Biosynthesis and Fabrication of Copper Oxide Thin Films as a P-Type Semiconductor for Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
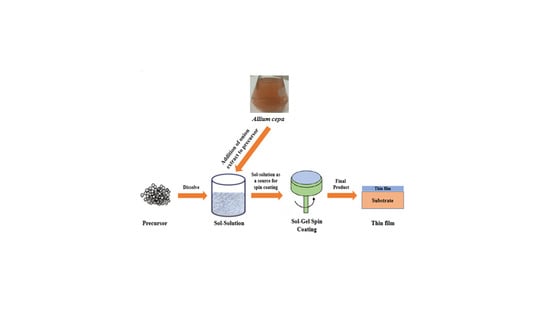
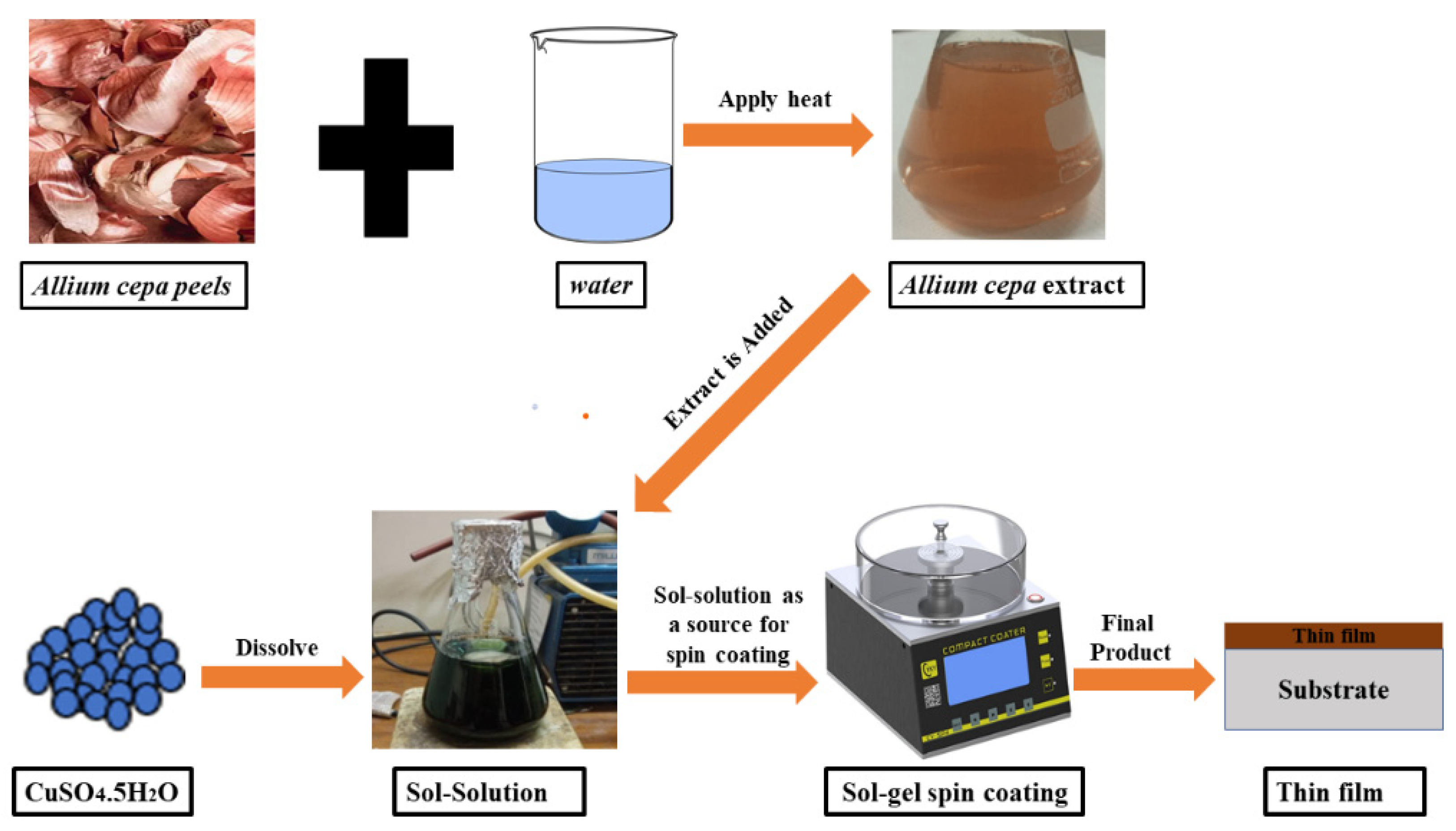
2.2. Preparation
2.3. Materials Characterization
3. Results and Discussion
3.1. Scanning Electron Microscope (SEM) Analysis
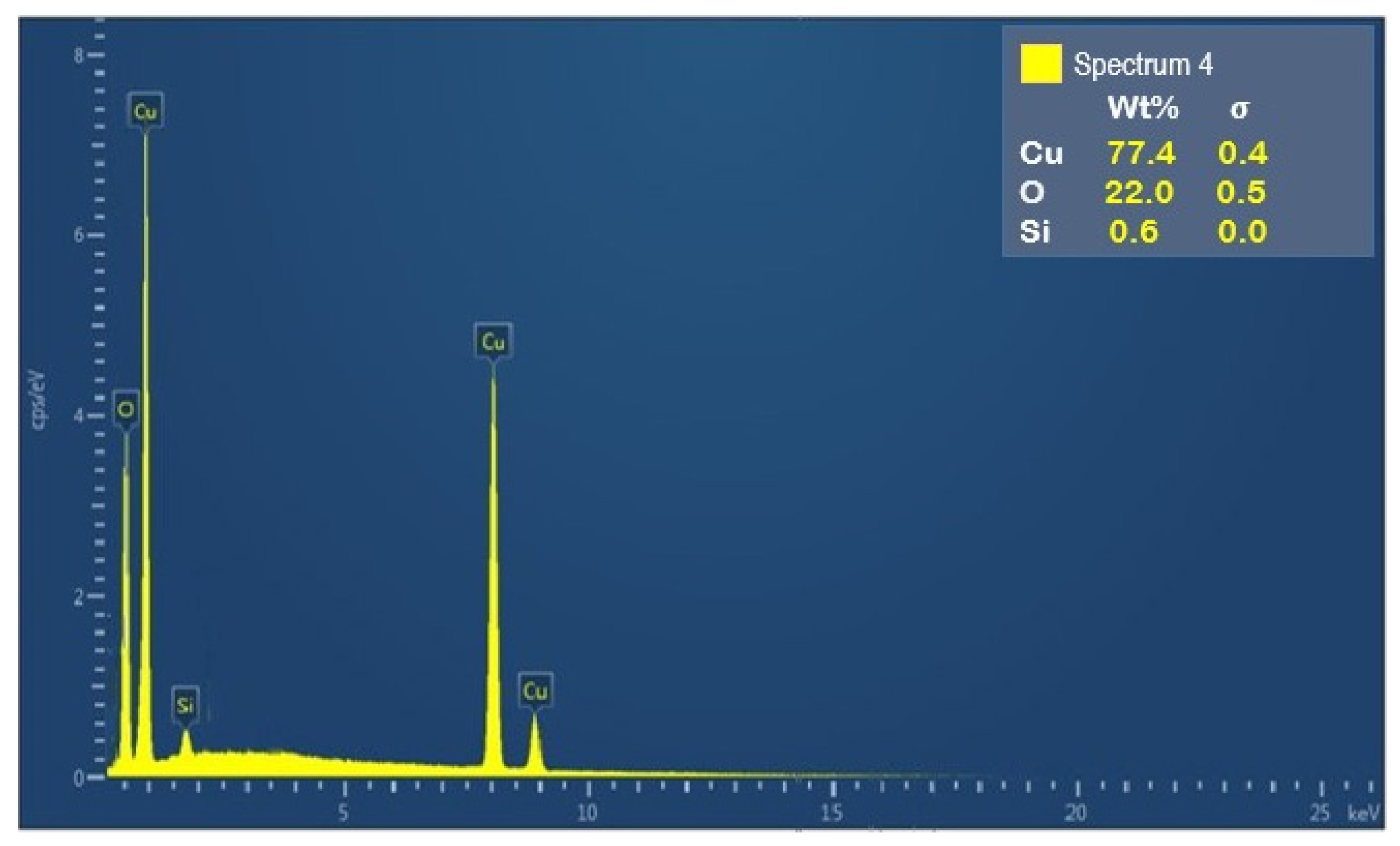
3.2. Elemental Analysis
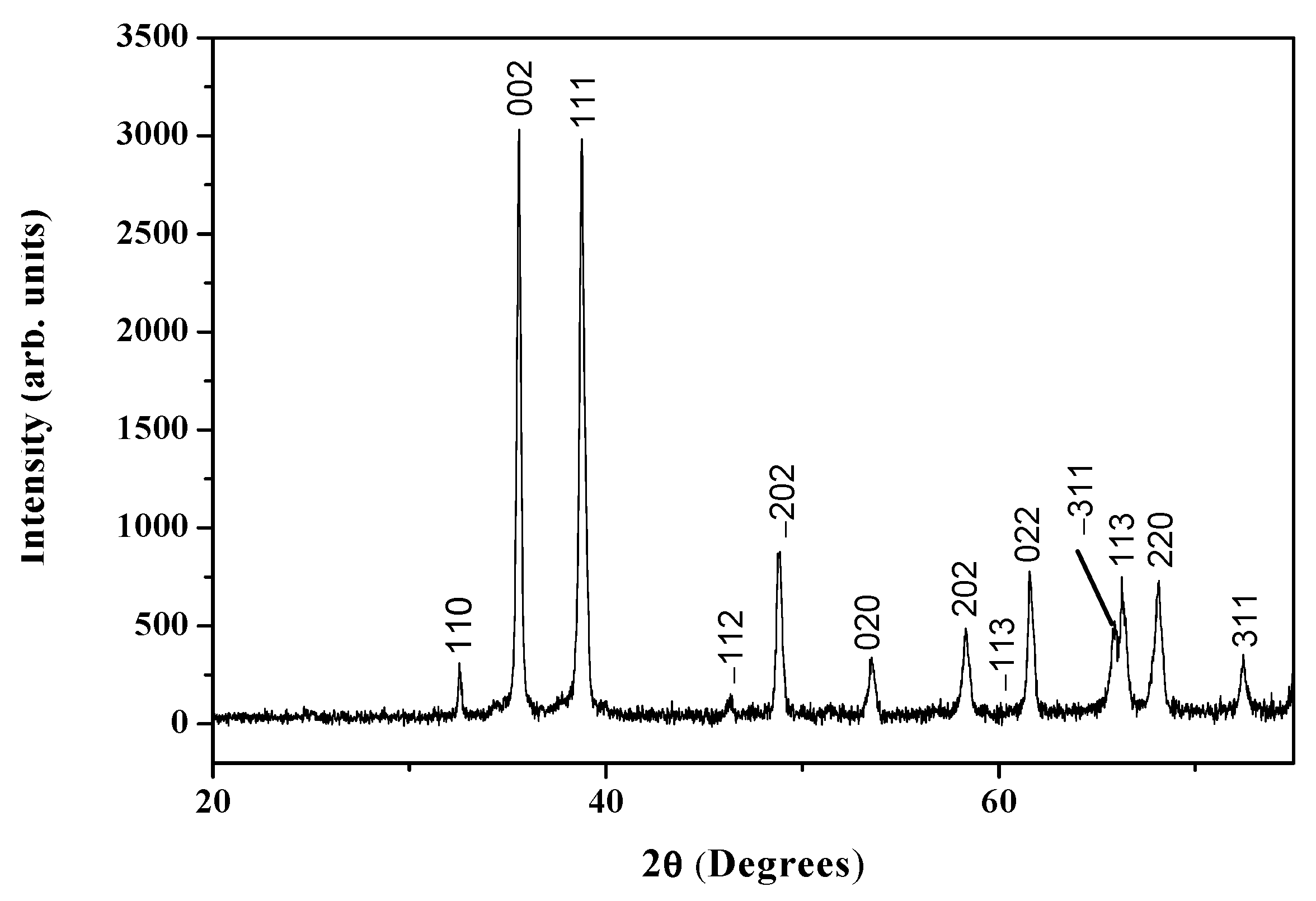
3.3. X-ray Diffraction Analysis (XRD)
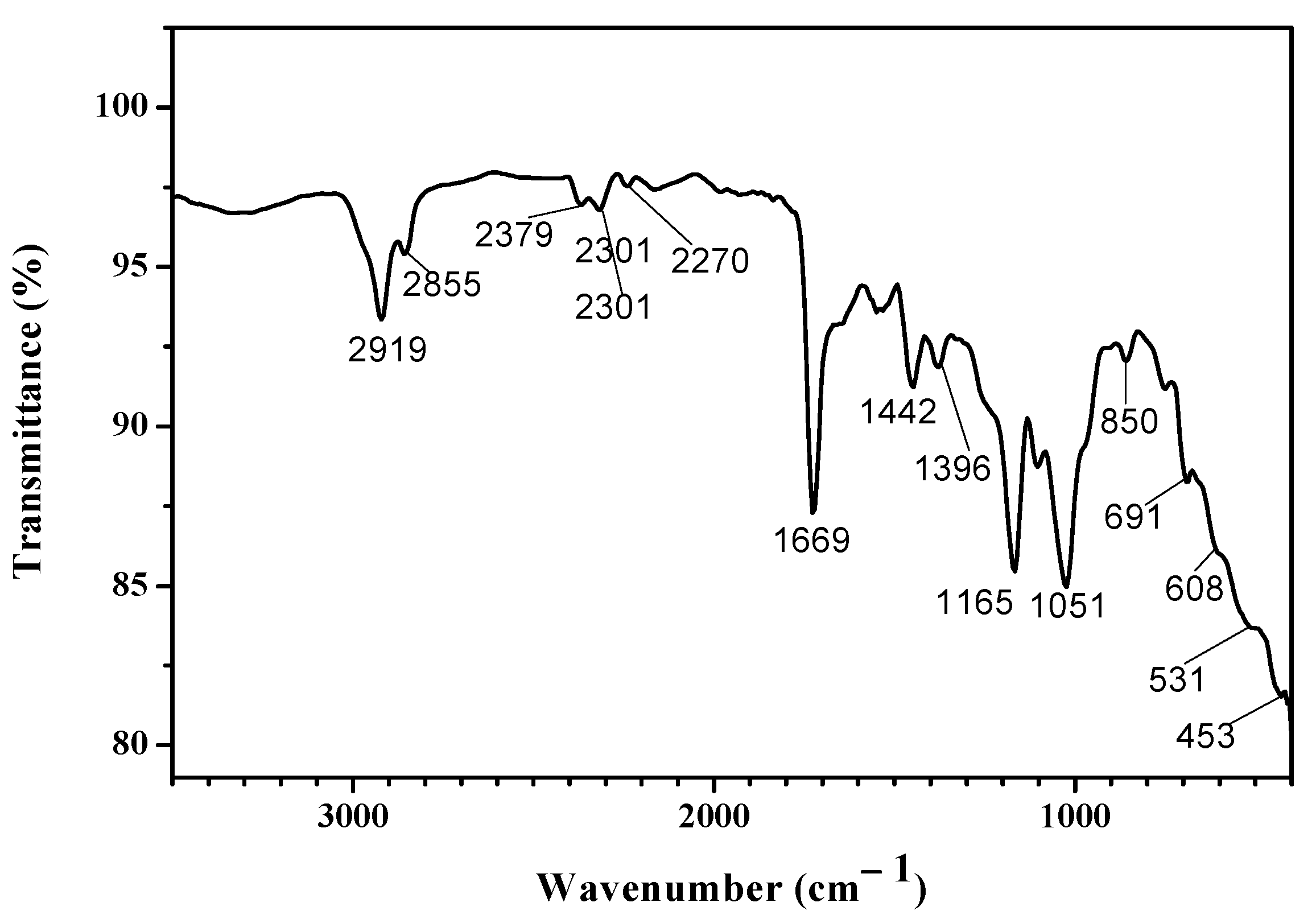
3.4. Fourier Transform Infrared Analysis (FTIR)
3.5. Uv-Vis Absorbance
3.6. Band Gap
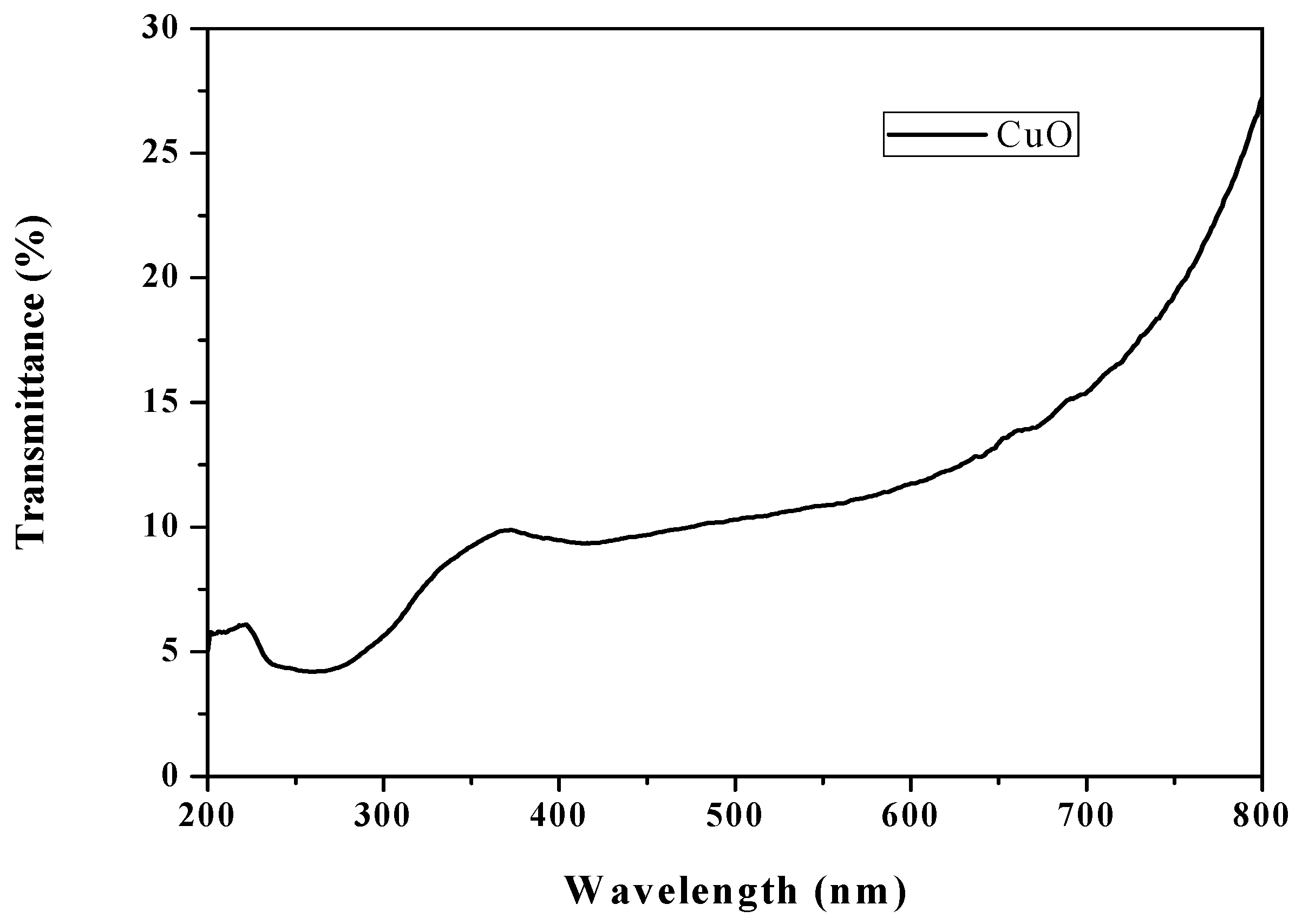
3.7. UV-Vis Transmittance
3.8. Electrical Properties
Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandan, D.; Zoppellaro, G.; Medřík, I.; Aparicio, C.; Kumar, P.; Petr, M.; Tomanec, O.; Gawande, M.B.; Varma, R.S.; Zbořil, R. Cobalt-entrenched N-, O-, and S-tridoped carbons as efficient multifunctional sustainable catalysts for base-free selective oxidative esterification of alcohols. Green Chem. 2018, 20, 3542–3556. [Google Scholar] [CrossRef]
- Dhaouadi, M.; Jlassi, M.; Sta, I.; Miled, I.B.; Mousdis, G.; Kompitsas, M.; Dimassi, W. Physical properties of copper oxide thin films prepared by sol–gel spin–coating method. Am. J. Phys. Appl. 2018, 6, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Mistry, V.H.; Mistry, B.V.; Modi, B.; Joshi, U. Effect of annealing on pulse laser deposition grown copper oxide thin film. AIP Conf. Proc. 2017, 1837, 040070. [Google Scholar]
- Asadi, M.; Rozati, S. Optical and structural properties of nanostructured copper oxide thin films as solar selective coating prepared by spray pyrolysis method. Mater. Sci. Pol. 2017, 35, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Prabu, R.D.; Valanarasu, S.; Ganesh, V.; Shkir, M.; AlFaify, S.; Kathalingam, A.; Srikumar, S.; Chandramohan, R. An effect of temperature on structural, optical, photoluminescence and electrical properties of copper oxide thin films deposited by nebulizer spray pyrolysis technique. Mater. Sci. Semicond. Process. 2018, 74, 129–135. [Google Scholar] [CrossRef]
