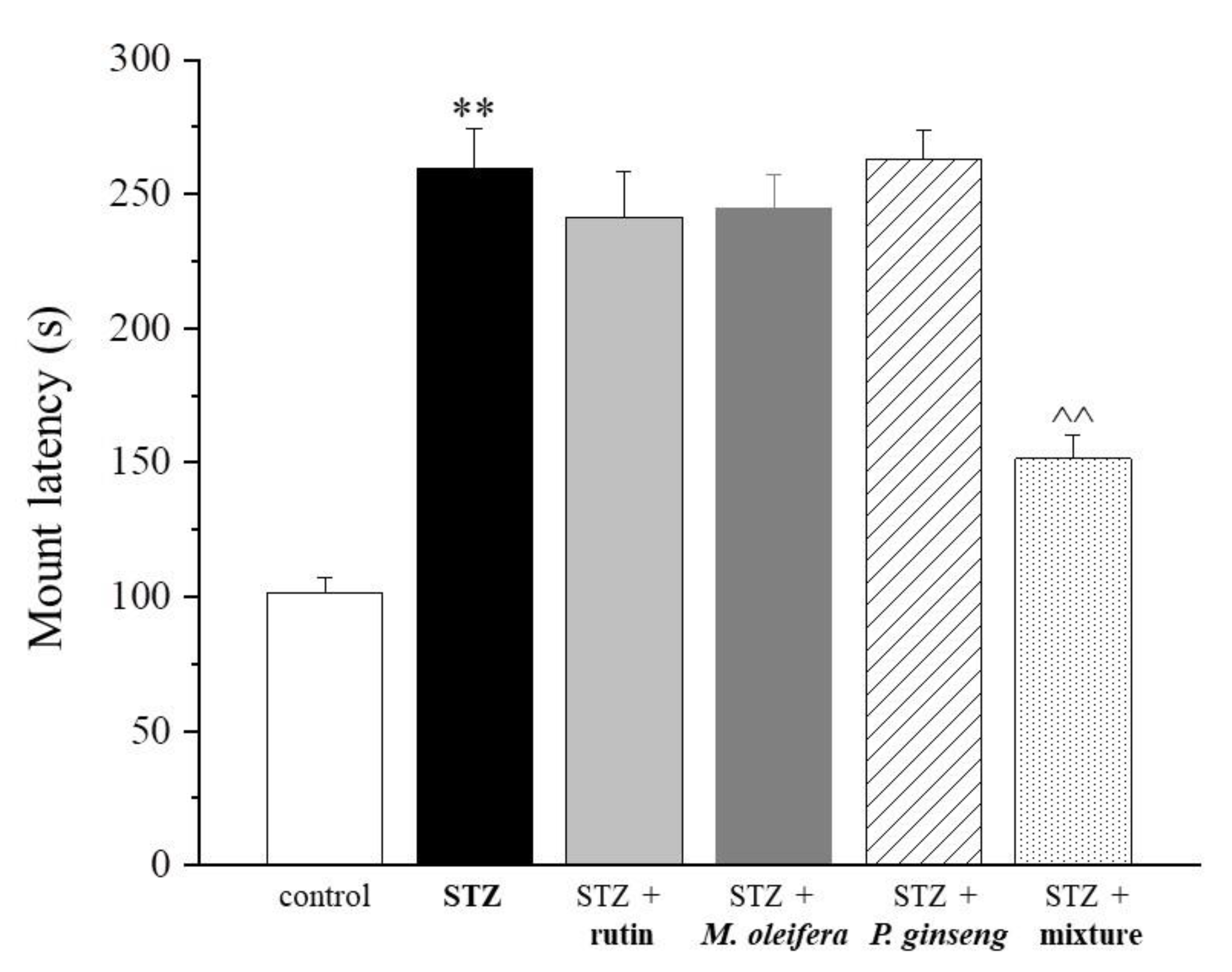
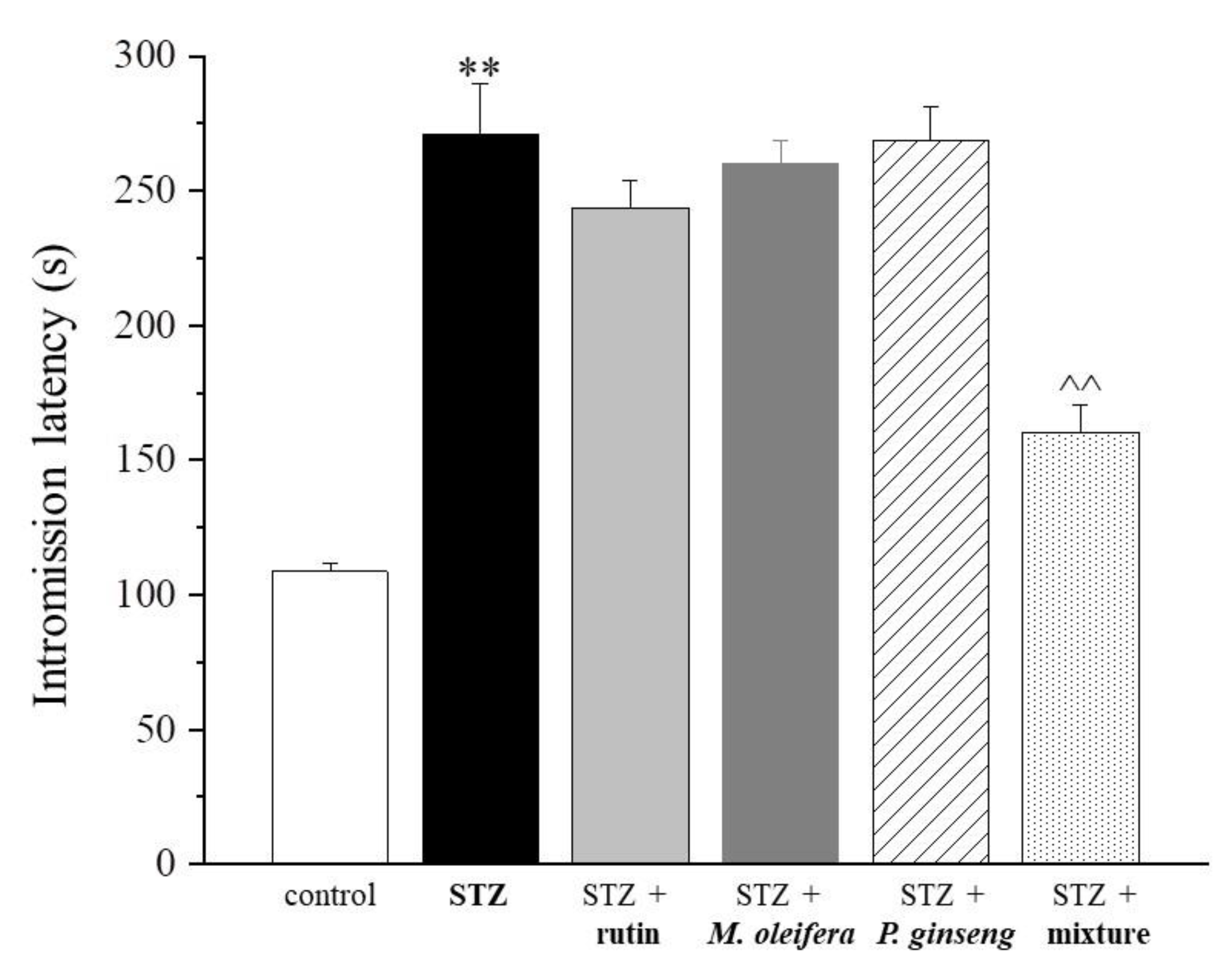
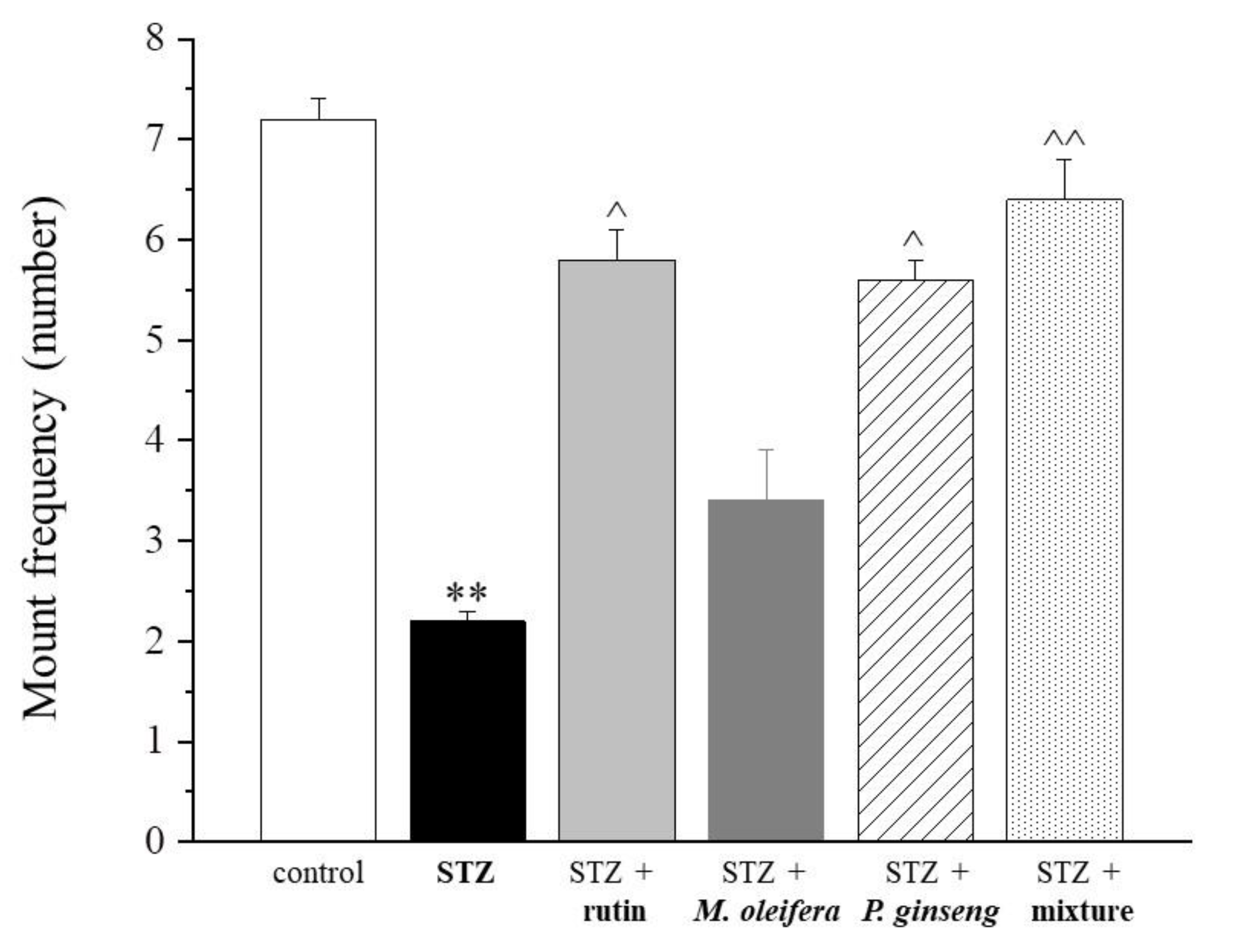

| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 108 ± 6.2 | 364.8 ± 23.5 ** | 375.3 ± 17.9 | 289.6 ± 26.2 ^ | 311.9 ± 15.9 ^ | 271.2 ± 28.4 ^ |
| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 101.3 ± 5.6 | 259.6 ± 14.5 ** | 241.4 ± 16.8 | 244.7 ± 12.6 | 263.2 ± 10.3 | 151.6 ± 8.4 ^^ |
| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 108.6 ± 3.3 | 271.1 ± 18.5 ** | 243.6 ± 10.1 | 260.0 ± 8.8 | 268.8 ± 12.6 | 160.4 ± 10.1 ^^ |
| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 7.2 ± 0.2 | 2.2 ± 0.1 ** | 5.8 ± 0.3 ^ | 3.4 ± 0.5 | 5.6 ± 0.2 ^ | 6.4 ± 0.4 ^^ |
| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 17.5 ± 3.1 | 6.2 ± 1.1 ** | 6.5 ± 0.8 | 8.2 ± 2.3 | 8.4 ± 2.6 | 13.8 ± 3.6 ^^ |
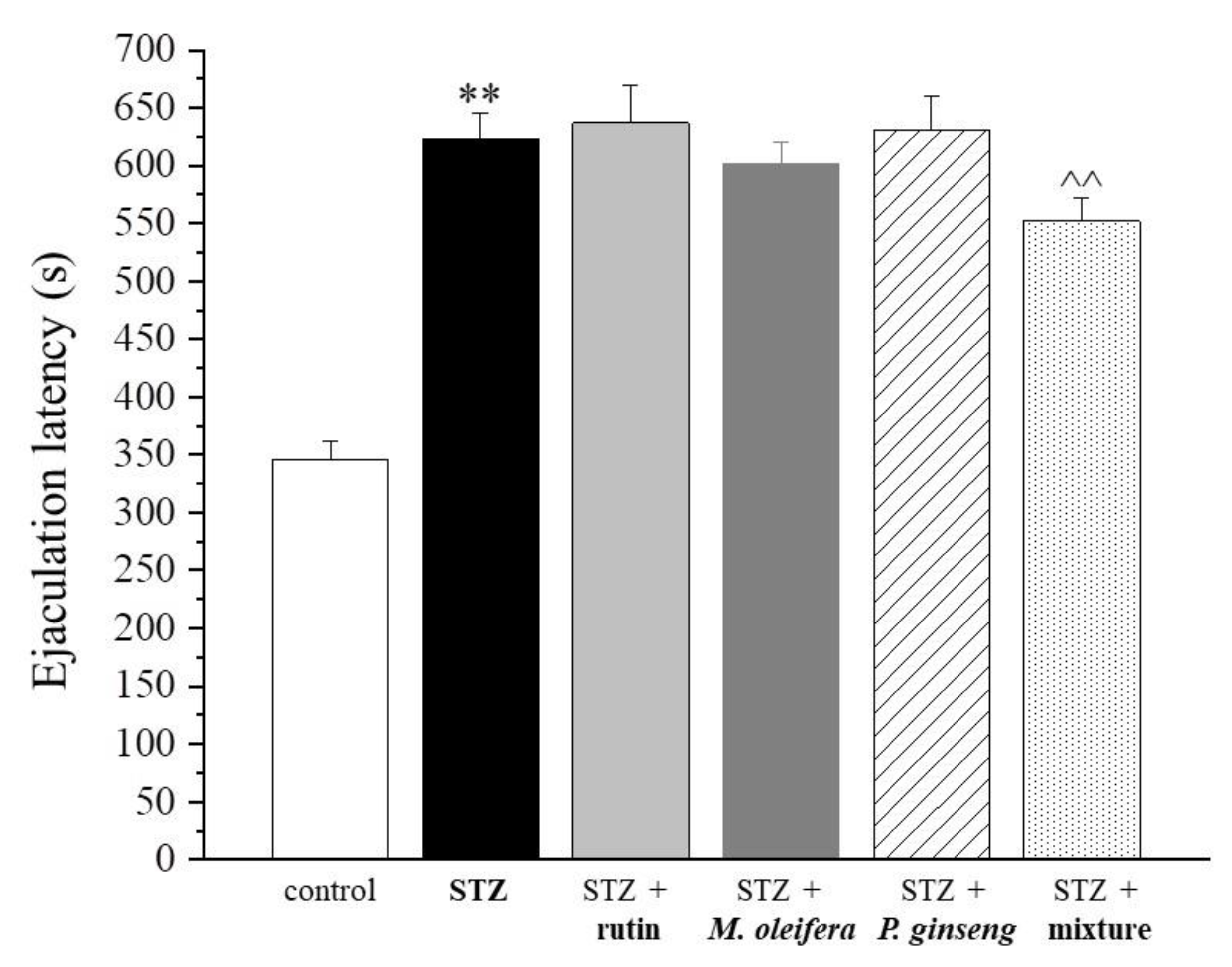
| Control | STZ | STZ + Rutin | STZ + M. oleifera Extract | STZ + P. ginseng Extract | STZ + Mixture |
