Low Urinary Iodine Concentration Is Associated with Increased Risk for Elevated Plasma Glucose in Females: An Analysis of NHANES 2011–12
Abstract
:1. Introduction
2. Materials and Methods
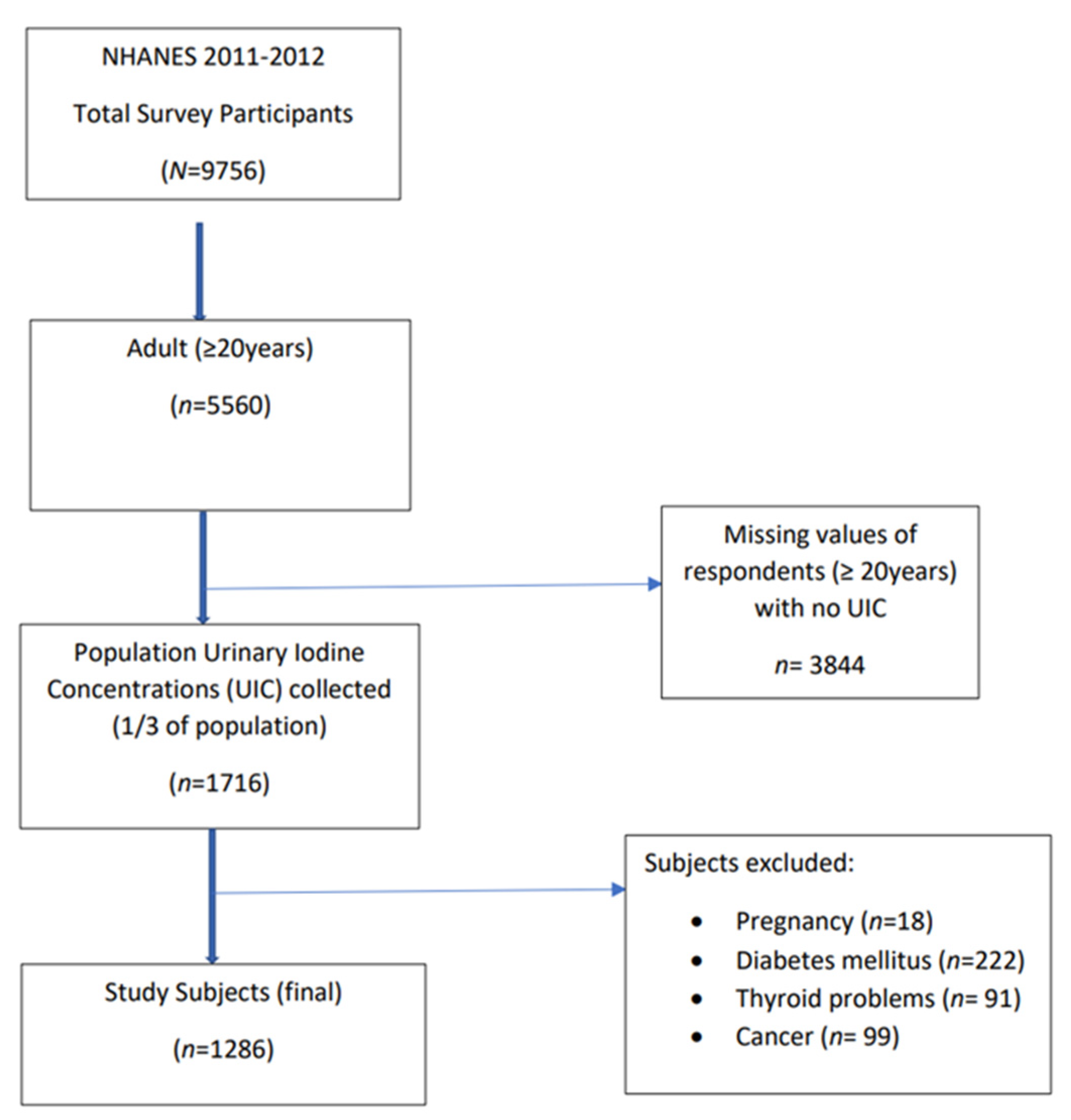
2.1. Data Source and Study Sample
2.2. Iodine Status
2.3. Markers of Insulin Resistance
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics by Urinary Iodine Concentration
3.2. Analysis of Markers of Insulin Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Maalouf, J.; Barron, J.; Gunn, J.P.; Yuan, K.; Perrine, C.G.; Cogswell, M.E. Iodized salt sales in the United States. Nutrients 2015, 7, 1691–1695. [Google Scholar] [CrossRef] [Green Version]
- Ershow, A.G.; Skeaff, S.A.; Merkel, J.M.; Pehrsson, P.R. Development of Databases on Iodine in Foods and Dietary Supplements. Nutrients 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. Iodine: Fact Sheet for Health Professionals. 2019. Available online: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/ (accessed on 1 August 2019).