- Aghayan, M.; Hussainova, I.; Kirakosyan, K.; Rodríguez, M.A. The template-assisted wet-combustion synthesis of copper oxide nanoparticles on mesoporous network of alumina nanofibers. Mater. Chem. Phys. 2017, 192, 138–146. [Google Scholar]
- ÖZMENTEŞ, R. Effect of thermal annealing time on the optical characteristics of CuO thin films. Yüzüncü Yıl Üniversitesi Fen Bilimleri Enstitüsü Derg 2019, 24, 176–182. [Google Scholar]
- Mikami, K.; Kido, Y.; Akaishi, Y.; Quitain, A.; Kida, T. Synthesis of Cu2O/CuO nanocrystals and their application to H2S sensing. Sensors 2019, 19, 211. [Google Scholar] [CrossRef] [Green Version]
- Mazhir, S.N.; Yasen, H.; Salih, M.M.; Mohamed, G.H. Structural and solar cell properties of CuO doped TiO2 thin films prepared by laser induced plasma. J. Eng. Appl. Sci. 2018, 13, 3555–3561. [Google Scholar]
- Krishnaprabha, M.; Venu, M.P.; Pattabi, M. Copper oxide thin films anchored on glass substrate by sol gel spin coating technique. AIP Conf. Proc. 2018, 1953, 100075. [Google Scholar]
- Nakate, U.T.; Lee, G.H.; Ahmad, R.; Patil, P.; Hahn, Y.-B.; Yu, Y.; Suh, E.-k. Nano-bitter gourd like structured CuO for enhanced hydrogen gas sensor application. Int. J. Hydrogen Energy 2018, 43, 22705–22714. [Google Scholar] [CrossRef]
- Xu, Z.; Buehler, M.J. Hierarchical nanostructures are crucial to mitigate ultrasmall thermal point loads. Nano Lett. 2009, 9, 2065–2072. [Google Scholar] [CrossRef]
- Paladugu, M.; Zou, J.; Guo, Y.N.; Zhang, X.; Joyce, H.J.; Gao, Q.; Tan, H.H.; Jagadish, C.; Kim, Y. Formation of hierarchical InAs nanoring/GaAs nanowire heterostructures. Angew. Chem. Int. Ed. 2009, 48, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2010, 144, 220–225. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, K. CuO hollow nanostructures catalyze [3 + 2] cycloaddition of azides with terminal alkynes. Chem. Commun. 2010, 46, 439–441. [Google Scholar]
- Ray, S.C. Preparation of copper oxide thin film by the sol–gel-like dip technique and study of their structural and optical properties. Sol. Energy Mater. Sol. Cells 2001, 68, 307–312. [Google Scholar] [CrossRef]
- Oral, A.; Menşur, E.; Aslan, M.; Başaran, E. The preparation of copper (II) oxide thin films and the study of their microstructures and optical properties. Mater. Chem. Phys. 2004, 83, 140–144. [Google Scholar] [CrossRef]
