Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization
Abstract
1. Introduction
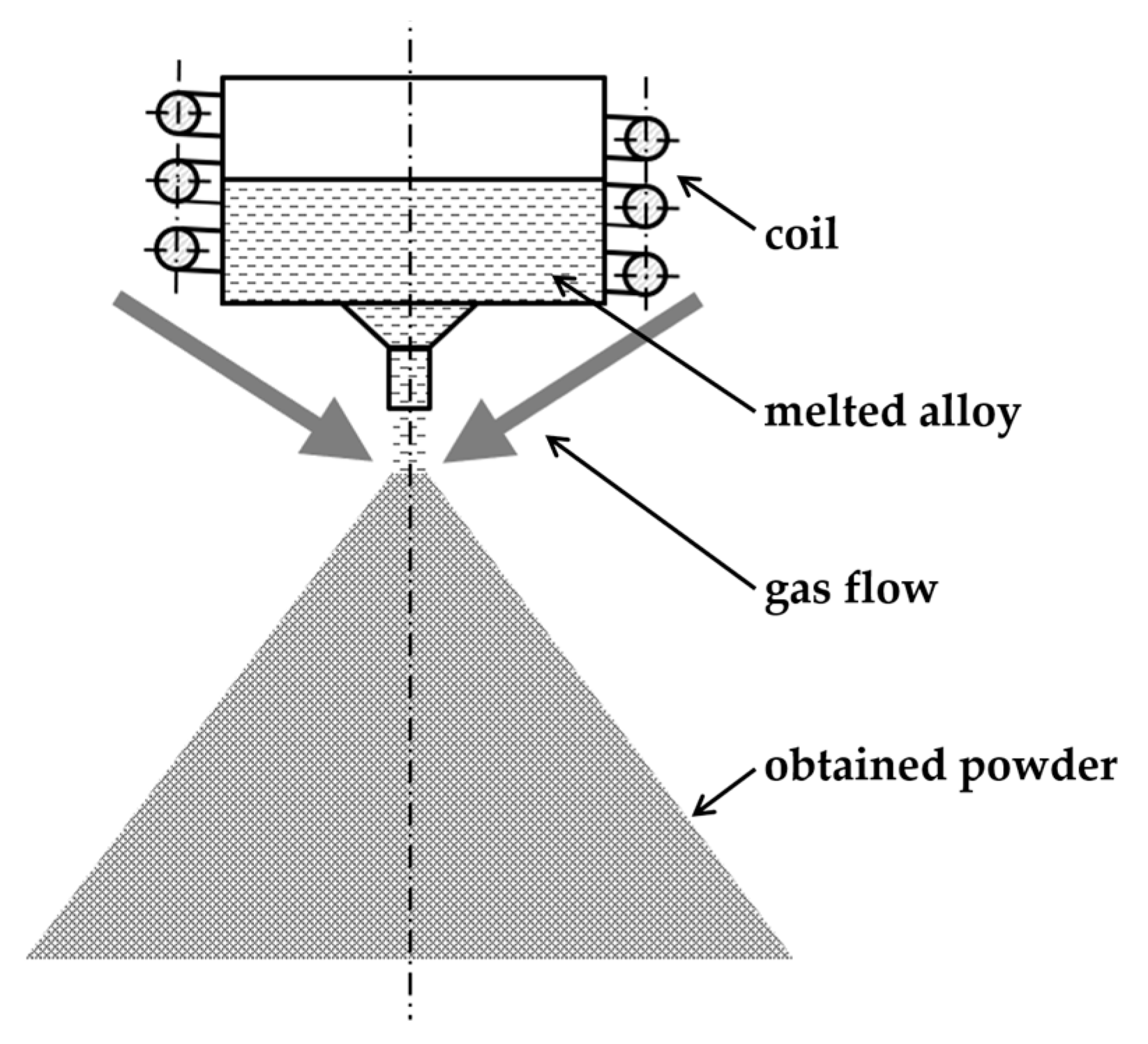
2. Materials and Methods
3. Results
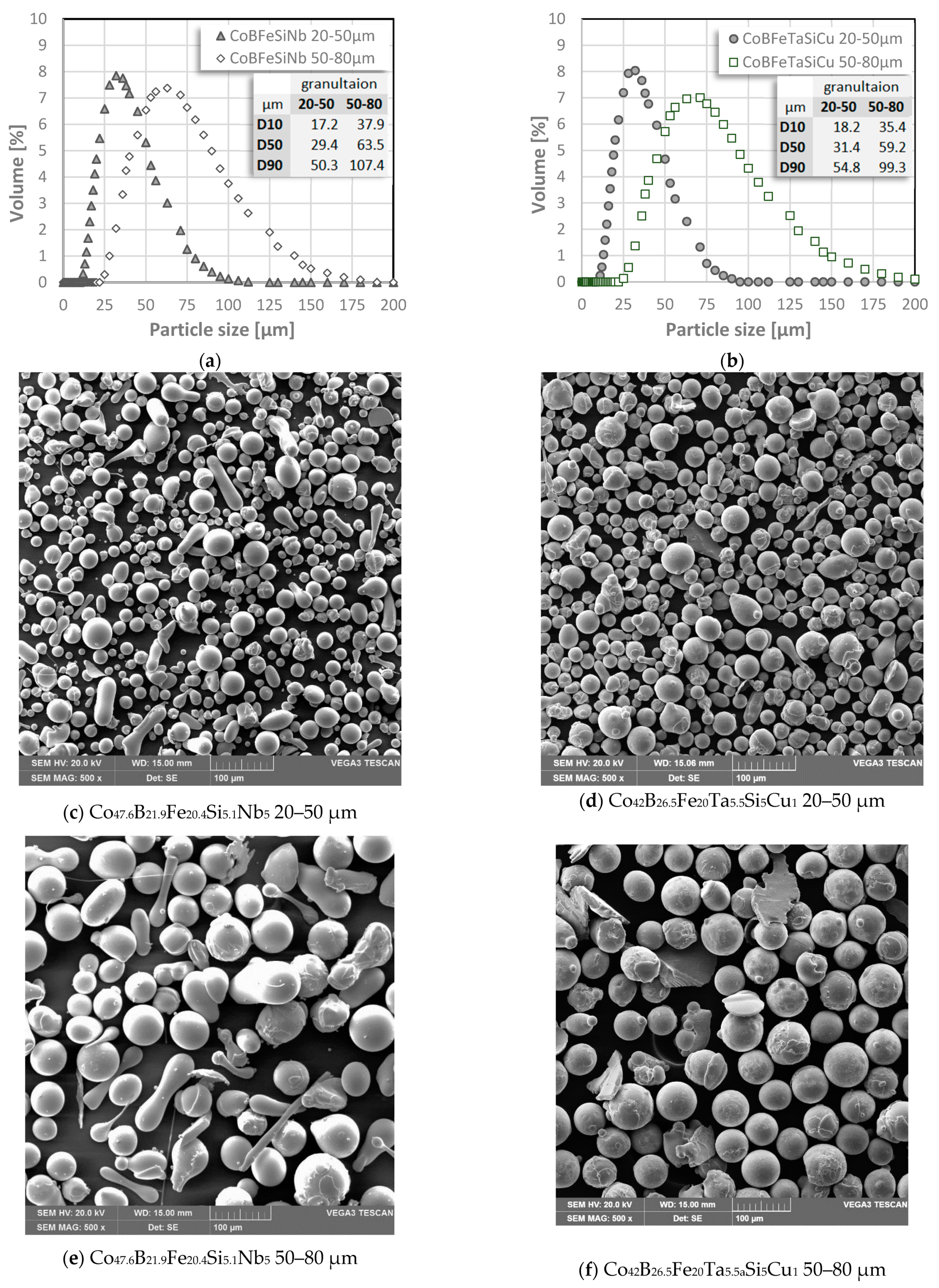
3.1. Particle Size Distribution
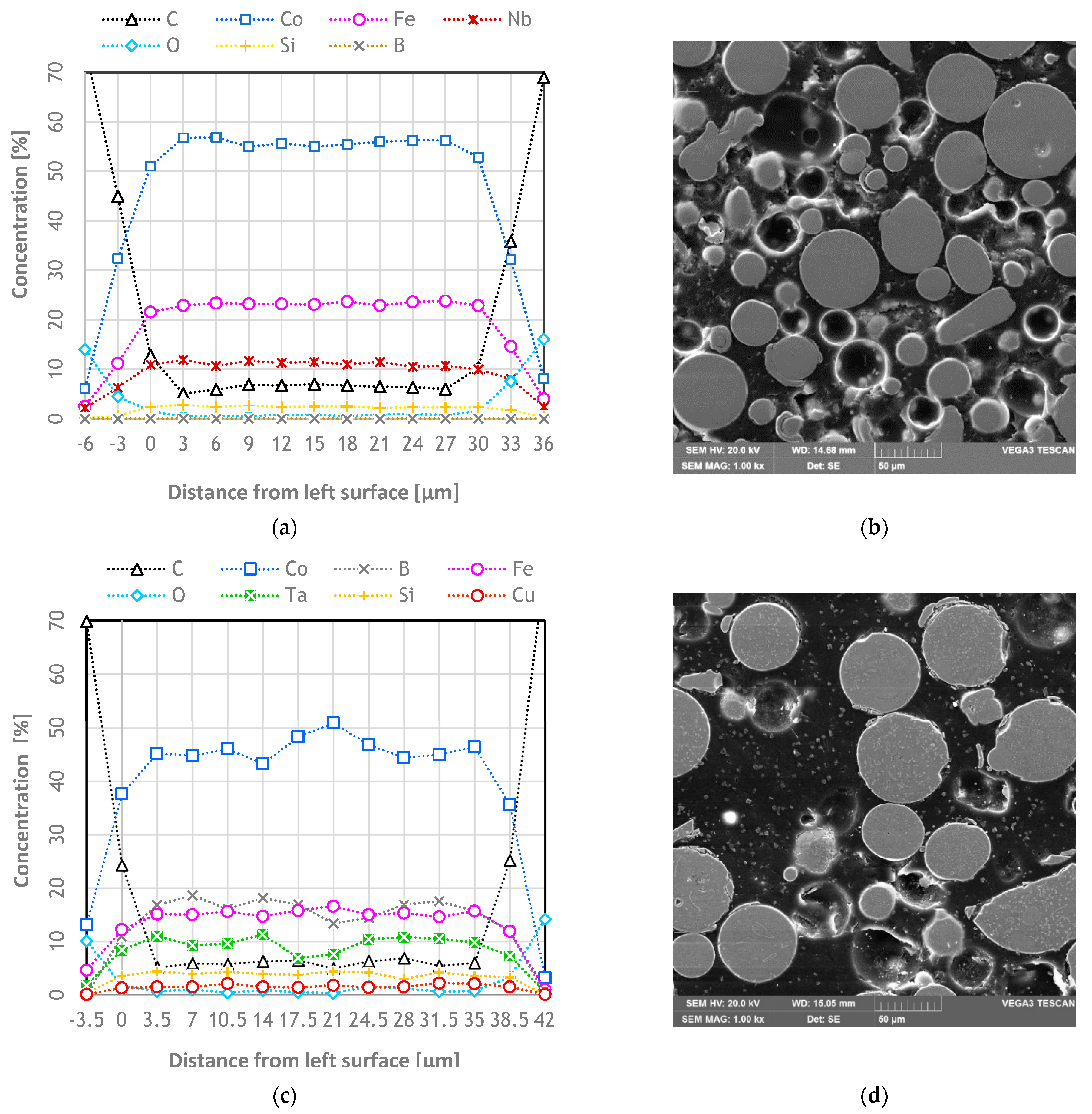
3.2. EDS Analyzes/Chemical Composition Homogeneity of Particles