- Karwowska, P.; Breda, J. The Role of the World Health Organization in Eliminating Iodine Deficiency Worldwide. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 138–142. [Google Scholar] [CrossRef]
- Delange, F. Iodine deficiency. In Werner and Ingbar’s The Thyroid: A Fundamental and Clincal Text, 8th ed.; Braverman, L.E., Utiger, R.D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 295–316. [Google Scholar]
- Wang, C.Y.; Yu, T.Y.; Shih, S.R.; Huang, K.C.; Chang, T.C. Low total and free triiodothyronine levels are associated with insulin resistance in non-diabetic individuals. Sci. Rep. 2018, 8, 10685. [Google Scholar] [CrossRef]
- Aceves, C.; Anguiano, B.; Delgado, G. Is iodine a gatekeeper of the integrity of the mammary gland? J. Mammary. Gland Biol. Neoplasia 2015, 10, 189–196. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Aeberli, I.; Melse-Boonstra, A.; Grimci, L.; Bridson, J.; Chaouki, N.; Mbhenyane, X.; Jooste, P.L. Iodine treatment in children with subclinical hypothyroidism due to chronic iodine deficiency decreases thyrotropin and C-peptide concentrations and improves the lipid profile. Thyroid 2009, 19, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pergola, G.; Ciampolillo, A.; Paolotti, S.; Trerotoli, P.; Giorgino, R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin. Endocrinol. 2007, 67, 265–269. [Google Scholar] [CrossRef]
- Duntas, L.H.; Wartofsky, L. Cardiovascular risk and subclinical hypothyroidism: Focus on lipids and new emerging risk factors. What is the evidence? Thyroid 2007, 17, 1075–1084. [Google Scholar] [CrossRef]
- Reinehr, T. Obesity and thyroid function. Mol. Cell Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef]
- Roef, G.; Lapauw, B.; Goemaere, S.; Zmierczak, H.G.; Toye, K.; Kaufman, J.M.; Taes, Y. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur. J. Endocrinol. 2012, 167, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Brenta, G. The effect of thyroid disorders on lipid levels and metabolism. Med. Clin. N. Am. 2012, 96, 269–281. [Google Scholar] [CrossRef]
- Duntas, L.H.; Orgiazzi, J.; Brabant, G. The interface between thyroid and diabetes mellitus. Clin. Endocrinol. 2011, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Sanchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, R.R. Iodine metabolism and thyroid physiology: Current concepts. Thyroid 1997, 7, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Associations between the levels of thyroid hormones and lipid/lipoprotein levels: Data from National Health and Nutrition Examination Survey 2007–2012. Environ. Toxicol. Pharmacol. 2017, 53, 133–144. [Google Scholar] [CrossRef]
- Le, T.N.; Celi, F.S.; Wickham, E.P., III. Thyrotropin Levels Are Associated with Cardiometabolic Risk Factors in Euthyroid Adolescents. Thyroid 2016, 26, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Shin, D.; Song, W.O. Low Urinary Iodine Concentrations Associated with Dyslipidemia in US Adults. Nutrients 2016, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention; National Center for Health Statistics. About the National Health and Nutrition Examination Survey; National Center for Health Statistics: Hyattsville, MD, USA, 2017. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 1 August 2019).
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey Methods and Analytic Guidelines; National Center for Health Statistics: Hyattsville, MD, USA, 2018. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 1 August 2019).
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital. Health Stat. 2014, 2, 1–33. [Google Scholar]
- Centers for Disease Control and Prevention; National Center for Health Statistics. Laboratory Procedure Manual: Iodine and Mercury in Urine; National Center for Health Statistics: Hyattsville, MD, USA, 2012. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011–2012/labmethods/uiouhg_g_met_iodine_mercury.pdf (accessed on 1 August 2019).
- World Health Organization. Urinary Iodine Concentrations for Determining Iodine Status Deficiency in Populations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2013.