- Mårtensson, N. Optical properties of silica-copper oxide thin films prepared by spin coating. Appl. Opt. 2011, 42. Available online: http://www.diva-portal.org/smash/get/diva2:445710/FULLTEXT02.pdf (accessed on 23 November 2021).
- Kumar, D.A.; Xavier, F.P.; Shyla, J.M. Investigation on the variation of conductivity and photoconductivity of CuO thin films as a function of layers of coating. Arch. Appl. Sci. Res. 2012, 4, 2174–2183. [Google Scholar]
- Xiang, J.; Tu, J.; Zhang, L.; Zhou, Y.; Wang, X.; Shi, S. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–319. [Google Scholar] [CrossRef]
- Nair, M.; Guerrero, L.; Arenas, O.L.; Nair, P. Chemically deposited copper oxide thin films: Structural, optical and electrical characteristics. Appl. Surf. Sci. 1999, 150, 143–151. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Wu, Y.; Bai, X.; Guo, D. Cupric oxide nanoflowers synthesized with a simple solution route and their field emission. J. Cryst. Growth 2008, 310, 3125–3130. [Google Scholar] [CrossRef]
- Teng, F.; Yao, W.; Zheng, Y.; Ma, Y.; Teng, Y.; Xu, T.; Liang, S.; Zhu, Y. Synthesis of flower-like CuO nanostructures as a sensitive sensor for catalysis. Sens. Actuators B 2008, 134, 761–768. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.R.; Guha, A.K.; Naskar, N. Bio-inspired fabrication of silver nanoparticles on nanostructured silica: Characterization and application as a highly efficient hydrogenation catalyst. Green Chem. 2013, 15, 2548–2557. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Tabet, N.; Daud, A.R. Synthesis and optical properties of CuO nanostructures obtained via a novel thermal decomposition method. J. Alloys Compd. 2011, 509, 8761–8769. [Google Scholar]
- Nwanna, E.C.; Imoisili, P.E.; Jen, T.-C. Fabrication and synthesis of SnO X thin films: A review. Int. J. Adv. Manuf. Technol. 2020, 111, 2809–2831. [Google Scholar] [CrossRef]
- Boudrioua, A.; Chakaroun, M.; Fischer, A. An Introduction to Organic Lasers; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Devi, H.S.; Singh, T.D. Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv. Electron. Electr. Eng. 2014, 4, 83–88. [Google Scholar]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Johan, M.R.; Suan, M.S.M.; Hawari, N.L.; Ching, H.A. Annealing effects on the properties of copper oxide thin films prepared by chemical deposition. Int. J. Electrochem. Sci 2011, 6, 6094–6104. [Google Scholar]