|---|---|---|---|---|---|
| 345.8 ± 15.6 | 623.4 ± 22.6 ** | 636.8 ± 32.1 | 601.8 ± 18.9 | 630.5 ± 29.4 | 552.1 ± 19.7 ^^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobili, S.; Lucarini, E.; Murzilli, S.; Vanelli, A.; Di Cesare Mannelli, L.; Ghelardini, C. Efficacy Evaluation of Plant Products in the Treatment of Erectile Dysfunction Related to Diabetes. Nutrients 2021, 13, 4520. https://doi.org/10.3390/nu13124520
Nobili S, Lucarini E, Murzilli S, Vanelli A, Di Cesare Mannelli L, Ghelardini C. Efficacy Evaluation of Plant Products in the Treatment of Erectile Dysfunction Related to Diabetes. Nutrients. 2021; 13(12):4520. https://doi.org/10.3390/nu13124520
Chicago/Turabian StyleNobili, Stefania, Elena Lucarini, Stefania Murzilli, Arianna Vanelli, Lorenzo Di Cesare Mannelli, and Carla Ghelardini. 2021. "Efficacy Evaluation of Plant Products in the Treatment of Erectile Dysfunction Related to Diabetes" Nutrients 13, no. 12: 4520. https://doi.org/10.3390/nu13124520