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey: 2011–2012 Data Documentation, Codebook, and Frequencies: Plasma Fasting Glucose and Insulin; National Center for Health Statistics: Hyattsville, MD, USA, 2014. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/GLU_G.htm (accessed on 1 August 2019).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey: 2011–2012 Data Documentation, Codebook, and Frequencies: Glycohemoglobin; National Center for Health Statistics: Hyattsville, MD, USA, 2013. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/GHB_G.htm (accessed on 1 August 2019).
- Anonymous. Summary of revisions for the 2010 Clinical Practice Recommendations. Diabetes Care 2010, 33 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar]
- Minich, D.M.; Bland, J.S. Dietary management of the metabolic syndrome beyond macronutrients. Nutr. Rev. 2008, 66, 429–444. [Google Scholar] [CrossRef]
- Chubb, S.A.; Davis, W.A.; Inman, Z.; Davis, T.M. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: The Fremantle Diabetes Study. Clin. Endocrinol. 2005, 62, 480–486. [Google Scholar] [CrossRef]
- Song, F.; Bao, C.; Deng, M.; Xu, H.; Fan, M.; Paillard-Borg, S.; Xu, W.; Qi, X. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine 2017, 55, 179–185. [Google Scholar] [CrossRef]
- Potenza, M.; Via, M.A.; Yanagisawa, R.T. Excess thyroid hormone and carbohydrate metabolism. Endocr. Pract. 2009, 15, 254–262. [Google Scholar] [CrossRef]
- Weinstein, S.P.; O’Boyle, E.; Haber, R.S. Thyroid hormone increases basal and insulin-stimulated glucose transport in skeletal muscle. The role of GLUT4 glucose transporter expression. Diabetes 1994, 43, 1185–1189. [Google Scholar] [CrossRef]
- Teixeira, S.S.; Tamrakar, A.K.; Goulart-Silva, F.; Serrano-Nascimento, C.; Klip, A.; Nunes, M.T. Triiodothyronine acutely stimulates glucose transport into L6 muscle cells without increasing surface GLUT4, GLUT1, or GLUT3. Thyroid 2012, 22, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, Y.; Liu, X.; Li, M.; Wu, B.; Li, Y.; Liang, Y.; Shao, X.; Holthofer, H.; Zou, H. Insulin resistance and metabolic syndrome in normal-weight individuals. Endocrine 2014, 6, 496–504. [Google Scholar] [CrossRef]
- Dvorak, R.V.; DeNino, W.F.; Ades, P.A.; Poehlman, E.T. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes 1999, 48, 2210–2214. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kobayashi, Y. Leaner Women with Impaired Insulin Secretion Accounts for about 40% of Gestational Diabetes Mellitus in Japan. J. Pregnancy 2019, 2019, 7578403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujral, U.P.; Weber, M.B.; Staimez, L.R.; Narayan, K.M.V. Diabetes Among Non-Overweight Individuals: An Emerging Public Health Challenge. Curr. Diabetes Rep. 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Allison, G.; Abbasi, F.; Lamendola, C.; Reaven, G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism 2004, 53, 495–499. [Google Scholar] [CrossRef]