- Yıldırım, M.A.; Ateş, A. Influence of films thickness and structure on the photo-response of ZnO films. Opt. Commun. 2010, 283, 1370–1377. [Google Scholar] [CrossRef]
- Bonnot, K.; Doblas, D.; Schnell, F.; Schlur, L.; Spitzer, D. Chip calorimetry for the sensitive identification of hexogen and pentrite from their decomposition inside copper oxide nanoparticles. Anal. Chem. 2015, 87, 9494–9499. [Google Scholar] [CrossRef]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Lai, G.; Lan, H.; Lin, S.; Qu, Y.; Lai, F. Optical properties of the oxidation of Cu thin films prepared by thermal evaporation. Surf. Rev. Lett. 2013, 20, 1350011. [Google Scholar] [CrossRef]
- Shariffudin, S.; Khalid, S.; Sahat, N.; Sarah, M.; Hashim, H. Preparation and characterization of nanostructured CuO thin films using sol-gel dip coating. IOP Conf. Ser. Mater. Sci. Eng. 2015, 99, 012007. [Google Scholar]
- Lee, W.-J.; Wang, X.-J. Structural, optical, and electrical properties of copper oxide films grown by the silar method with post-annealing. Coatings 2021, 11, 864. [Google Scholar] [CrossRef]
- Prasad, K.S.; Patra, A.; Shruthi, G.; Chandan, S. Aqueous extract of Saraca indica leaves in the synthesis of copper oxide nanoparticles: Finding a way towards going green. J. Nanotechnol. 2017, 2017, 7502610. [Google Scholar] [CrossRef]
- Monadi, N.; Saeednia, S.; Iranmanesh, P.; Ardakani, M.H.; Sinaei, S. Preparation and characterization of copper oxide nanoparticles through solid state thermal decomposition of an aqua nitrato copper (II) complex with a tridentate schiff-base ligand as a new precursor. Nanosci. Nanotechnol.-Asia 2019, 9, 92–100. [Google Scholar]
- Saghatforoush, L.A.; Mehdizadeh, R.; Chalabian, F. Synthesis of CuO nanoparticles from a new nano-structured copper (II) Schiff base complex by ultrasonic and solvothermal methods: Structural, thermal and antibacterial studies. J. Chem. Pharm. Res. 2011, 3, 691–702. [Google Scholar]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design and synthesis of coordination polymers with chelated units and their application in nanomaterials science. RSC Adv. 2017, 7, 42242–42288. [Google Scholar] [CrossRef] [Green Version]