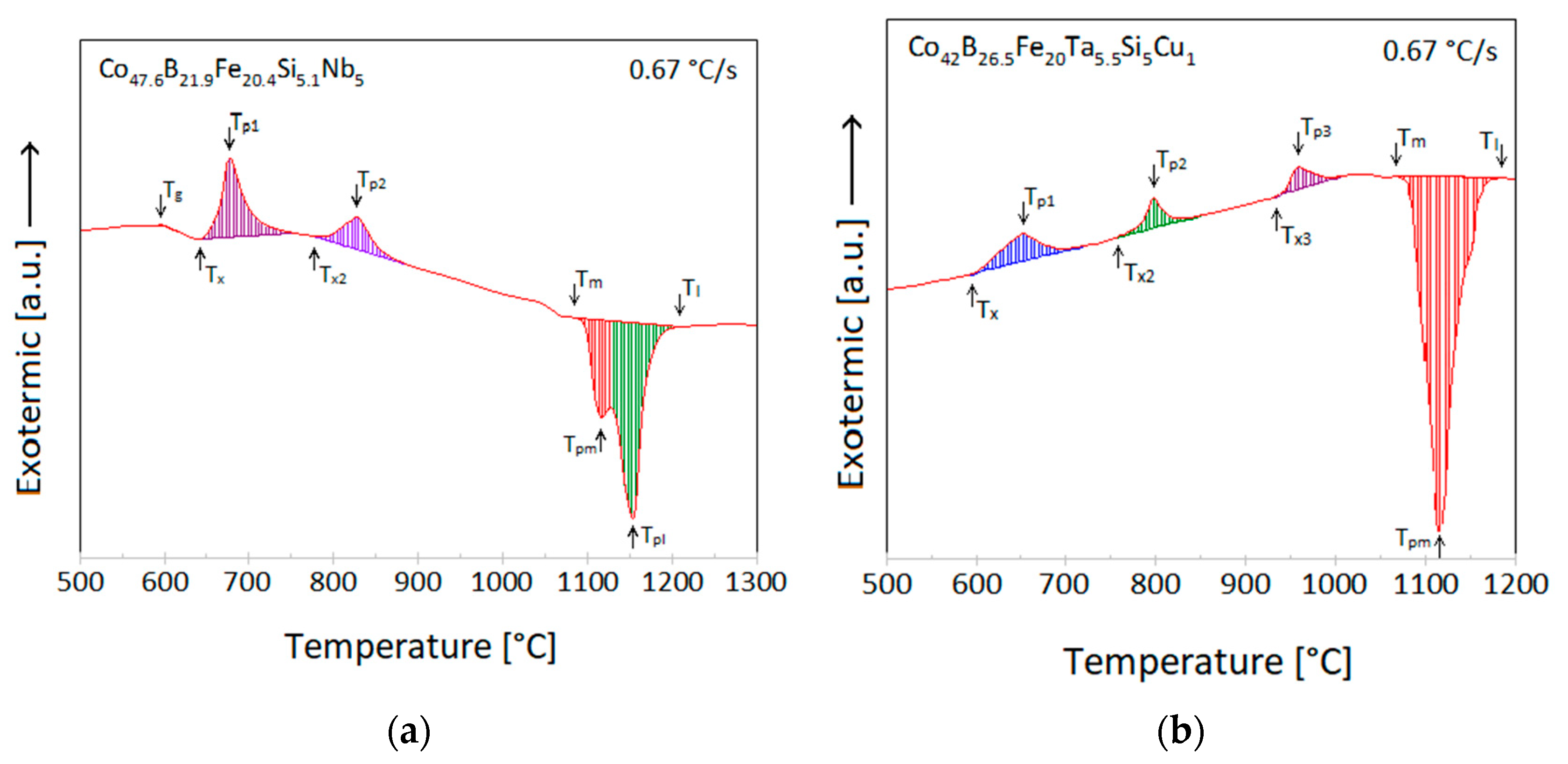
3.3. Differential Thermal Analysis
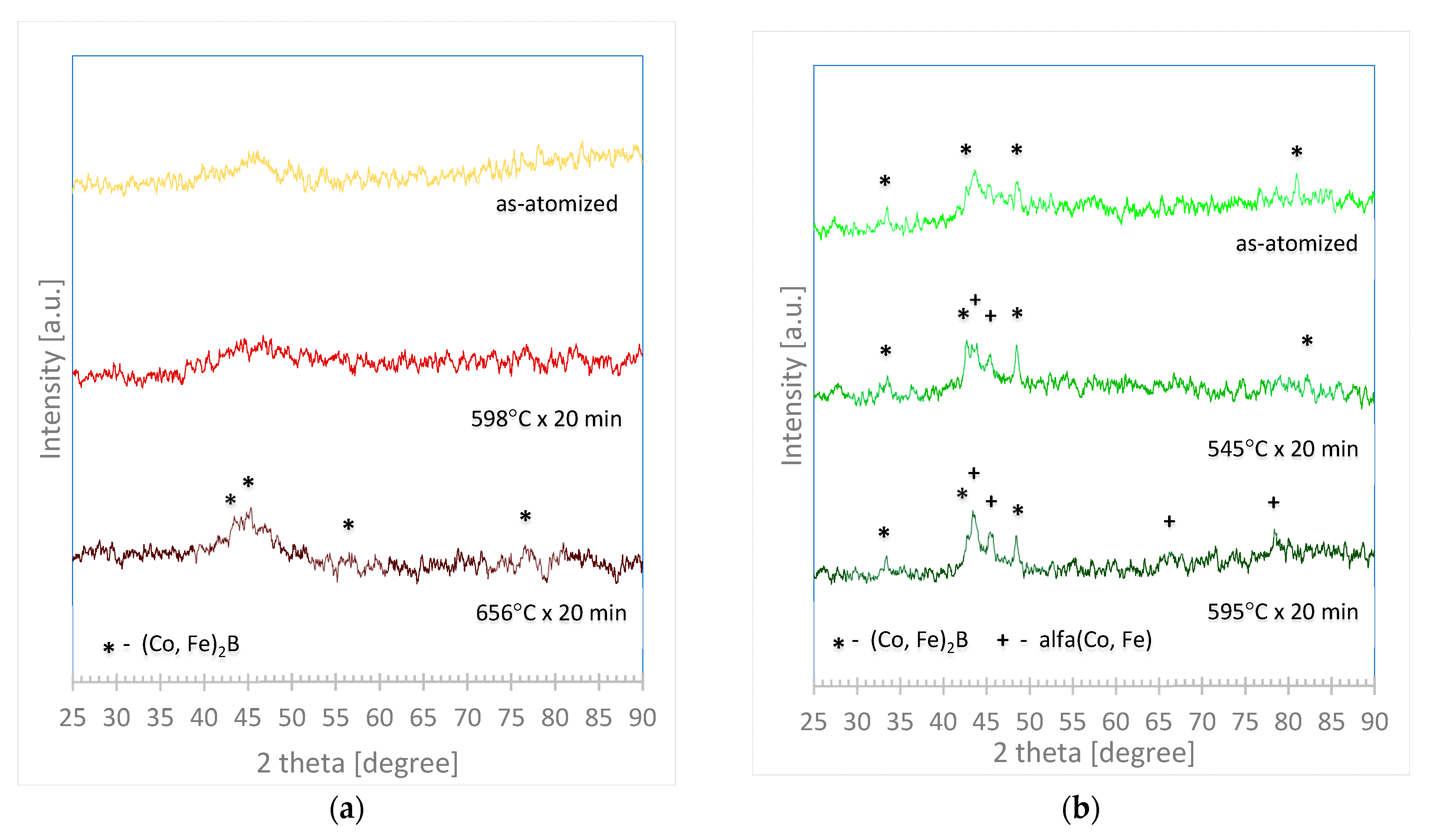
3.4. Annealing and X-ray Diffraction Test (of As-Atomized and Annealed Samples)
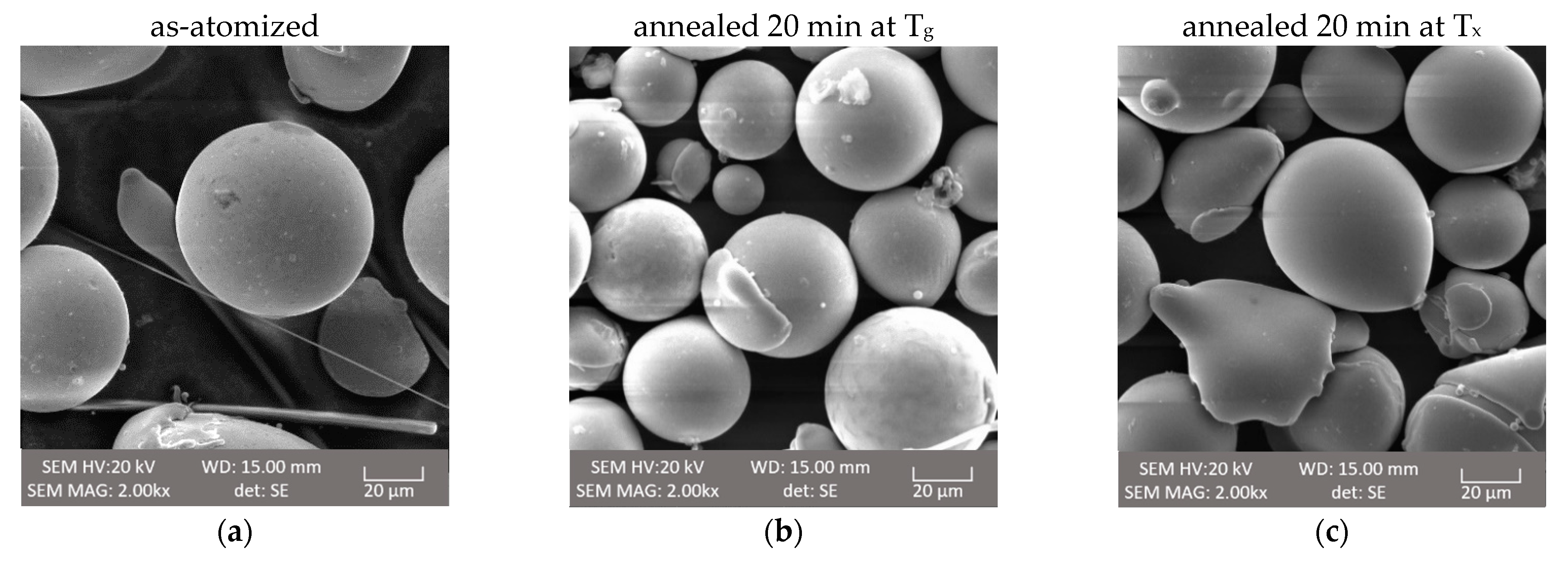
3.5. SEM Analysis
3.6. Nanoindetation
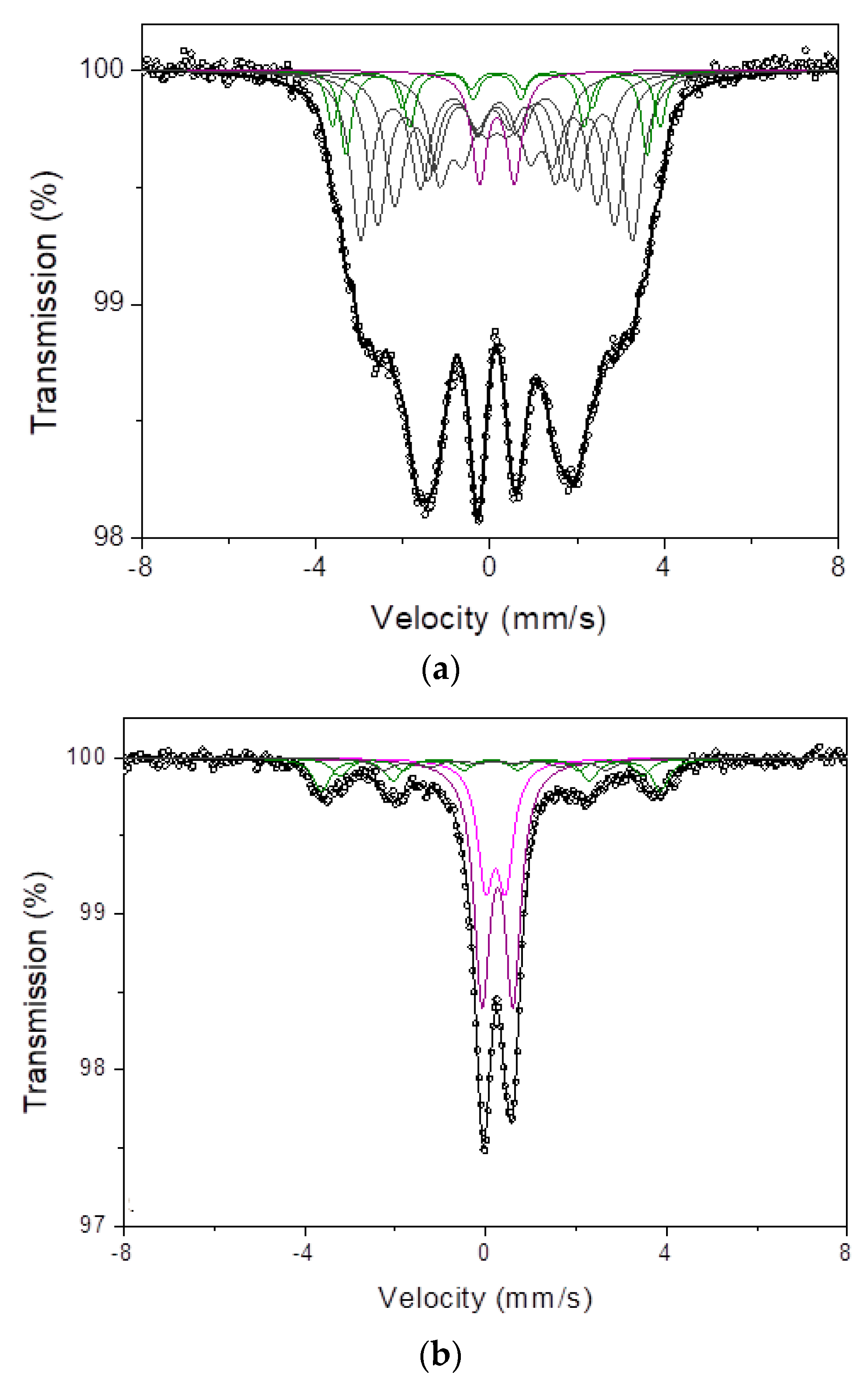
3.7. Mössbauer Studies
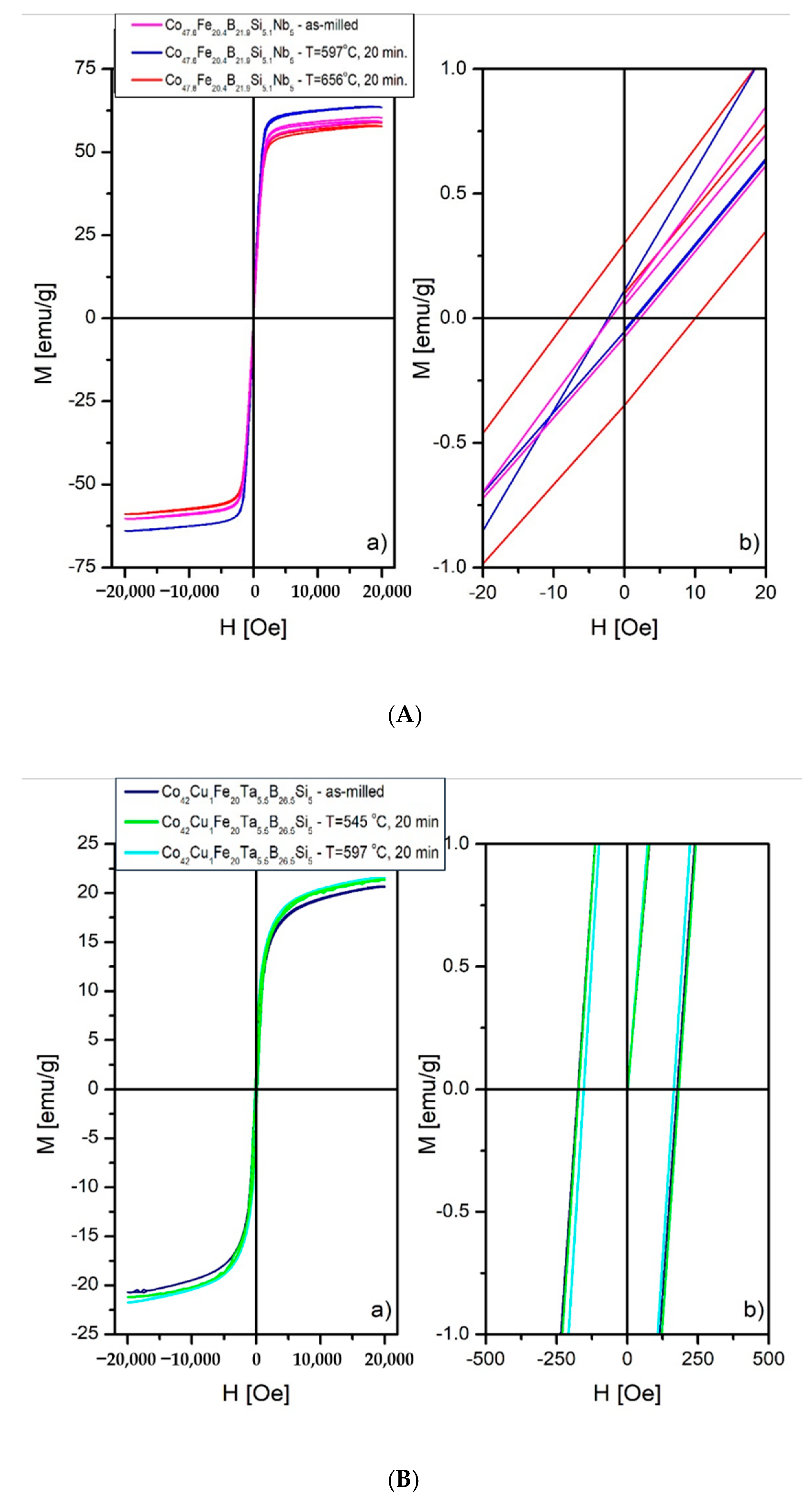
3.8. Magnetic Properties
4. Discussion
5. Conclusions
- ▪
- Co47.6B21.9Fe20.4Si5.1Nb5% at. and Co42B26.5Fe20Ta5.5Si5Cu1% at. are suitable alloys to produce powder by atomization and the obtained particles have a chemical homogeneity concentration. However, the second alloy had a slightly smaller homogeneity than the first alloy.
- ▪
- The atomized powder 50–80 µm of Co47.6Fe20.4B21.9Si5.1Nb5 alloy exhibits a Tg = 620 °C and Tx = 663 °C on a DTA research. Co42Cu1Fe20Ta5.5B26.5Si5 exhibits the smaller Tx (then first alloy), namely 595 °C and does not show any Tg sights. Those temperatures are lower than the temperature taken from the literature, which can be caused by a different form of tested samples (powders vs. rods).
- ▪
- The surface of the first and second alloy did not visibly change after annealing at Tg nor Tx.
- ▪
- The annealing for 20 min at Tg and the annealing at the Tx powder samples of Co47.6B21.9Fe20.4Si5.1Nb5and Co42B26.5Fe20Ta5.5Si5Cu1 causes an increase of the hardness of the indentation and increase of the elastic indentation module.