- Fox, C.S.; Pencina, M.J.; D’Agostino, R.B.; Murabito, J.M.; Seely, E.W.; Pearce, E.N.; Vasan, R.S. Relations of thyroid function to body weight: Cross-sectional and longitudinal observations in a community-based sample. Arch. Intern. Med. 2008, 168, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bulow, I.; Perrild, H.; Ovesen, L.; Jorgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Vega-Vega, O.; Fonseca-Correa, J.I.; Mendoza-De la Garza, A.; Rincon-Pedrero, R.; Espinosa-Cuevas, A.; Baeza-Arias, Y.; Dary, O.; Herrero-Bervera, B.; Nieves-Anaya, I.; Correa-Rotter, R. Contemporary Dietary Intake: Too Much Sodium, Not Enough Potassium, yet Sufficient Iodine: The SALMEX Cohort Results. Nutrients 2018, 10, 816. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Grandinetti, A.; Matsuura, G.; Sharma, S.; Mau, M.; Henderson, B.E.; Kolonel, L.N. Diabetes prevalence and body mass index differ by ethnicity: The Multiethnic Cohort. Ethn. Dis. 2009, 19, 49–55. [Google Scholar] [PubMed]
- Misra, A.; Sattar, N.; Tandon, N.; Shrivastava, U.; Vikram, N.K.; Khunti, K.; Hills, A.P. Clinical management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018, 6, 979–991. [Google Scholar] [CrossRef]
- Lee, J.W.; Brancati, F.L.; Yeh, H.C. Trends in the prevalence of type 2 diabetes in Asians versus whites: Results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 2011, 34, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Gujral, U.P.; Mohan, V.; Pradeepa, R.; Deepa, M.; Anjana, R.M.; Narayan, K.M. Ethnic differences in the prevalence of diabetes in underweight and normal weight individuals: The CARRS and NHANES studies. Diabetes Res. Clin. Pract. 2018, 146, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Herrick, K.A.; Perrine, C.G.; Aoki, Y.; Caldwell, K.L. Iodine Status and Consumption of Key Iodine Sources in the U.S. Population with Special Attention to Reproductive Age Women. Nutrients 2018, 10, 874. [Google Scholar] [CrossRef] [Green Version]
- Borucki Castro, S.I.; Berthiaume, R.; Laffey, P.; Fouquet, A.; Beraldin, F.; Robichaud, A.; Lacasse, P. Iodine concentration in milk sampled from Canadian farms. J. Food Prot. 2010, 73, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110 (Suppl. S3), 349–353. [Google Scholar] [CrossRef] [Green Version]
- Teas, J.; Braverman, L.E.; Kurzer, M.S.; Pino, S.; Hurley, T.G.; Hebert, J.R. Seaweed and soy: Companion foods in Asian cuisine and their effects on thyroid function in American women. J. Med. Food 2007, 10, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.; Byth, K.; Eastman, C.J. Iodine Intake and Thyroid Function in Pregnant Women in a Private Clinical Practice in Northwestern Sydney before Mandatory Fortification of Bread with Iodised Salt. J. Thyroid Res. 2012, 2012, 798963. [Google Scholar] [CrossRef] [PubMed]