- Ergin, B.; Ketenci, E.; Atay, F. Characterization of ZnO films obtained by ultrasonic spray pyrolysis technique. Int. J. Hydrogen Energy 2009, 34, 5249–5254. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Joint Committee for Powder Diffraction Standards. Powder Diffraction File No. 01-087-0712; JCPDS International Center for Diffraction Data: Swarthmore, PA, USA, 1991. [Google Scholar]
- Volanti, D.; Keyson, D.; Cavalcante, L.; Simões, A.Z.; Joya, M.; Longo, E.; Varela, J.A.; Pizani, P.; Souza, A. Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave. J. Alloys Compd. 2008, 459, 537–542. [Google Scholar] [CrossRef]
- Caroling, G.; Priyadharshini, M.N.; Vinodhini, E.; Ranjitham, A.M.; Shanthi, P. Biosynthesis of copper nanoparticles using aqueous guava extract-characterisation and study of antibacterial effects. Int. J. Pharm. Biol. Sci. 2015, 5, 25–43. [Google Scholar]
- Mittu, R. Synthesis, characterization of copper nanoparticles-a review. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 37–40. [Google Scholar]
- Manjunath, A.; Irfan, M.; Anushree, K.P.; Vinutha, K.M.; Yamunarani, N. Synthesis and characterization of CuO nanoparticles and CuO doped PVA nanocomposites. Adv. Mater. Phys. Chem. 2016, 6, 263. [Google Scholar] [CrossRef] [Green Version]
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach. Green Process. Synth 2019, 8, 691–702. [Google Scholar] [CrossRef]
- Mariello, M.; Fachechi, L.; Guido, F.; De Vittorio, M. Multifunctional sub-100 µm thickness flexible piezo/triboelectric hybrid water energy harvester based on biocompatible AlN and soft parylene C-PDMS-Ecoflex™. Nano Energy 2021, 83, 105811. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Greer, A. Making metallic glasses plastic by control of residual stress. Nat. Mater. 2006, 5, 857. [Google Scholar]
- Espinosa, H.; Prorok, B.; Fischer, M. A methodology for determining mechanical properties of freestanding thin films and MEMS materials. J. Mech. Phys. Solids 2003, 51, 47–67. [Google Scholar]