- ▪
- Magnetic research shows that the Co47.6B21.9Fe20.4Si5.1Nb5alloy is a soft magnetic material, both in the atomized state and after annealing state. Co42B26.5Fe20Ta5.5Si5Cu1 shows semi-hard magnetic material properties in both states.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilarczyk, W. Struktura i Właściwości Masywnych Szkieł Metalicznych w Stanie Po Wytworzeniu i Po Procesie Spawania Laserowego; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2017. [Google Scholar]
- Stoica, M. Fe-Based Bulk Metallic Glasses: Understanding the Influence of Impurities on Glass Formation; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-658-17018-9. [Google Scholar]
- Żrodowski, Ł.; Wróblewski, R.; Choma, T.; Morończyk, B.; Ostrysz, M.; Leonowicz, M.; Łacisz, W.; Błyskun, P.; Wróbel, J.S.; Cieślak, G.; et al. Novel Cold Crucible Ultrasonic Atomization Powder Production Method for 3D Printing. Materials 2021, 14, 2541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bi, J.; Meng, Z.; Li, R.; Li, Y.; Zhang, T. Tribological Behaviors of High-Hardness Co-Based Amorphous Coatings Fabricated by Laser Cladding. Tribol. Int. 2021, 162, 107142. [Google Scholar] [CrossRef]
- Lin, T.-J.; Sheu, H.-H.; Lee, C.-Y.; Lee, H.-B. The Study of Mechanical Properties and Corrosion Behavior of the Fe-Based Amorphous Alloy Coatings Using High Velocity Oxygen Fuel Spraying. J. Alloy Compd. 2021, 867, 159132. [Google Scholar] [CrossRef]
- Ciftci, N.; Yodoshi, N.; Armstrong, S.; Mädler, L.; Uhlenwinkel, V. Processing Soft Ferromagnetic Metallic Glasses: On Novel Cooling Strategies in Gas Atomization, Hydrogen Enhancement, and Consolidation. J. Mater. Sci. Technol. 2020, 59, 26–36. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Si, J.J.; Song, J.G.; Yang, Q.; Hui, X.D. Synthesis of Mg–Zn–Ca Metallic Glasses by Gas-Atomization and Their Excellent Capability in Degrading Azo Dyes. Mater. Sci. Eng. B 2014, 181, 46–55. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, W.; Sun, W.; Zhang, S.; Yang, B.; Wang, J. Impact of Gas Pressure on Particle Feature in Fe-Based Amorphous Alloy Powders via Gas Atomization: Simulation and Experiment. J. Mater. Sci. Technol. 2022, 105, 203–213. [Google Scholar] [CrossRef]
- Katz-Demyanetz, A.; Koptyug, A.; Popov, V.V. In-Situ Alloying as a Novel Methodology in Additive Manufacturing. In Proceedings of the 2020 IEEE 10th International Conference on Nanomaterials: Applications Properties (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 02SAMA05-1–02SAMA05-4. [Google Scholar]
- Deng, L.; Wang, S.; Wang, P.; Kühn, U.; Pauly, S. Selective Laser Melting of a Ti-Based Bulk Metallic Glass. Mater. Lett. 2018, 212, 346–349. [Google Scholar] [CrossRef]
- Nong, X.D.; Zhou, X.L.; Ren, Y.X. Fabrication and Characterization of Fe-Based Metallic Glasses by Selective Laser Melting. Opt. Laser Technol. 2019, 109, 20–26. [Google Scholar] [CrossRef]
- Popov, V.V.; Grilli, M.L.; Koptyug, A.; Jaworska, L.; Katz-Demyanetz, A.; Klobčar, D.; Balos, S.; Postolnyi, B.O.; Goel, S. Powder Bed Fusion Additive Manufacturing Using Critical Raw Materials: A Review. Materials 2021, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Mao, A.; Zhang, X.; Jin, X.; Wang, B.; Liu, M.; Gu, X. Preparation, Characterization and Properties of Multicomponent AlCoCrFeNi2.1 Powder by Gas Atomization Method. J. Alloy Compd. 2017, 721, 609–614. [Google Scholar] [CrossRef]
- Yang, T.; Cai, B.; Shi, Y.; Wang, M.; Zhang, G. Preparation of Nanostructured CoCrFeMnNi High Entropy Alloy by Hot Pressing Sintering Gas Atomized Powders. Micron 2021, 147, 103082. [Google Scholar] [CrossRef]
- Han, C.; Fang, Q.; Shi, Y.; Tor, S.B.; Chua, C.K.; Zhou, K. Recent Advances on High-Entropy Alloys for 3D Printing. Adv. Mater. 2020, 32, 1903855. [Google Scholar] [CrossRef] [PubMed]
- Sumita, M.; Hanawa, T.; Ohnishi, I.; Yoneyama, T. 9.04—Failure Processes in Biometallic Materials. In Comprehensive Structural Integrity; Milne, I., Ritchie, R.O., Karihaloo, B., Eds.; Pergamon: Oxford, UK, 2003; pp. 131–167. ISBN 978-0-08-043749-1. [Google Scholar]
- Klement, W.; Willens, R.H.; Duwez, P. Non-Crystalline Structure in Solidified Gold–Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Msetra, Z.; Khitouni, N.; Suñol, J.J.; Khitouni, M.; Chemingui, M. Characterization and Thermal Analysis of New Amorphous Co60Fe18Ta8B14alloy Produced by Mechanical Alloying. Mater. Lett. 2021, 292, 129532. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Huo, J.; Yang, W.; Chang, L.; Chang, C.; Sun, Y. Near Room-Temperature Magnetocaloric Effect of Co-Based Bulk Metallic Glass. J. Magn. Magn. Mater. 2018, 446, 162–165. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Zeng, Q.; Zhang, G.; Yin, K.; Liang, T.; Yang, W.; Stoica, M.; Sun, L.; Shen, B. Ductile Co-Based Bulk Metallic Glass with Superhigh Strength and Excellent Soft Magnetic Properties Induced by Modulation of Structural Heterogeneity. Materialia 2020, 9, 100561. [Google Scholar] [CrossRef]