- Brucker-Davis, F.; Panaia-Ferrari, P.; Gal, J.; Fenichel, P.; Hieronimus, S. Iodine Supplementation throughout Pregnancy Does Not Prevent the Drop in FT4 in the Second and Third Trimesters in Women with Normal Initial Thyroid Function. Eur. Thyroid J. 2013, 2, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinoer, D.; De, N.P.; Delange, F.; Lemone, M.; Toppet, V.; Spehl, M.; Grun, J.P.; Kinthaert, J.; Lejeune, B. A randomized trial for the treatment of mild iodine deficiency during pregnancy: Maternal and neonatal effects. J. Clin. Endocrinol. Metab. 1995, 80, 258–269. [Google Scholar] [PubMed] [Green Version]
- Gregory, C.O.; Serdula, M.K.; Sullivan, K.M. Use of supplements with and without iodine in women of childbearing age in the United States. Thyroid 2009, 19, 1019–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, K.; Yap, C.; Mina, A.; Gunton, J.E. Iodine deficiency in women of childbearing age: Not bread alone? Asia Pac. J. Clin. Nutr. 2018, 27, 853–859. [Google Scholar] [PubMed]
- De, L.S.; Pearce, E.N.; Braverman, L.E. Iodine Supplementation in Women During Preconception, Pregnancy, and Lactation: Current Clinical Practice by U.S. Obstetricians and Midwives. Thyroid 2017, 27, 434–439. [Google Scholar]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.J.; Hunt, A.E.; Steinberg, H.O.; Paradisi, G.; Hook, G.; Katz, A.; Quon, M.J.; Baron, A.D. Repeatability characteristics of simple indices of insulin resistance: Implications for research applications. J. Clin. Endocrinol. Metab. 2001, 86, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Methods to assess iron and iodine status. Br. J. Nutr. 2008, 99 (Suppl. S3), S2–S9. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | Low UIC (<100 µg/L) | Normal UIC (≥100 µg/L) | Chi-Square | |||
|---|---|---|---|---|---|---|---|
| n | wt’d% | n | wt’d% | n | wt’d% | 2p-Value | |
| All | 1286 | 100 | 490 | 37.99 | 796 | 62.01 | |
| Sex | |||||||
| Male | 666 | 50.62 | 235 | 16.40 | 431 | 34.22 | 0.0015 ** |
| Female | 620 | 49.38 | 255 | 21.59 | 365 | 27.79 | |
| Age | |||||||
| 20–39 years | 579 | 44.62 | 235 | 17.48 | 344 | 27.14 | 0.0160 * |
| 40–59 years | 424 | 38.58 | 175 | 16.38 | 249 | 22.20 | |
| ≥60 years | 283 | 16.80 | 80 | 4.13 | 203 | 12.67 | |
| Race | |||||||
| NHW | 429 | 63.21 | 164 | 24.06 | 265 | 39.15 | 0.9865 |
| NHB | 338 | 12.15 | 128 | 4.53 | 210 | 7.62 | |
| NHA | 221 | 5.80 | 86 | 2.27 | 135 | 3.53 | |
| Hispanics | 298 | 18.83 | 112 | 7.13 | 186 | 11.70 | |
| Education | |||||||
| <High School | 278 | 15.74 | 103 | 5.87 | 175 | 9.87 | 0.1321 |
| High School | 248 | 18.91 | 92 | 6.14 | 156 | 12.77 | |
| >High School | 760 | 65.35 | 295 | 25.99 | 465 | 39.37 | |
| Poverty Income Ratio 3 | |||||||
| Low | 670 | 41.08 | 244 | 14.31 | 426 | 26.77 | 0.3670 |
| Medium | 250 | 21.84 | 100 | 8.71 | 150 | 13.13 | |
| High | 366 | 37.08 | 146 | 14.97 | 220 | 22.11 | |
| Smoking 4 | |||||||
| Low | 410 | 37.63 | 149 | 13.75 | 261 | 23.88 | 0.3520 |
| Medium | 595 | 40.11 | 230 | 14.68 | 365 | 25.43 | |