- Lee, M.L.; Fitzgerald, E.A.; Bulsara, M.T.; Currie, M.T.; Lochtefeld, A. Strained Si, SiGe, and Ge channels for high-mobility metal-oxide-semiconductor field-effect transistors. J. Appl. Phys. 2005, 97, 1. [Google Scholar] [CrossRef]
- Bodade, A.; Taiwade, M.; Chaudhari, G. Bioelectrode based chitosan-nano copper oxide for application to lipase biosensor. J. Appl. Pharm. Res. 2017, 5, 30–39. [Google Scholar]
- Zheng, L.; Liu, X. Solution-phase synthesis of CuO hierarchical nanosheets at near-neutral pH and near-room temperature. Mater. Lett. 2007, 61, 2222–2226. [Google Scholar] [CrossRef]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Bitire, S.O.; Jen, T.-C.; Belaid, M. Transesterification of parsley seed oil using a green catalyst: Considering the optimization process and modeling. Int. J. Sustain. Energy 2021, 40, 977–1001. [Google Scholar] [CrossRef]
- Iqbal, J.; Jan, T.; Ul-Hassan, S.; Ahmed, I.; Mansoor, Q.; Umair Ali, M.; Abbas, F.; Ismail, M. Facile synthesis of Zn doped CuO hierarchical nanostructures: Structural, optical and antibacterial properties. AIP Adv. 2015, 5, 127112. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K. Structural, optical and antibacterial efficacy of pure and zinc-doped copper oxide against pathogenic bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef]
- Butte, S.; Waghuley, S. Optical properties of Cu2O and CuO. AIP Conf. Proc. 2020, 2220, 020093. [Google Scholar]
- Dubale, A.A.; Pan, C.-J.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Wang, H.; Tam, F.; Grady, N.K.; Halas, N.J. Cu nanoshells: Effects of interband transitions on the nanoparticle plasmon resonance. J. Phys. Chem. B 2005, 109, 18218–18222. [Google Scholar] [CrossRef]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Lin, Y.; Xu, J.; He, J.; Wang, T.; Yu, G.; Shao, D.; Wang, W.-H.; Lu, F.; Li, L. Surface plasmon resonance enhanced visible-light-driven photocatalytic activity in Cu nanoparticles covered Cu2O microspheres for degrading organic pollutants. Appl. Surf. Sci. 2016, 366, 120–128. [Google Scholar] [CrossRef]
- Jillani, S.; Jelani, M.; Hassan, N.U.; Ahmad, S.; Hafeez, M. Synthesis, characterization and biological studies of copper oxide nanostructures. Mater. Res. Express 2018, 5, 045006. [Google Scholar] [CrossRef]