- Kim, J.T.; Hong, S.H.; Kim, Y.S.; Park, H.J.; Maity, T.; Chawake, N.M.; Prashanth, K.G.; Park, J.M.; Song, K.K.; Wang, W.M.; et al. Co-Cr-Mo-C-B Metallic Glasses with Wide Supercooled Liquid Region Obtained by Systematic Adjustment of the Metalloid Ratio. J. Non-Cryst. Solids 2019, 505, 310–319. [Google Scholar] [CrossRef]
- Dastani, M.M.; AL-Ali, M.H.; Moradi, M. Influence of Current Annealing on the Magneto-Impedance Response of Co-Based Ribbons Arising from Surface Structural Improvement. J. Non-Cryst. Solids 2019, 516, 9–13. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, L.; Li, X.; Ma, L.; Liu, Q.; Wang, J. Optimization of Magnetoimpedance Effect in Co-Based Ribbon by Laser Patterning for Sensor Arrays Application. J. Phys. D Appl. Phys. 2018, 51, 045005. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Bao, F.; Zhu, Z.; Zhang, H.; Zhang, W. Roles of Y and Fe Contents on Glass-Forming Ability, Thermal Stability, and Magnetic Properties of Co-Based Co–Fe–Y–B Bulk Metallic Glasses. Intermetallics 2021, 132, 107135. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Yue, S.; Cheng, R.; Wang, A.; He, A.; Dong, Y.; Ni, H.; Liu, C.-T. Preparation of Non-Magnetic and Ductile Co-Based Bulk Metallic Glasses with High GFA and Hardness. Intermetallics 2019, 107, 47–52. [Google Scholar] [CrossRef]
- Murray, C.; Sun, S.; Gaschler, W.; Doyle, H.; Betley, T.; Kagan, C. Colloidal Synthesis of Nanocrystals and Nanocrystal Superlattices. IBM J. Res. Dev. 2001, 45, 47–56. [Google Scholar] [CrossRef]
- Yang, H.T.; Su, Y.K.; Shen, C.M.; Yang, T.Z.; Gao, H.J. Synthesis and Magnetic Properties of ε-Cobalt Nanoparticles. Surf. Interface Anal. 2004, 36, 155–160. [Google Scholar] [CrossRef]
- Ackland, K.; Masood, A.; Kulkarni, S.; Stamenov, P. Ultra-Soft Magnetic Co-Fe-B-Si-Nb Amorphous Alloys for High Frequency Power Applications. AIP Adv. 2018, 8, 056129. [Google Scholar] [CrossRef]
- Pinzón-Escobar, E.; Montiel, H.; García, A.; Alvarez, G. Magnetic and Electrical Properties of Vitrovac/Au/Vitrovac Multilayered Obtained by Means of Magnetron Sputtering. J. Phys. Conf. Ser. 2021, 1723, 012025. [Google Scholar] [CrossRef]
- Kekalo, I.; Stolyarov, V.; Taranichev, V. The Effect of Annealing on the Laws of Magnetization and Magnetization Reversal in Amorphous Alloys; Amorphous Metal Alloys, Metallurgy: Moscow, Russia, 1983. [Google Scholar]
- Nosenko, A.V.; Kyrylchuk, V.V.; Semen’ko, M.P.; Nowicki, M.; Marusenkov, A.; Mika, T.M.; Semyrga, O.M.; Zelinska, G.M.; Nosenko, V.K. Soft Magnetic Cobalt Based Amorphous Alloys with Low Saturation Induction. J. Magn. Magn. Mater. 2020, 515, 167328. [Google Scholar] [CrossRef]
- Rezaei-Shahreza, P.; Seifoddini, A.; Hasani, S. Microstructural and Phase Evolutions: Their Dependent Mechanical and Magnetic Properties in a Fe-Based Amorphous Alloy during Annealing Process. J. Alloy Compd. 2018, 738, 197–205. [Google Scholar] [CrossRef]
- Hasani, S.; Rezaei-Shahreza, P.; Seifoddini, A. Effect of Cu Presence on Evolution of Mechanical and Magnetic Properties in a Novel Fe-Based Bulk Metallic Glass during Partial Annealing Process. Met. Mater. Trans. A 2019, 50, 63–71. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Shao, L.; Ge, Y.; Lin, P.; Chu, C.; Shen, B. Effects of Structural Relaxation on the Dye Degradation Ability of FePC Amorphous Alloys. J. Non-Cryst. Solids 2019, 525, 119671. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Xi, Y.-C.; Zhao, Y.-H.; Liu, S.; Bai, S.-L.; Liu, Z.-D. Effects of Laser Re-Melting and Annealing on Microstructure, Mechanical Property and Corrosion Resistance of Fe-Based Amorphous/Crystalline Composite Coating. Mater. Charact. 2017, 127, 239–247. [Google Scholar] [CrossRef]
- Xie, C.; Yang, Y.; Zhong, S.; Li, S.; Deng, S. Formation, Magnetic Properties and Bending Deformation of Fe-Based Amorphous Alloy without Metalloids. J. Alloy Compd. 2017, 695, 877–880. [Google Scholar] [CrossRef]
- Onodera, R.; Kimura, S.; Watanabe, K.; Yokoyama, Y.; Makino, A.; Koyama, K. Nucleation Control for Fine Nano Crystallization of Fe-Based Amorphous Alloy by High-Magnetic-Field Annealing. J. Alloy Compd. 2015, 637, 213–218. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Sneddon, I.N. The Relation between Load and Penetration in the Axisymmetric Boussinesq Problem for a Punch of Arbitrary Profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Q.; Ji, Y.; Li, R.; Pang, S.; Wang, J.; Xu, T. Centimeter-Scale-Diameter Co-Based Bulk Metallic Glasses with Fracture Strength Exceeding 5000 MPa. Chin. Sci. Bull. 2011, 56, 3972–3977. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guan, S.; Zhu, S.; Li, R.; Zhang, T. Effects of Boron Content on the Glass-Forming Ability and Mechanical Properties of Co–B–Ta Glassy Alloys. J. Alloy Compd. 2014, 617, 7–11. [Google Scholar] [CrossRef]
