Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review
Abstract
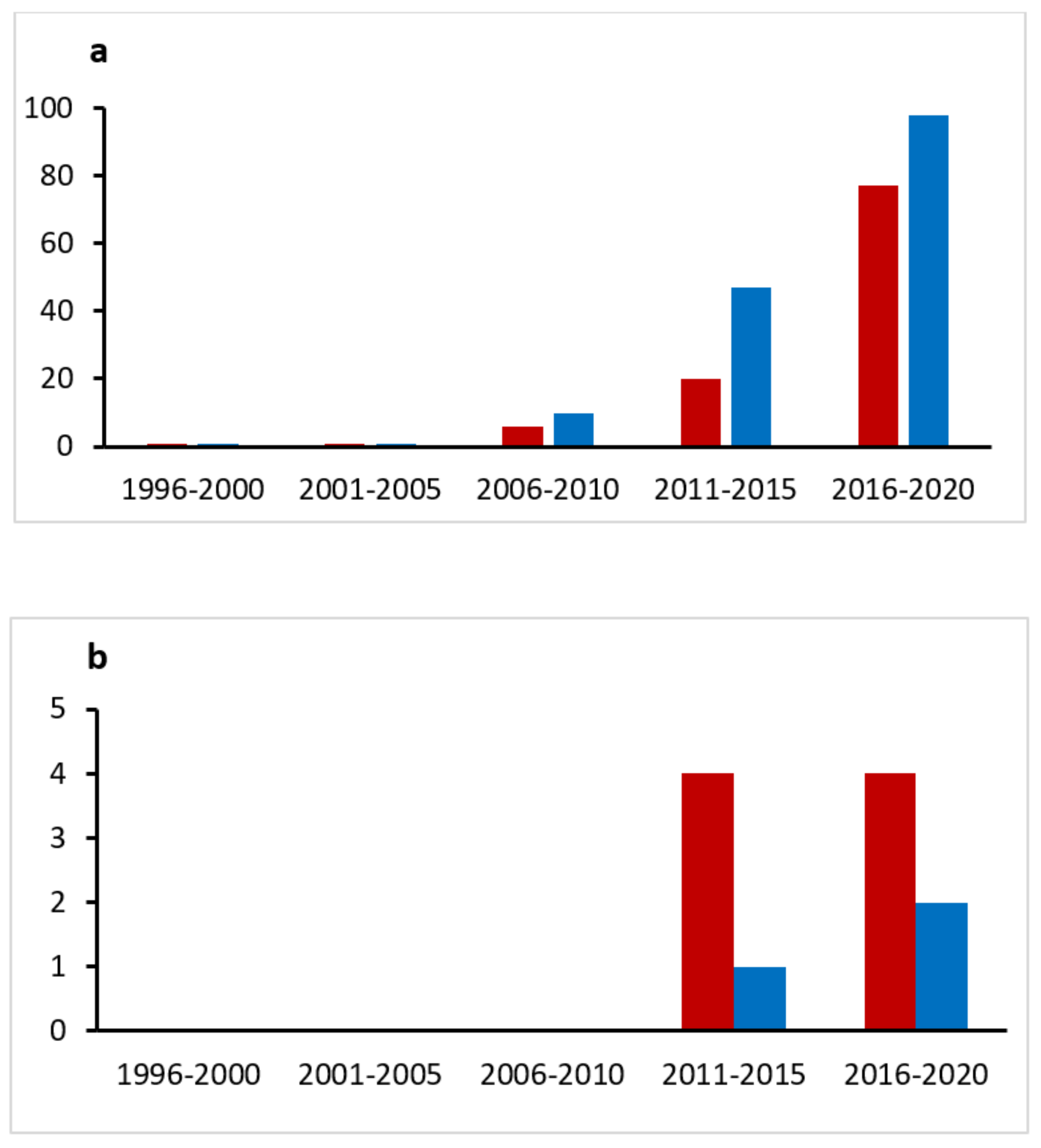
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection of Studies
2.2.1. Inclusion Criteria
- The article includes a description of the procedure to make LF composites;
- The article includes applications of LF composites related to medicine;
- The article includes applications of LF composites related to foods;
- Review articles that help to identify articles related to the topics for review.
2.2.2. Exclusion Criteria
- Articles not providing detailed information on the preparation of LF composites;
- Articles not providing detailed information on the applications of LF composites;
- Articles published before the year 1999.