- Yoon, K.H.; Choi, W.J.; Kang, D.H. Photoelectrochemical properties of copper oxide thin films coated on an n-Si substrate. Thin Solid Films 2000, 372, 250–256. [Google Scholar] [CrossRef]
- Ashour, A.; Kaid, M.; El-Sayed, N.; Ibrahim, A. Physical properties of ZnO thin films deposited by spray pyrolysis technique. Appl. Surf. Sci. 2006, 252, 7844–7848. [Google Scholar] [CrossRef]
- Wanjala, K.; Njoroge, W.; Ngaruiya, J. Optical and electrical characterization of ZnS: Sn thin films for solar cell application. Int. J. Energy Eng. 2016, 6, 1–7. [Google Scholar]
- Choi, D.-w.; Park, J.-S. Highly conductive SnO2 thin films deposited by atomic layer deposition using tetrakis-dimethyl-amine-tin precursor and ozone reactant. Surf. Coat. Technol. 2014, 259, 238–243. [Google Scholar] [CrossRef]
- Keikhaei, M.; Ichimura, M. Fabrication of Copper Oxide thin films by galvanostatic deposition from weakly acidic solutions. Int. J. Electrochem. Sci. 2018, 13, 9931–9941. [Google Scholar] [CrossRef]
- Farhad, S.F.U.; Hossain, M.A.; Tanvir, N.I.; Akter, R.; Patwary, M.A.M.; Shahjahan, M.; Rahman, M.A. Structural, optical, electrical, and photoelectrochemical properties of cuprous oxide thin films grown by modified SILAR method. Mater. Sci. Semicond. Process. 2019, 95, 68–75. [Google Scholar] [CrossRef]
- Farhad, S.F.U. Copper Oxide Thin Films Grown by Pulsed Laser Deposition for Photovoltaic Applications. Ph.D. Thesis, University of Bristol, Bristol, UK, 2016. [Google Scholar]
- Beena, D.; Lethy, K.; Vinodkumar, R.; Pillai, V.M.; Ganesan, V.; Phase, D.; Sudheer, S. Effect of substrate temperature on structural, optical and electrical properties of pulsed laser ablated nanostructured indium oxide films. Appl. Surf. Sci. 2009, 255, 8334–8342. [Google Scholar] [CrossRef]
- Meyer, B.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Choi, G.M. Nonstoichiometry and electrical conduction of CuO. J. Phys. Chem. Solids 1996, 57, 81–84. [Google Scholar] [CrossRef]
- Gopalakrishna, D.; Vijayalakshmi, K.; Ravidhas, C. Effect of annealing on the properties of nanostructured CuO thin films for enhanced ethanol sensitivity. Ceram. Int. 2013, 39, 7685–7691. [Google Scholar] [CrossRef]