| High | 281 | 22.26 | 111 | 9.56 | 170 | 12.70 | |
| BMI 5 | |||||||
| Underweight | 46 | 3.08 | 22 | 1.32 | 24 | 1.76 | 0.3296 |
| Normal | 418 | 32.32 | 173 | 13.61 | 245 | 18.70 | |
| Overweight | 412 | 33.10 | 151 | 11.52 | 261 | 21.58 | |
| Obese | 410 | 31.50 | 144 | 11.54 | 266 | 19.96 | |
| Supplement 6 | |||||||
| Yes | 217 | 18.03 | 68 | 5.68 | 149 | 12.36 | 0.1056 |
| No | 1069 | 81.97 | 422 | 32.31 | 647 | 49.65 | |
| Alcohol | |||||||
| None | 486 | 31.27 | 176 | 10.94 | 310 | 20.32 | 0.1274 |
| >0 to 1 drinks/day | 263 | 21.34 | 102 | 8.34 | 161 | 13.01 | |
| >1 to 2 drinks/day | 211 | 19.31 | 90 | 9.27 | 121 | 10.05 | |
| >2 drinks/day | 326 | 28.08 | 122 | 9.44 | 204 | 18.63 | |
| Physical Activity 7 | |||||||
| None | 745 | 51.64 | 276 | 19.93 | 469 | 31.71 | 0.8763 |
| 0 to <500 | 249 | 22.15 | 97 | 8.09 | 152 | 14.06 | |
| 500 to <1000 | 161 | 14.09 | 65 | 5.61 | 96 | 8.48 | |
| ≥1000 | 131 | 12.12 | 52 | 4.35 | 79 | 7.77 | |
| Waist Circumference | |||||||
| Male | |||||||
| ≤102 cm | 426 | 60.85 | 157 | 19.75 | 269 | 41.10 | 0.9767 |
| >102 cm | 240 | 39.15 | 78 | 12.64 | 162 | 26.50 | |
| Female | |||||||
| ≤88 cm | 260 | 43.70 | 119 | 20.62 | 141 | 23.08 | 0.1804 |
| >88 cm | 360 | 56.30 | 136 | 23.11 | 224 | 33.20 | |
| Characteristic | mUIC µg/L | (95% CI) | |
|---|---|---|---|
| Overall | 126.6 | (111.9, 141.2) | |
| Sex | Male | 145.6 | (124.1, 167.1) |
| Female | 111.2 * | (96.4, 126.0) | |
| Age | 20–39 years | 120.1 | (98.1, 142.1) |
| 40–59 years | 113.9 * | (94.6, 133.3) | |
| ≥60 years | 157.3 | (133.8, 180.9) | |
| Race | NHW | 120.2 | (98.9, 141.4) |
| NHB | 135.1 | (109.8, 160.4) | |
| NHA | 128.9 | (114.0, 143.8) | |
| Hispanics | 136.1 | (113.9, 158.2) | |
| Education | <High School | 136.4 | (113.4, 159.5) |
| High School | 127.8 | (96.6, 159.1) | |
| >High School | 121.4 | (103.3, 139.5) | |
| PIR 2 | Low | 141.0 | (125.9, 156.1) |
| Medium | 120.4 | (97.7, 143.1) | |
| High | 117.1 * | (97.7, 136.6) | |
| Smoking 3 | Low | 129.9 | (106.7, 153.1) |
| Medium | 130.1 | (108.1, 151.9) | |
| High | 113.8 | (90.9, 136.8) | |
| BMI 4 | Underweight | 118.6 | (71.5, 165.6) |
| Normal | 111.0 * | (95.4, 126.6) | |
| Overweight | 138.8 | (115.5, 162.1) | |
| Obese | 139.2 | (115.3, 163.1) | |
| Supplement 5 | Yes | 148.4 | (124.5, 172.3) |
| No | 121.8 * | (106.7, 136.9) | |
| Alcohol | None | 133.7 | (101.0, 166.5) |
| <0 to 1 drinks/d | 121.3 | (96.3, 146.3) | |
| >1 to 2 drinks/d | 101.0 | (72.1, 129.9) | |
| >2 drinks/d | 140.3 | (121.1, 159.4) | |
| Physical Activity 6 | No Activity | 129.8 | (107.7, 151.6) |
| 0 to <500 | 119.5 | (87.2, 151.8) | |
| 500 to <1000 | 119.1 | (95.3, 142.8) | |
| ≥1000 | 138.7 | (113.3, 163.9) | |
| Waist Circumference | Male | ||
| ≤102 cm | 146.6 | (124.0, 169.2) | |
| >102 cm | 143.6 | (114.0, 173.2) | |
| Female | |||
| ≤88 cm | 104.1 * | (87.3, 120.8) | |
| >88 cm | 116.8 | (97.9, 135.7) |
| FPG | Insulin | Hb1Ac | HOMA- IR | ||