3. Results
3.1. Encapsulation of Lactoferrin
3.1.1. Encapsulation of Lactoferrin with Milk Proteins
- Whey proteins
- Caseins
3.1.2. Encapsulation of Lactoferrin with Other Proteins
3.1.3. Encapsulation of Lactoferrin with Polysaccharides
- Alginate
- Chondroitin sulphate
- Galactomannans
- Gum arabic
- Pectin
- Chitosan
3.2. Nanocarriers Coated with Lactoferrin
3.3. Edible and Active Film Composites with Lactoferrin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Brock, J.H. The physiology of lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Pérez, M.D.; Conesa, C.; Calvo, M.; Sánchez, L. Effect of technological treatments on bovine lactoferrin: An overview. Food Res. Int. 2018, 106, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Jyonotsuka, T.; Kamemori, N.; Kawano, G.; Shimizu, H.; Ando, K.; Harada, E. Enteric-formulated lactoferrin was more effectively transported into blood circulation from gastrointestinal tract in adult rats. Exp. Physiol. 2006, 91, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Rajabzadeh, G.; Zibaei, S.; Jafari, S.M.; Sani, A.M. Nano-encapsulation of isolated lactoferrin from camel milk by calcium alginate and evaluation of its release. Int. J. Biol. Macromol. 2015, 79, 669–673. [Google Scholar] [CrossRef]
- Braim, S.; Śpiewak, K.; Brindell, M.; Heeg, D.; Alexander, C.; Monaghan, T. Lactoferrin-Loaded Alginate Microparticles to Target Clostridioides difficile Infection. J. Pharm. Sci. 2019, 108, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Costa, C.I.; Matos, C.M.; Moutinho, C.G.; Amorim, M.; Pintado, M.E.; Gomes, A.P.; Vila, M.M.; Teixeira, J.A. Nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013, 32, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lu, J.; Ye, A.; Xu, Q.; Tian, M.; Kong, Y.; Wei, F.; Han, J. Comparative performances of lactoferrin-loaded liposomes under in vitro adult and infant digestion models. Food Chem. 2018, 258, 366–373. [Google Scholar] [CrossRef]
- El-Fakharany, E.M. Nanoformulation of lactoferrin potentiates its activity and enhances novel biotechnological applications. Int. J. Biol. Macromol. 2020, 165, 970–984. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Vargas, E.R.; Rodriguez, M.L.R.M.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent advances in microencapsulation of natural sources of antimicrobial compounds used in food—A review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Mohammadian, M.; Waly, M.I.; Moghadam, M.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Nanostructured food proteins as efficient systems for the encapsulation of bioactive compounds. Food Sci. Hum. Wellness 2020, 9, 199–213. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.S.; Banarjee, S.K.; Gaikwad, D.D.; Jadhav, S.L.; Thorat, R.M. Microencapsulation: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 38–43. [Google Scholar]
- Maresca, D.; De Prisco, A.; La Storia, A.; Cirillo, T.; Esposito, F.; Mauriello, G. Microencapsulation of nisin in alginate beads by vibrating technology: Preliminary investigation. LWT Food Sci. Technol. 2016, 66, 436–443. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-based delivery systems for the nanoencapsulation of food ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release–a review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Gao, Q.; Tang, C.H.; Ge, G.; Zhao, M.; Sun, W. Heteroprotein complex formation of soy protein isolate and lactoferrin: Thermodynamic formation mechanism and morphologic structure. Food Hydrocoll. 2020, 100, 105415. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The nutraceutical bioavailability classification scheme: Classifying nutraceuticals according to factors limiting their oral bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- McClements, D.J. The future of food colloids: Next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Delboni, L.A.; Da Silva, F.L.B. On the complexation of whey proteins. Food Hydrocoll. 2016, 55, 89–99. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Teo, A.; Goh, K.K.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.S.; Lee, S.J. Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: Effect of temperature, pH and salt. Food Chem. 2016, 197, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; de Kruif, C.G.K. Complex coacervates of lactotransferrin and α-lactoglobulin. J. Colloid Interface Sci. 2014, 430, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Chapeau, A.L.; Tavares, G.M.; Hamon, P.; Croguennec, T.; Poncelet, D.; Bouhallab, S. Spontaneous co-assembly of lactoferrin and β-lactoglobulin as a promising biocarrier for vitamin B9. Food Hydrocoll. 2016, 57, 280–290. [Google Scholar] [CrossRef]