- Suda, S.; Fujitsu, S.; Koumoto, K.; Yanagida, H. The effect of atmosphere and doping on electrical conductivity of CuO. Jpn. J. Appl. Phys. 1992, 31, 2488. [Google Scholar] [CrossRef]
- Kamble, D.L.; Harale, N.S.; Patil, V.L.; Patil, P.S.; Kadam, L.D. Characterization and NO2 gas sensing properties of spray pyrolyzed SnO2 thin films. J. Anal. Appl. Pyrolysis 2017, 127, 38–46. [Google Scholar] [CrossRef]
- Choi, G.; Satyanarayana, L.; Park, J. Effect of process parameters on surface morphology and characterization of PE-ALD SnO2 thin films for gas sensing. Appl. Surf. Sci. 2006, 252, 7878–7883. [Google Scholar] [CrossRef]
- Shao, P.; Deng, S.; Chen, J.; Chen, J.; Xu, N. Study of field emission, electrical transport, and their correlation of individual single CuO nanowires. J. Appl. Phys. 2011, 109, 023710. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Musselman, K.P.; Marin, A.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. Incompatible length scales in nanostructured Cu2O solar cells. Adv. Funct. Mater. 2012, 22, 2202–2208. [Google Scholar] [CrossRef] [Green Version]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-Zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef] [Green Version]
- Viet Pham, T.; Rao, M.; Andreasson, P.; Peng, Y.; Wang, J.; Jinesh, K. Photocarrier generation in CuxO thin films deposited by radio frequency sputtering. Appl. Phys. Lett. 2013, 102, 032101. [Google Scholar] [CrossRef]


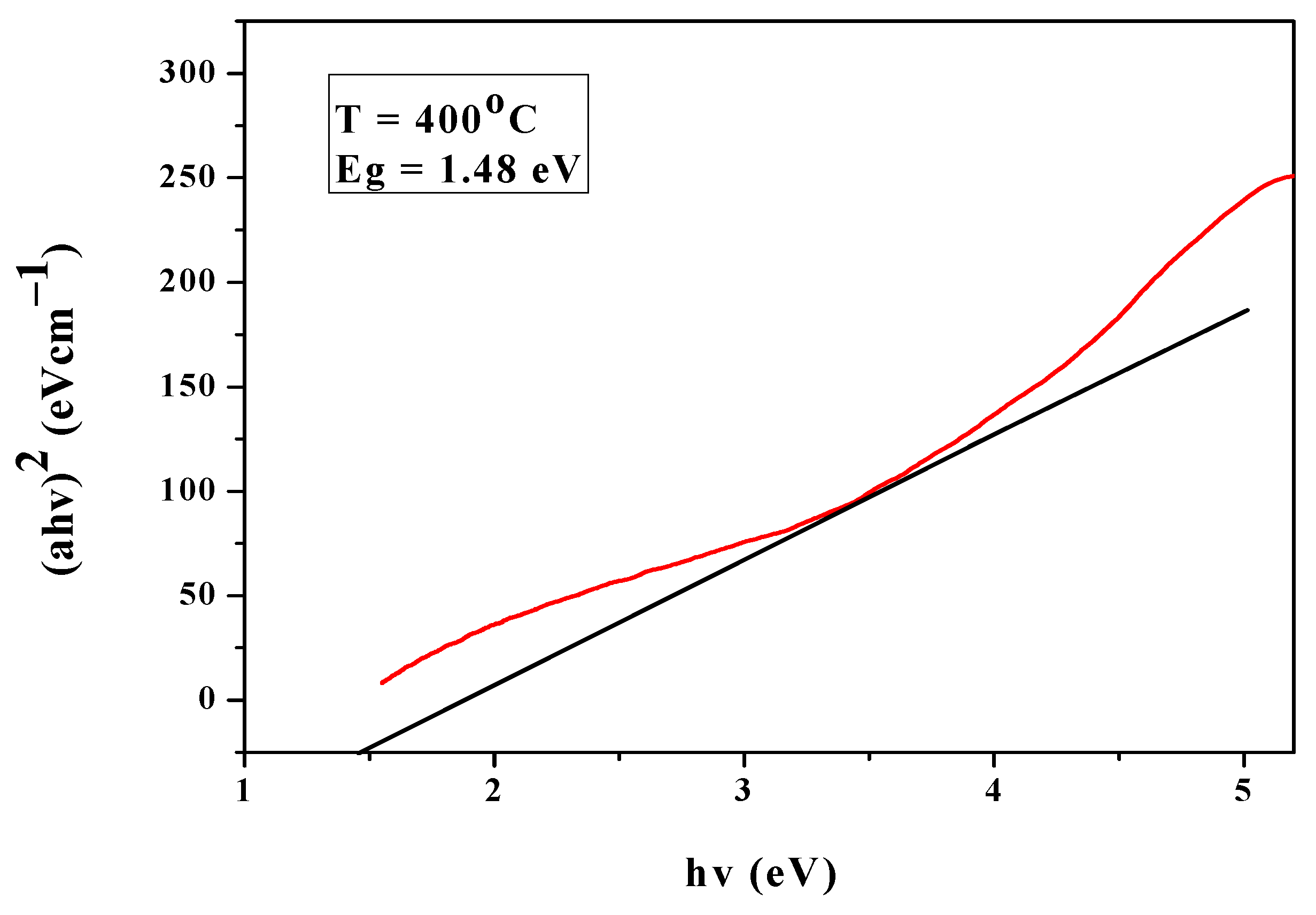

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwanna, E.C.; Imoisili, P.E.; Bitire, S.O.; Jen, T.-C. Biosynthesis and Fabrication of Copper Oxide Thin Films as a P-Type Semiconductor for Solar Cell Applications. Coatings 2021, 11, 1545. https://doi.org/10.3390/coatings11121545
Nwanna EC, Imoisili PE, Bitire SO, Jen T-C. Biosynthesis and Fabrication of Copper Oxide Thin Films as a P-Type Semiconductor for Solar Cell Applications. Coatings. 2021; 11(12):1545. https://doi.org/10.3390/coatings11121545
Chicago/Turabian StyleNwanna, Emeka Charles, Patrick Ehi Imoisili, Sarah Oluwabunmi Bitire, and Tien-Chien Jen. 2021. "Biosynthesis and Fabrication of Copper Oxide Thin Films as a P-Type Semiconductor for Solar Cell Applications" Coatings 11, no. 12: 1545. https://doi.org/10.3390/coatings11121545