|---|---|---|---|---|---|
| Characteristic | mmol/L | μU/mL | % | ||
| Sex | Male | 5.7 ± 0.07 | 13.5 ± 0.70 | 5.5 ± 0.03 | 3.5 ± 0.20 |
| Female | 5.4 ± 0.07 | 12.0 ± 0.70 | 5.4 ± 0.03 | 2.9 ± 0.13 | |
| r2, % | <1 | <1 | <1 | <1 | |
| Age | 20–39 years | 5.4 ± 0.05 * | 12.7 ± 0.60 | 5.3 ± 0.03 ** | 3.1 ± 0.16 |
| 40–59 years | 5.6 ± 0.10 | 13.3 ± 1.02 | 5.5 ± 0.03 | 3.5 ± 0.31 | |
| ≥60 years | 5.7 ± 0.15 | 11.6 ± 1.15 | 5.7 ± 0.06 | 3.1 ± 0.30 | |
| r2, % | 2.8 | <1 | 7 | <1 | |
| Race | NHW | 5.5 ± 0.04 | 12.4 ± 0.68 | 5.4 ± 0.03 | 3.1 ± 0.17 |
| NHB | 5.5 ± 0.08 | 15.4 ± 0.52 ** | 5.6 ± 0.02 ** | 3.9 ± 0.13 ** | |
| NHA | 5.4 ± 0.09 | 12.2 ± 1.21 | 5.5 ± 0.06 | 3.0 ± 0.35 | |
| Hispanics | 5.6 ± 0.09 | 12.9 ± 1.39 | 5.5 ± 0.03 | 3.4 ± 0.39 | |
| r2, % | <1 | <1 | 1.3 | <1 | |
| Education | <High School | 5.8 ± 0.11 ** | 13.9 ± 1.20 | 5.6 ± 0.03 ** | 3.7 ± 0.30 |
| High School | 5.5 ± 0.06 | 13.6 ± 0.90 | 5.4 ± 0.03 | 3.4 ± 0.25 | |
| >High School | 5.4 ± 0.04 | 12.2 ± 0.60 | 5.4 ± 0.03 | 3.0 ± 0.15 | |
| r2, % | 1.5 | <1 | 2.5 | 1 | |
| PIR 2 | Low | 5.5 ± 0.07 | 14.4 ± 0.79 * | 5.5 ± 0.03 * | 3.6 ± 0.24 * |
| Medium | 5.6 ± 0.06 | 12.3 ± 0.88 | 5.4 ± 0.04 | 3.2 ± 0.27 | |
| High | 5.5 ± 0.08 | 10.9 ± 0.87 | 5.4 ± 0.04 | 2.7 ± 0.21 | |
| r2, % | <1 | 2.5 | <1 | 2 | |
| Smoking 3 | Low | 5.5 ± 0.07 | 11.5 ± 0.73 | 5.4 ± 0.03 | 2.9 ± 0.17 |
| Medium | 5.6 ± 0.09 | 12.9 ± 0.91 | 5.5 ± 0.03 | 3.3 ± 0.25 | |
| High | 5.6 ± 0.05 | 13.9 ± 0.77 * | 5.5 ± 0.02 * | 3.5 ± 0.18 * | |
| r2, % | <1 | <1 | <1 | <1 | |
| BMI 4 | Underweight | 5.3 ± 0.11 | 16.7 ± 5.16 | 5.3 ± 0.05 | 4.1 ± 1.32 |
| Normal | 5.3 ± 0.08 | 8.3 ± 0.68 | 5.3 ± 0.04 | 2.0 ± 0.16 | |
| Overweight | 5.5 ± 0.06 | 11.3 ± 0.80 | 5.4 ± 0.03 | 2.8 ± 0.19 | |
| Obese | 5.7 ± 0.07 ** | 18.3 ± 1.00 ** | 5.6 ± 0.04 ** | 4.8 ± 0.27 ** | |
| r2, % | 3.1 | 18 | 3.8 | 18.2 | |
| Supplement 5 | Yes | 5.5 ± 0.07 | 12.7 ± 1.53 | 5.4 ± 0.03 | 3.2 ± 0.43 |
| No | 5.6 ± 0.04 | 12.8 ± 0.53 | 5.4 ± 0.03 | 3.2 ± 0.13 | |
| r2, % | <1 | <1 | <1 | <1 | |
| Alcohol | None | 5.4 ± 0.06 * | 13.1 ± 1.01 | 5.5 ± 0.03 | 3.3 ± 0.26 |
| >0 to 1 drinks/d | 5.5 ± 0.15 | 10.5 ± 0.82 ** | 5.5 ± 0.06 | 2.6 ± 0.22 ** | |
| >1 to 2 drinks/d | 5.7 ± 0.11 | 13.2 ± 1.44 | 5.3 ± 0.04 | 3.5 ± 0.42 | |
| ≥2 drinks/d | 5.6 ± 0.06 | 13.7 ± 0.59 | 5.4 ± 0.03 | 3.5 ± 0.14 | |
| r2, % | 1.3 | 1.4 | 1.1 | 1.5 | |
| Physical Activity 6 | No activity | 5.5 ± 0.03 | 14.3 ± 0.58 | 5.5 ± 0.03 | 3.6 ± 0.10 |
| 0 to <500 | 5.6 ± 0.13 | 12.5 ± 1.02 | 5.4 ± 0.05 | 3.2 ± 0.30 | |
| 500 to <1000 | 5.5 ± 0.08 | 11.0 ± 0.83 | 5.4 ± 0.05 | 2.8 ± 0.20 | |
| ≥1000 | 5.4 ± 0.09 | 9.6 ± 1.22 ** | 5.3 ± 0.04 ** | 2.4 ± 0.30 ** | |
| r2, % | <1 | 3.3 | 4.7 | 2.9 | |
| Waist Circumference | Male | ||||
| ≤102 cm | 5.6 ± 0.07 | 10.3 ± 0.70 | 5.4 ± 0.02 | 2.6 ± 0.18 | |
| >102 cm | 5.8 ± 0.08 ** | 18.3 ± 1.30 ** | 5.6 ± 0.04 ** | 4.9 ± 0.39 ** | |
| r2, % | 1.1 | 14.8 | 4.4 | 14.2 | |
| Female | |||||