- Chapeau, A.L.; Hamon, P.; Rousseau, F.; Croguennec, T.; Poncelet, D.; Bouhllab, S. Scale-up production of vitamin loaded heteroprotein coacervates and their protective property. J. Food Eng. 2017, 206, 67–76. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Interaction between lactoferrin and whey proteins and its influence on the heat-induced gelation of whey proteins. Food Chem. 2018, 252, 92–98. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Hamon, P.; Carvalho, A.F.; Bouhallab, S. How the presence of a small molecule affects the complex coacervation between lactoferrin and β-lactoglobulin. Int. J. Biol. Macromol. 2017, 102, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, K.K.; Karagiannis, T.C.; Hughes, J.G.; Small, D.M.; Hung, A. High temperature induced structural changes of apo-lactoferrin and interactions with β-lactoglobulin and α-lactalbumin for potential encapsulation strategies. Food Hydrocoll. 2020, 105, 105817. [Google Scholar] [CrossRef]
- De Figueiredo Furtado, G.; da Silva Carvalho, A.G.; Hubinger, M.D. Model infant formulas: Influence of types of whey proteins and oil composition on emulsion and powder properties. J. Food Eng. 2021, 292, 110256. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Liyanaarachchi, W.S.; Chandrapala, J.; Dissanayake, M.; Vasiljevic, T. Utilizing unique properties of caseins and the casein micelle for delivery of sensitive food ingredients and bioactives. Trends Food Sci. Technol. 2016, 57, 178–187. [Google Scholar] [CrossRef]
- Anema, S.G.; de Kruif, C.G.K. Interaction of lactoferrin and lysozyme with casein micelles. Biomacromolecules 2011, 12, 3970–3976. [Google Scholar] [CrossRef]
- Anema, S.G.; de Kruif, C.G.K. Co-acervates of lactoferrin and caseins. Soft Matter 2012, 8, 4471–4478. [Google Scholar] [CrossRef] [Green Version]
- Anema, S.G.; de Kruif, C.G.K. Lactoferrin binding to transglutaminase cross-linked casein micelles. Int. Dairy J. 2012, 26, 83–87. [Google Scholar] [CrossRef]
- Anema, S.G.; de Kruif, C.G.K. Phase separation and composition of coacervates of lactoferrin and caseins. Food Hydrocoll. 2016, 52, 670–677. [Google Scholar] [CrossRef]
- Anema, S.G. Spontaneous interaction of lactoferrin with casein micelles or individual caseins. J. R. Soc. N. Z. 2018, 48, 89–110. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Formation of lactoferrin/sodium caseinate complexes and their adsorption behaviour at the air/water interface. Food Chem. 2017, 232, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. 2020, 60, 64–83. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends Food Sci. Technol. 2017, 60, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, S.; Li, J.; McClements, D.J.; Liu, X. Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: Complexes, emulsions, and nanoparticles. Trends Food Sci. Technol. 2018, 79, 67–77. [Google Scholar] [CrossRef]
- Kilic, E.; Novoselova, M.V.; Lim, S.H.; Pyataev, N.A.; Pinyaev, S.I.; Kulikov, O.A.; Sindeeva, O.A.; Mayorova, O.A.; Murney, R.; Antipina, M.N.; et al. Formulation for oral delivery of lactoferrin based on bovine serum albumin and tannic acid multilayer microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adal, E.; Sadeghpour, A.; Connell, S.; Rappolt, M.; Ibanoglu, E.; Sarkar, A. Heteroprotein complex formation of bovine lactoferrin and pea protein isolate: A multiscale structural analysis. Biomacromolecules 2017, 18, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Abbas, S.; Karangwa, E.; Zhang, X.; Xia, S.; Feng, B.; Jia, C. Stable nanoparticles prepared by heating electrostatic complexes of whey protein isolate–dextran conjugate and chondroitin sulfate. J. Agric. Food Chem. 2015, 63, 4179–4189. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, A.V.; Kurek, D.V.; Zubareva, A.A.; Il’in, M.M., Jr.; Mestechkina, N.M.; Varlamov, V.P. Preparation and characterization of biopolymer nanoparticles based on lactoferrin-polysaccharide complexes. React. Funct. Polym. 2016, 102, 33–38. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; Wang, Y.; Chen, J.; Zhong, Y.; Liang, R.; Peng, S.; McClements, D.J.; Liu, W. Tunable high internal phase emulsions (HIPEs) formulated using lactoferrin-gum Arabic complexes. Food Hydrocoll. 2021, 113, 106445. [Google Scholar] [CrossRef]
- Gulao, E.D.S.; de Souza, C.J.; da Silva, F.A.; Coimbra, J.S.; Garcia-Rojas, E.E. Complex coacervates obtained from lactoferrin and gum arabic: Formation and characterization. Food Res. Int. 2014, 65, 367–374. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Dos Santos, C.H.C.; de Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of the black pepper (Piper nigrum L.) essential oil by lactoferrin-sodium alginate complex coacervates: Structural characterization and simulated GIT conditions. Food Chem. 2020, 316, 126345. [Google Scholar] [CrossRef]
- Avendaño-Romero, G.; López-Malo, A.; Paolu, E. Propiedades del alginato y aplicaciones en alimentos. Temas Sel. Ing. Aliment. 2013, 7, 87–96. [Google Scholar]
- Wang, B.; Blanch, E.; Barrow, C.J.; Adhikari, B. Preparation and study of digestion behavior of lactoferrin-sodium alginate complex coacervates. J. Funct. Foods 2017, 37, 97–106. [Google Scholar] [CrossRef]
- Yucel Falco, C.; Amadei, F.; Dhayal, S.K.; Cárdenas, M.; Tanaka, M.; Risbo, J. Hybrid coating of alginate microbeads based on protein-biopolymer multilayers for encapsulation of probiotics. Biotechnol. Prog. 2019, 35, e2806. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel alginate-enclosed chitosan–calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine 2012, 7, 1521–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Abdelfattah, E.Z.A.; Fang, J.Y.; Samaha, M.W.; Freag, M.S. Inhalable lactoferrin/chondroitin-functionalized monoolein nanocomposites for localized lung cancer targeting. ACS Biomater. Sci. Eng. 2020, 6, 1030–1042. [Google Scholar] [CrossRef]
- Albuquerque, P.B.; Cerqueira, M.A.; Vicente, A.A.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Immobilization of bioactive compounds in Cassia grandis galactomannan-based films: Influence on physicochemical properties. Int. J. Biol. Macromol. 2017, 96, 727–735. [Google Scholar] [CrossRef]
- Mathur, V.; Mathur, N.K. Fenugreek and other lesser known legume galactomannan-polysaccharides: Scope for developments. J. Sci. Ind. Res. 2005, 64, 475–481. [Google Scholar]
- Il’ina, A.V.; Mestechkina, N.M.; Kurek, D.V.; Levov, A.N.; Semenyuk, P.I.; Orlov, V.N.; Shcherbukhin, V.D.; Varlamov, V.P. Preparing, studying, and prospects of using nanoparticles based on chitosan and galactomannan. Nanotechnol. Russ. 2011, 6, 154–160. [Google Scholar] [CrossRef]
- Santos, M.B.; Dos Santos, C.H.C.; de Carvalho, M.G.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Physicochemical, thermal and rheological properties of synthesized carboxymethyl tara gum (Caesalpinia spinosa). Int. J. Biol. Macromol. 2019, 134, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.B.; de Carvalho, M.G.; Garcia-Rojas, E.E. Carboxymethyl tara gum-lactoferrin complex coacervates as carriers for vitamin D3: Encapsulation and controlled release. Food Hydrocoll. 2021, 112, 106347. [Google Scholar] [CrossRef]
- Bengoechea, C.; Jones, O.G.; Guerrero, A.; McClements, D.J. Formation and characterization of lactoferrin/pectin electrostatic complexes: Impact of composition, pH and thermal treatment. Food Hydrocoll. 2011, 25, 1227–1232. [Google Scholar] [CrossRef]
- Raei, M.; Shahidi, F.; Farhoodi, M.; Jafari, S.M.; Rafe, A. Application of whey protein-pectin nano-complex carriers for loading of lactoferrin. Int. J. Biol. Macromol. 2017, 105, 281–291. [Google Scholar] [CrossRef]
- Niu, Z.; Loveday, S.M.; Barbe, V.; Thielen, I.; He, Y.; Singh, H. Protection of native lactoferrin under gastric conditions through complexation with pectin and chitosan. Food Hydrocoll. 2019, 93, 120–130. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Lyalina, T.; Zubareva, A.; Varlamov, V.; Svirshchevskaya, E. Cross-presentation of lactoferrin encapsulated into chitosan-based nanoparticles. Nanobiomedicine 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Vicente, A.A. Influence of chitosan coating on protein-based nanohydrogels properties and in vitro gastric digestibility. Food Hydrocoll. 2016, 60, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Vicente, A.A. In vitro digestion of lactoferrin-glycomacropeptide nanohydrogels incorporating bioactive compounds: Effect of a chitosan coating. Food Hydrocoll. 2018, 84, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.-Y.; Wen, J. Oral delivery of bovine lactoferrin using pectin- and chitosan-modified liposomes and solid lipid particles: Improve-ment of stability of lactoferrin. Chem. Biol. Drug Des. 2015, 86, 466–475. [Google Scholar] [CrossRef]
- Agwa, M.M.; Sabra, S. Lactoferrin coated or conjugated nanomaterials as an active targeting approach in nanomedicine. Int. J. Biol. Macromol. 2020, 167, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Curtis, A.S. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials 2004, 25, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhu, Y.; Jiang, W.; Zhou, Q.; Yang, H.; Gu, N.; Zhang, Y.; Xu, H.; Xu, H.; Yang, X. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 2011, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Jia, Q.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood–brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef]
- Fang, J.H.; Chiu, T.L.; Huang, W.C.; Lai, Y.H.; Hu, S.H.; Chen, Y.Y.; Chen, S.Y. Dual-Targeting Lactoferrin-Conjugated Polymerized Magnetic Polydiacetylene-Assembled Nanocarriers with Self-Responsive Fluorescence/Magnetic Resonance Imaging for In Vivo Brain Tumor Therapy. Adv. Healthc. Mater. 2016, 5, 688–695. [Google Scholar] [CrossRef]
- Song, M.M.; Xu, H.L.; Liang, J.X.; Xiang, H.H.; Liu, R.; Shen, Y.X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater. Sci. Eng. C 2017, 77, 904–911. [Google Scholar] [CrossRef]
- Sharifi, M.; Rezayat, S.M.; Akhtari, K.; Hasan, A.; Falahati, M. Fabrication and evaluation of anti-cancer efficacy of lactoferrin-coated maghemite and magnetite nanoparticles. J. Biomol. Struct. Dyn. 2020, 38, 2945–2954. [Google Scholar] [CrossRef]
- Ali, O.M.; Bekhit, A.A.; Khattab, S.N.; Helmy, M.W.; Abdel-Ghany, Y.S.; Teleb, M.; Elzoghby, A.O. Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy. Colloids Surf. B 2020, 188, 110824. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Cheng, S.J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mahidhara, G.; Kanwar, R.K.; Roy, K.; Kanwar, J.R. Oral administration of iron-saturated bovine lactoferrin–loaded ceramic nanocapsules for breast cancer therapy and influence on iron and calcium metabolism. Int. J. Nanomed. 2015, 10, 4081–4098. [Google Scholar]