| ≤88 cm | 5.1 ± 0.05 | 9.1 ± 1.21 | 5.3 ± 0.03 | 2.1 ± 0.28 | |
| >88 cm | 5.6 ± 0.12 ** | 14.0 ± 0.61 ** | 5.5 ± 0.04 ** | 3.5 ± 0.12 ** | |
| r2, % | 6 | 6.9 | 5 | 8.7 |
| Elevated IR Marker | Model | Low UIC (<100 µg/L) | Normal UIC (≥100 µg/L) | 2p-Value |
|---|---|---|---|---|
| OR (95% CI) | Referent | |||
| FPG ≥ 5.6 mmol/L | 1 | 1.08 (0.72–1.62) | 1 | 0.7036 |
| 2 | 1.11 (0.77–1.59) | 1 | 0.5630 | |
| Insulin > 9 μU/mL | 1 | 0.99 (0.60–1.65) | 1 | 0.1877 |
| 2 | 0.99 (0.59–1.67) | 1 | 0.9926 | |
| HOMA-IR ≥ 2.6 | 1 | 0.86 (0.55–1.34) | 1 | 0.4744 |
| 2 | 0.83 (0.56–1.25) | 1 | 0.3489 | |
| HbA1c ≥ 5.7% | 1 | 0.83 (0.57–1.21) | 1 | 0.3091 |
| 2 | 0.91 (0.64–1.28) | 1 | 0.5502 |
| Elevated IR Marker | Covariates | p-Value |
|---|---|---|
| FPG ≥ 5.6 mmol/L | Age | 0.0412 * |
| Smoking | 0.1767 | |
| BMI | 0.0810 | |
| Waist circumference | 0.0360 * | |
| Insulin >9 μU/mL | Race | 0.4766 |
| Smoking | 0.0996 | |
| BMI | 0.8079 | |
| Waist circumference | 0.0496 | |
| HOMA-IR ≥ 2.6 | Smoking | 0.2743 |
| Alcohol Use | 0.1018 | |
| BMI | 0.0003 ** | |
| Waist circumference | 0.0012 ** | |
| Iodine supplement use | 0.8537 | |
| HbA1C ≥ 5.7% | Age | 0.0006 ** |
| Education | 0.9270 | |
| BMI | 0.0022 ** | |
| Waist circumference | 0.0205 * | |
| Physical activity | 0.4364 |
| Elevated IR Marker | Model | Low UIC (<100 µg/L) | Normal UIC (≥100 µg/L) | 2p-Value |
|---|---|---|---|---|
| OR (95% CI) | Referent | |||
| FPG ≥ 5.6 mmol/L | 1 | 1.52 (0.94–2.44) | 1 | 0.0839 |
| 2 | 1.73 (1.09–2.72) | 1 | 0.0211 * | |
| Insulin > 9 μU/mL | 1 | 1.04 (0.58–1.87) | 1 | 0.8873 |
| 2 | 1.08 (0.54–2.16) | 1 | 0.8120 | |
| HOMA-IR ≥ 2.6 | 1 | 0.91 (0.46–1.80) | 1 | 0.7734 |
| 2 | 0.56 (0.32–0.99) | 1 | 0.0478 * | |
| HbA1c ≥ 5.7% | 1 | 0.56 (0.34–0.90) | 1 | 0.0208 * |
| 2 | 0.58 (0.34–1.02) | 1 | 0.0563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezemaduka Okoli, C.B.; Woldu, H.G.; Peterson, C.A. Low Urinary Iodine Concentration Is Associated with Increased Risk for Elevated Plasma Glucose in Females: An Analysis of NHANES 2011–12. Nutrients 2021, 13, 4523. https://doi.org/10.3390/nu13124523
Ezemaduka Okoli CB, Woldu HG, Peterson CA. Low Urinary Iodine Concentration Is Associated with Increased Risk for Elevated Plasma Glucose in Females: An Analysis of NHANES 2011–12. Nutrients. 2021; 13(12):4523. https://doi.org/10.3390/nu13124523
Chicago/Turabian StyleEzemaduka Okoli, Chisom B., Henok G. Woldu, and Catherine A. Peterson. 2021. "Low Urinary Iodine Concentration Is Associated with Increased Risk for Elevated Plasma Glucose in Females: An Analysis of NHANES 2011–12" Nutrients 13, no. 12: 4523. https://doi.org/10.3390/nu13124523
APA StyleEzemaduka Okoli, C. B., Woldu, H. G., & Peterson, C. A. (2021). Low Urinary Iodine Concentration Is Associated with Increased Risk for Elevated Plasma Glucose in Females: An Analysis of NHANES 2011–12. Nutrients, 13(12), 4523. https://doi.org/10.3390/nu13124523