- Roy, K.; Patel, Y.S.; Kanwar, R.K.; Rajkhowa, R.; Wang, X.; Kanwar, J.R. Biodegradable Eri silk nanoparticles as a delivery vehicle for bovine lactoferrin against MDA-MB-231 and MCF-7 breast cancer cells. Int. J. Nanomed. 2016, 11, 25. [Google Scholar]
- Kanwar, J.R.; Kamalapuram, S.K.; Krishnakumar, S.; Kanwar, R.K. Multimodal iron oxide (Fe3O4)-saturated lactoferrin nanocapsules as nanotheranostics for real-time imaging and breast cancer therapy of claudin-low, triple-negative (ER-/PR-/HER2). Nanomedicine 2016, 11, 249–268. [Google Scholar] [CrossRef] [PubMed]
- AbdElhamid, A.S.; Zayed, D.G.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zein-El-Dein, E.A.; El-Gizawy, S.A.; Elzoghby, A.O. Lactoferrin-tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. Nanomedicine 2018, 13, 2637–2656. [Google Scholar] [CrossRef]
- El-Lakany, S.A.; Elgindy, N.A.; Helmy, M.W.; Abu-Serie, M.M.; Elzoghby, A.O. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin. Drug Deliv. 2018, 15, 835–850. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef]
- Sabra, S.A.; Sheweita, S.A.; Haroun, M.; Ragab, D.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Elzoghby, A.O.; Rohani, S. Magnetically guided self-assembled protein micelles for enhanced delivery of dasatinib to human triple-negative breast cancer cells. J. Pharm. Sci. 2019, 108, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.B.M.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef]
- Pandey, V.; Gajbhiye, K.R.; Soni, V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 2015, 22, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, J.R.; Mahidhara, G.; Roy, K.; Sasidharan, S.; Krishnakumar, S.; Prasad, N.; Sehgal, R.; Kanwar, R.K. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine 2015, 10, 35–55. [Google Scholar] [CrossRef]
- Roy, K.; Kanwar, R.K.; Kanwar, J.R. LNA aptamer based multi-modal, Fe3O4-saturated lactoferrin (Fe3O4-bLf) nanocarriers for triple positive (EpCAM, CD133, CD44) colon tumor targeting and NIR, MRI and CT imaging. Biomaterials 2015, 71, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Kumari, S.; Kondapi, A.K. Evaluation of antiproliferative activity, safety and biodistribution of oxaliplatin and 5-fluorouracil loaded lactoferrin nanoparticles for the management of colon adenocarcinoma: An in vitro and an in vivo study. Pharm. Res. 2018, 35, 178. [Google Scholar] [CrossRef]
- Miao, D.; Jiang, M.; Liu, Z.; Gu, G.; Hu, Q.; Kang, T.; Song, Q.; Yao, L.; Li, W.; Gao, X.; et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol. Pharm. 2014, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chen, Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin-and folic acid-grafted poly (lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef]
- Mo, X.; Zheng, Z.; He, Y.; Zhong, H.; Kang, X.; Shi, M.; Liu, T.; Jiao, Z.; Huang, Y. Antiglioma via regulating oxidative stress and remodeling tumor-associated macrophage using lactoferrin-mediated biomimetic codelivery of simvastatin/fenretinide. J. Control. Release 2018, 287, 12–23. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, K.; Patel, S.; Singh, R.; Patel, K.; Sawant, K. Hyaluronic acid tethered pH-responsive alloy-drug nanoconjugates for multimodal therapy of glioblastoma: An intranasal route approach. Mater. Sci. Eng. C 2019, 98, 419–436. [Google Scholar] [CrossRef]
- Pandey, A.; Kulkarni, S.; Vincent, A.P.; Nannuri, S.H.; George, S.D.; Mutalik, S. Hyaluronic acid-drug conjugate modified core-shell MOFs as pH responsive nanoplatform for multimodal therapy of glioblastoma. Int. J. Pharm. 2020, 588, 119735. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, T.; Yang, Z.; Zhou, Z.; Tang, W.; Fan, W.; Liu, Y.; Mu, J.; Li, L.; Bregadze, V.I.; et al. Small-sized gadolinium oxide based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma. Biomaterials 2020, 235, 119783. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. pH/temperature sensitive magnetic nanogels conjugated with Cy5. 5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef]
- Su, Z.; Xing, L.; Chen, Y.; Xu, Y.; Yang, F.; Zhang, C.; Ping, Q.; Xiao, Y. Lactoferrin-modified poly (ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol. Pharm. 2014, 11, 1823–1834. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Gandhi, S.; Krishnan, K.M. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale 2015, 7, 16890–16898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Mu, K.; Jiang, L.; Xie, H.; Liu, W.; Li, Z.; Qi, H.; Liang, S.; Xu, H.; Zhu, Y.; et al. Glioma-targeting micelles for optical/magnetic resonance dual-mode imaging. Int. J. Nanomed. 2015, 10, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.; Lamprecht, A. Nuclear and cytoplasmic delivery of lactoferrin in glioma using chitosan nanoparticles: Cellular location dependent-action of lactoferrin. Eur. J. Pharm. Biopharm. 2018, 129, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Xu, Y.; Zou, Q.; Tu, L.; Tang, C.; Xu, T.; Deng, L.; Wu, C. Hepatocellular carcinoma targeting effect of PEGylated liposomes modified with lactoferrin. Eur. J. Pharm. Sci. 2012, 46, 131–141. [Google Scholar] [CrossRef]
- Wei, M.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Xu, Y.; Wu, C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 5123. [Google Scholar]
- Wei, M.Y.; Zou, Q.; Wu, C.B.; Xu, Y.H. In vitro targeting effect of lactoferrin modified PEGylated liposomes for hepatoma cells. Acta Pharm. Sin. 2015, 50, 1272–1279. [Google Scholar]
- Abdelmoneem, M.A.; Mahmoud, M.; Zaky, A.; Helmy, M.W.; Sallam, M.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Decorating protein nanospheres with lactoferrin enhances oral COX-2 inhibitor/herbal therapy of hepatocellular carcinoma. Nanomedicine 2018, 13, 2377–2395. [Google Scholar] [CrossRef]
- Abdelmoneem, M.A.; Elnaggar, M.A.; Hammady, R.S.; Kamel, S.M.; Helmy, M.W.; Abdulkader, M.A.; Zaky, A.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-targeted lactoferrin shell-oily core nanocapsules for synergistic targeted/herbal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 26731–26744. [Google Scholar] [CrossRef] [PubMed]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 2018, 13, 2015–2035. [Google Scholar] [CrossRef] [PubMed]
- Kabary, D.M.; Helmy, M.W.; Elkhodairy, K.A.; Fang, J.Y.; Elzoghby, A.O. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf. B 2018, 169, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.G.; Elzoghby, A.O.; Helmy, M.W.; Khalil, R.; Khairy, H.; Omar, S. Dual therapeutic targeting of lung infection and carcinoma using lactoferrin-based green nanomedicine. ACS Biomater. Sci. Eng. 2020, 6, 5685–5699. [Google Scholar] [CrossRef]
- Etman, S.M.; Abdallah, O.Y.; Mehanna, R.A.; Elnaggar, Y.S. Lactoferrin/Hyaluronic acid double-coated lignosulfonate nanoparticles of quinacrine as a controlled release biodegradable nanomedicine targeting pancreatic cancer. Int. J. Pharm. 2020, 578, 119097. [Google Scholar] [CrossRef]
- Etman, S.M.; Abdallah, O.Y.; Elnaggar, Y.S. Novel fucoidan based bioactive targeted nanoparticles from Undaria pinnatifida for treatment of pancreatic cancer. Int. J. Biol. Macromol. 2020, 145, 390–401. [Google Scholar] [CrossRef]
- Ahmed, F.; Ali, M.J.; Kondapi, A.K. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int. J. Biol. Macromol. 2014, 70, 572–582. [Google Scholar] [CrossRef]
- Akilo, O.D.; Kumar, P.; Choonara, Y.E.; Pradeep, P.; du Toit, L.C.; Pillay, V. Hypothesis: Apo-lactoferrin–Galantamine Proteo-alkaloid Conjugate for Alzheimer’s disease Intervention. J. Cell. Mol. Med. 2018, 22, 1957–1963. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705. [Google Scholar] [CrossRef] [Green Version]
- Gopalan, D.; Pandey, A.; Udupa, N.; Mutalik, S. Receptor specific, stimuli responsive and subcellular targeted approaches for effective therapy of Alzheimer: Role of surface engineered nanocarriers. J. Control. Release 2020, 319, 183–200. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s disease in mice. Bioconjugate Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, Z.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Pillay, V. A review of the potential role of nano-enabled drug delivery technologies in amyotrophic lateral sclerosis: Lessons learned from other neurodegenerative disorders. J. Pharm. Sci. 2015, 104, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gomis, J.; Fernández-Solanas, A.; Vinas, M.; González, P.; Planas, M.E.; Sánchez, S. Effects of topical application of free and liposome-encapsulated lactoferrin and lactoperoxidase on oral microbiota and dental caries in rats. Arch. Oral Biol. 1999, 44, 901–906. [Google Scholar] [CrossRef]
- Halder, A.; Shukla, D.; Das, S.; Roy, P.; Mukherjee, A.; Saha, B. Lactoferrin-modified Betulinic Acid-loaded PLGA nanoparticles are strong anti-leishmanials. Cytokine 2018, 110, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin adsorbed onto biomimetic hydroxyapatite nanocrystals controlling-in vivo-the Helicobacter pylori infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Lakshmi, Y.S.; Kondapi, A.K. Triple drug combination of zidovudine, efavirenz and lamivudine loaded lactoferrin nanoparticles: An effective nano first-line regimen for HIV therapy. Pharm. Res. 2017, 34, 257–268. [Google Scholar] [CrossRef]
- Senapathi, J.; Bommakanti, A.; Mallepalli, S.; Mukhopadhyay, S.; Kondapi, A.K. Sulfonate modified Lactoferrin nanoparticles as drug carriers with dual activity against HIV-1. Colloids Surf. B 2020, 191, 110979. [Google Scholar] [CrossRef]
- Hadidi, N.; Saffari, M.; Faizi, M. Optimized transferosomal bovine lactoferrin (BLF) as a promising novel non-invasive topical treatment for genital warts caused by human papiluma virus (HPV). Iran. J. Pharm. Res. 2018, 17, 12. [Google Scholar]
- Anand, N.; Sehgal, R.; Kanwar, R.K.; Dubey, M.L.; Vasishta, R.K.; Kanwar, J.R. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int. J. Nanomed. 2015, 10, 6355. [Google Scholar]
- Anand, N.; Kanwar, R.K.; Sehgal, R.; Kanwar, J.R. Antiparasitic and immunomodulatory potential of oral nanocapsules encapsulated lactoferrin protein against Plasmodium berghei. Nanomedicine 2016, 11, 47–62. [Google Scholar] [CrossRef]
- Samarasinghe, R.M.; Kanwar, R.K.; Kanwar, J.R. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials 2014, 35, 7522–7534. [Google Scholar] [CrossRef]
- Huang, R.; Han, L.; Li, J.; Ren, F.; Ke, W.; Jiang, C.; Pei, Y. Neuroprotection in a 6-hydroxydopamine-lesioned Parkinson model using lactoferrin-modified nanoparticles. J. Gene Med. 2009, 11, 754–763. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.C.; Rajesh, R. Current development of nanocarrier delivery systems for Parkinson’s disease pharmacotherapy. J. Taiwan Inst. Chem. Eng. 2018, 87, 15–25. [Google Scholar] [CrossRef]
- Tang, S.; Wang, A.; Yan, X.; Chu, L.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Li, Z.; Liu, Y.; Wang, Q.; Luo, J.; Chen, X.; Xie, Z.; Zhang, Y.; Zhang, H.; Chen, T. Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson’s disease. Biomaterials 2020, 260, 120339. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, S.; Kim, J.G.; Song, M.H.; Shim, K.S.; Lim, Y.M.; Kim, H.J.; Park, K.; Kim, S.E. Enhanced tendon restoration effects of anti-inflammatory, lactoferrin-immobilized, heparin-polymeric nanoparticles in an Achilles tendinitis rat model. Carbohydr. Polym. 2020, 241, 116284. [Google Scholar] [CrossRef]
- Coughlan, K.; Shaw, N.B.; Kerry, J.F.; Kerry, J.P. Combined effects of proteins and polysaccharides on physical properties of whey protein concentrate-based edible films. J. Food Sci. 2004, 69, E271–E275. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Min, S.; Krochta, J.M. Inhibition of Penicillium commune by edible whey protein films incorporating lactoferrin, lacto-ferrin hydrolysate, and lactoperoxidase systems. J. Food Sci. 2005, 70, M87–M94. [Google Scholar] [CrossRef]
- Barbiroli, A.; Farris, S.; Rollini, M. Combinational approaches for antimicrobial packaging: Lysozyme and lactoferrin. In Antimicrobial Food Packaging, 1st ed.; Barros-Velázquez, J., Ed.; Academic Press: London, UK, 2016; pp. 589–597. [Google Scholar]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.; Avides, M.C.; Quintas, M.A.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.A.; Wang, B.; Oh, J.H. Antimicrobial activity of lactoferrin against foodborne pathogenic bacteria incorporated into edible chitosan film. J. Food Prot. 2008, 71, 319–324. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control. 2012, 26, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Padrao, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Padrao, J.; Ribeiro, S.; Lanceros-Méndez, S.; Rodrigues, L.R.; Dourado, F. Effect of bacterial nanocellulose binding on the bactericidal activity of bovine lactoferrin. Heliyon 2020, 6, e04372. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.; Atarés, L.; Chiralt, A. Effect of the incorporation of antimicrobial/antioxidant proteins on the properties of potato starch films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Quintieri, L.; Pistillo, B.R.; Caputo, L.; Favia, P.; Baruzzi, F. Bovine lactoferrin and lactoferricin on plasma-deposited coating against spoilage Pseudomonas spp. Innov. Food Sci. Emerg. Technol. 2013, 20, 215–222. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wu, J.Y. Biocompatible polyelectrolyte complex nanoparticles from lactoferrin and pectin as potential vehicles for antioxidative curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Santos, S.F.; Bourbon, A.I.; Pinheiro, A.C.; González-Fernández, A.; Pastrana, L.M.; Cerqueira, M.A.; Vicente, A.A. Lactoferrin-based nanoparticles as a vehicle for iron in food applications–Development and release profile. Food Res. Int. 2016, 90, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Application | Type of Nanocarrier | Model of Study |
|---|---|---|
| Cancer therapy | ||
| Brain tumour | Magnetic nanocarriers | Rats [77] |
| Solid lipid nanoparticle | HBMECs, U87MG cells [81] | |
| Breast cancer | Polymeric nanoparticles | MDA-MB-231 cells, mice [82] |
| Silk nanoparticles | MDA-MB-231, MCF-7 cells [83] | |
| Protein nanocapsules | Mice [84] | |
| Quantum dots-based chondroitin sulphate nanocapsules | MCF-7, MDA-MB-231 cells, mice [85] | |
| Zein nanospheres | MCF-7, 4T1 cellsrats [86] | |
| Polymeric micelles | MDA-MB-231, MCF-7 cells [87,88] | |
| Silica nanoparticles | MCF-7 cells [80] | |
| Betulinic acid nanoparticles | MDA-MB-231 cells [89] | |
| Magnetic nanoparticles | 4T1 cells [79] | |
| Bronchogenic carcinoma | Solid lipid nanoparticles | BEAS-2B cells, rats [90] |
| Colon tumour imaging | Polymeric nanocarriers | Caco-2 cells, mice [56,91] |
| Polymeric nanocarriers | Caco-2 cells, mice [92] | |
| Protein nanoparticles | COLO-205 cells, rats [93] | |
| Glioblastoma | Polymeric nanoparticles | BCEC, C6 glioma cells, rats, mice [94] |
| Polymeric nanoparticles | U87MG, HBMEC, HA cells [95] | |
| Protein nanoparticles | U87MG, BCEC, HUVEC cells, mice [96] | |
| Protein, FePt nanoparticles | U87MG, U-373 MG, MDCKII cells, rats [97,98] | |
| Gadolinium oxide nanoparticles | U87MG, MCF-7 cells, mice [99] | |
| Glioma | Magnetic nanoparticles | HEK 293, C6 glioma, ECV 304 cells, rats [75] |
| Magnetic nanogels | C6 glioma, ECV 304 Rats [100] | |
| Protein nanoparticles | BCECs, C6 glioma cells, rats [101] | |
| Magnetic nanoparticles | C6 glioma cells [102] | |
| Magnetic nanoparticles | C6 glioma cells, rats [103] | |
| Polymeric nanoparticles | C6 glioma cells, rats [104] | |
| Magnetic nanocomposites | C6 glioma cells [78] | |
| Polymeric nanoparticles | BCECs, C6 glioma cells, mice [105] | |
| Polymeric nanoparticles | L929, glioma 261 cells [106] | |
| Hepatocellular carcinoma | PEGylated liposomes | HepG2, ECV304, BEL7402, NIH 3T3, SMMC7721 cells, mice [107,108] |
| PEGylated liposomes | HepG2 cells [109] | |
| Protein nanoparticles, nanoemulsion | HepG2 cells, mice [110,111] | |
| Laryngeal cancer | Betulinic acid nanoparticles | HEp-2 cells [89] |
| Lung carcinoma | Inhalable nanocomposites | A549 cells, mice [112] |
| Lipid nanocarriers | A549 cells, mice [113] | |
| Inhalable nanocomposites | A549 cells, mice [58] | |
| Polymeric nanoparticles | A549 cells, mice [114] | |
| Pancreatic cancer | Polymeric nanoparticles | PANC-1 cells, mice [115,116] |
| Retinoblastoma | Protein nanoparticles | Y79 cells [117] |
| Other pathologies | ||
| Alzheimer | Protein nanoparticles | In silico [118] |
| Polymeric nanoparticles | 16HBE, SH-SY5Y cells, mice [119] | |
| - | Review [120] | |
| Polymeric nanoparticles | bEnd3 cells, mice, rats [121] | |
| Amyotrophic lateral sclerosis | Polymeric nanoparticles | Review [122] |
| Dental caries | Liposomes | Rats [123] |
| Leishmaniosis | Polymeric nanoparticles | Mice [124] |
| Infection by Clostridioides | Polymeric nanoparticles | Caco-2, Vero cells [6] |
| Infection by Helicobacter pylori | Nanocrystals | Mice [125] |
| HIV | Nanosuspensions | U-937 cells, rats [126] |
| Protein nanoparticles | SupT1, HL2/3 cells [127] | |
| Infection by papilloma virus | Transferosomes | Hela cells [128] |
| Infection by Toxoplasma gondii | Protein nanocapsules | J7741 cells, mice [129] |
| Infection by Plasmodium berghei | Polymeric nanoparticles | Mice [130] |
| Osteoarthritis | Polymeric nanocarriers | Mice [131] |
| Parkinson | Protein nanoparticles | BCEC cells, rats [132,133] |
| Polymeric nanoparticles | bEnd.3 cells, mice [134] | |
| Polymeric nanoparticles | SH-SY5Y, 16HBE cells, mice [135] | |
| Polymer, solid lipid nanoparticles, liposomes, exosomes | Review [136] | |
| Polymeric nanoparticles | SH-SY5Y, 16HBE cells, rats [137] | |
| Phosphorus nanosheets | SH-SY5Y, bEnd.3 cells, mice, rats [138] | |
| Tendinitis | Polymeric nanoparticles | Tenocytes, rats [139] |
| System | Other Molecules Combined | Activity |
|---|---|---|
| Composite edible and active films | ||
| Chitosan films | Glycomacropeptide | Water vapour, oxygen and carbon dioxide permeability decrease [144] |
| Lysozyme | Antimicrobial activity against Listeria monocytogenes and Escherichia coli O157:H7 [145] | |
| Cellulose films | Lysozyme | Antimicrobial activity against Escherichia, Listeria and natural microbiota in veal meat [146] |
| -- | Antimicrobial activity against Escherichia coli and Staphylococcus aureus [147,148] | |
| Starch films | Lysozyme | Antimicrobial activity against E.coli and coliform microbiota of pork minced meatLard oxidation reduction [149] |
| Gelatin-based films with chitosan nanoparticles | Quercetin | Antimicrobial activityAntioxidantFilm degradation improvement [150] |
| Polyethylene microtubes | Lactoferricin B | Antimicrobial activity against Pseudomonas strains responsible for casein hydrolysis and cheese pigmentation in mozzarella [151] |
| LF nanoparticles | ||
| Cichoric acid (CA) | Antioxidant [104] | |
| Pectin and curcumin | Antioxidant [152] | |
| Iron | Iron carrier [153] | |
| Functional ingredients in commercial products [64] | ||
| Emulsions | ||
| Resveratrol | Emulsion stability and antioxidant activity [44] | |
| WPI or whey protein hydrolysates with oil mixture | Ingredient in powder formula to mimic fat composition of human milk [34] | |
| To deliver active compounds [43,45] | ||
| Coacervates | ||
| Whey proteins | Non-specified [28] | |
| B9 vitamin | For functional foods [29,30] | |
| Food systems, bioactive encapsulation [25,32] | ||
| Caseins | To deliver food ingredients and bioactive compounds [37,38,39,40] | |
| Pea protein isolate (PPI) | Food applications [47] | |
| Soy protein isolate (SPI) | Increase the thermal stability of LF [21] | |
| Gels | ||
| Whey protein isolate (WPI) | Improvement of gelled protein products [31] | |
| GMP | Curcumin and caffeine | Encapsulation to deliver compounds [69] |
| Polysaccharides | Food additive [54] | |
| To deliver compounds [5] | ||
| To encapsulate essential oils [52] | ||
| To encapsulate probiotics [55] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abad, I.; Conesa, C.; Sánchez, L. Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review. Materials 2021, 14, 7358. https://doi.org/10.3390/ma14237358
Abad I, Conesa C, Sánchez L. Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review. Materials. 2021; 14(23):7358. https://doi.org/10.3390/ma14237358
Chicago/Turabian StyleAbad, Inés, Celia Conesa, and Lourdes Sánchez. 2021. "Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review" Materials 14, no. 23: 7358. https://doi.org/10.3390/ma14237358
APA StyleAbad, I., Conesa, C., & Sánchez, L. (2021). Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review. Materials, 14(23), 7358. https://doi.org/10.3390/ma14237358