- Lai, L.; He, R.; Ding, K.; Liu, T.; Liu, R.; Chen, Y.; Guo, S. Ternary Co-Mo-B Bulk Metallic Glasses with Ultrahigh Strength and Good Ductility. J. Non-Cryst. Solids 2019, 524, 119657. [Google Scholar] [CrossRef]
- Yazici, Z.; Hitit, A.; Yalcin, Y.; Ozgul, M. Effects of Minor Cu and Si Additions on Glass Forming Ability and Mechanical Properties of Co-Fe-Ta-B Bulk Metallic Glass. Met. Mater. Int. 2016, 22, 50–57. [Google Scholar] [CrossRef]
- Dai, T.; Wang, N. Study on Magnetic Properties and Degradability of Gas Atomization Fe-Based (Fe-Si-B-P) Amorphous Powder. J. Supercond. Nov. Magn. 2019, 32, 3699–3702. [Google Scholar] [CrossRef]
- Alvarez, K.L.; Manuel Martin, J.; Ipatov, M.; Dominguez, L.; Gonzalez, J. Magnetic Properties of Annealed Amorphous Fe72.5Si12.5B15 Alloy Obtained by Gas Atomization Technique. IEEE Trans. Magn. 2018, 54, 2002405. [Google Scholar] [CrossRef]
- Ciftci, N.; Ellendt, N.; von Bargen, R.; Henein, H.; Maedler, L.; Uhlenwinkel, V. Atomization and Characterization of a Glass Forming Alloy {(Fe0.6Co0.4)0.75B0.2Si0.05}96Nb4. J. Non-Cryst. Solids 2014, 394, 36–42. [Google Scholar] [CrossRef]
- Miura, A.; Dong, W.; Fukue, M.; Yodoshi, N.; Takagi, K.; Kawasaki, A. Preparation of Fe-Based Monodisperse Spherical Particles with Fully Glassy Phase. J. Alloy Compd. 2011, 509, 5581–5586. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Q.; Yuan, C.; Yang, W.; Zhou, J.; Xue, L.; Hu, F.; Sun, B.; Shen, B. Effects of Cu Additions on Mechanical and Soft-Magnetic Properties of CoFeBSiNb Bulk Metallic Glasses. J. Alloy Compd. 2018, 737, 815–820. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, A.; Man, Q.; Shen, B. (Co1−xFex)68B21.9Si5.1Nb5 Bulk Glassy Alloys with High Glass-Forming Ability, Excellent Soft-Magnetic Properties and Superhigh Fracture Strength. Intermetallics 2012, 23, 63–67. [Google Scholar] [CrossRef]
- Li, L.; Sun, H.; Fang, Y.; Zheng, J. Co-Based Soft Magnetic Bulk Glassy Alloys Optimized for Glass-Forming Ability and Plasticity. Bull. Mater. Sci. 2016, 39, 691–695. [Google Scholar] [CrossRef][Green Version]
- Shen, B.; Chang, C.; Kubota, T.; Inoue, A. Superhigh Strength and Excellent Soft-Magnetic Properties of [(Co1−xFex)0.75B0.2Si0.05]96Nb4 Bulk Glassy Alloys. J. Appl. Phys. 2006, 100, 013515. [Google Scholar] [CrossRef]
- Shen, B.; Inoue, A. (Fe,Co,Ni)–B–Si–Nb Bulk Glassy Alloy With Super-High Strength and Some Ductility [Article Retracted]. J. Mater. Res. 2005, 20, 1–5. [Google Scholar] [CrossRef][Green Version]
- Pang, L.L.; Inoue, A.; Zanaeva, E.N.; Wang, F.; Bazlov, A.I.; Han, Y.; Kong, F.L.; Zhu, S.L.; Shull, R.B. Nanocrystallization, Good Soft Magnetic Properties and Ultrahigh Mechanical Strength for Fe82–85B13–16Si1Cu1 Amorphous Alloys. J. Alloy Compd. 2019, 785, 25–37. [Google Scholar] [CrossRef]
- Han, J.; Wang, C.; Kou, S.; Liu, X. Thermal Stability, Crystallization Behavior, Vickers Hardness and Magnetic Properties of Fe-Co-Ni-Cr-Mo-C-B-Y Bulk Metallic Glasses. Trans. Nonferrous Met. Soc. China 2013, 23, 148–155. [Google Scholar] [CrossRef]
- Błoch, K.; Nabiałek, M.; Postawa, P.; Sandu, A.V.; Śliwa, A.; Jeż, B. The Magnetisation Process of Bulk Amorphous Alloys: Fe36+xCo36−xY8B20, Where: X = 0, 3, 7, or 12. Materials 2020, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Nabiałek, M.; Jeż, B.; Pietrusiewicz, P.; Jeż, K.; Płoszaj, B.; Sandu, A.V.; Abdullah, M.M.A.B.; Wysłocki, J.; Kalwik, A.; Postawa, P.; et al. Effect of Chemical Composition on Curie Temperature of FeCoB Alloys. Acta Phys. Pol. A 2021, 139, 491–494. [Google Scholar] [CrossRef]
- Olekšáková, D.; Kollár, P.; Füzer, J.; Onderko, F.; Dobák, S.; Viňáš, J.; Fáberová, M.; Bureš, R. Magnetic Properties of Sintered Fe_{50}Co_{50} Powder Cores. Acta Phys. Pol. A 2017, 131, 807–809. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.; Elkins, J.; Liu, J.P. Hard and Semi-Hard Magnetic Materials Based on Cobalt and Cobalt Alloys. J. Alloy Compd. 2020, 824, 153874. [Google Scholar] [CrossRef]
- Nematov, M.G.; Baraban, I.; Yudanov, N.A.; Rodionova, V.; Qin, F.X.; Peng, H.-X.; Panina, L.V. Evolution of the Magnetic Anisotropy and Magnetostriction in Co-Based Amorphous Alloys Microwires Due to Current Annealing and Stress-Sensory Applications. J. Alloy Compd. 2020, 837, 155584. [Google Scholar] [CrossRef]
- Zhukov, A.; Talaat, A.; Ipatov, M.; Blanco, J.M.; Zhukova, V. Tailoring of Magnetic Properties and GMI Effect of Co-Rich Amorphous Microwires by Heat Treatment. J. Alloy Compd. 2014, 615, 610–615. [Google Scholar] [CrossRef]


| Elements | |||||||
|---|---|---|---|---|---|---|---|
| First Alloy | Cobalt | Iron | Boron | Silicon | Niobium | Copper | Tantalum |
| % at. | 47.60 | 20.40 | 21.90 | 5.10 | 5.00 | 0.00 | 0.00 |
| % wg. | 58.58 | 23.79 | 4.94 | 2.99 | 9.70 | 0.00 | 0.00 |
| Second Alloy | - | - | - | - | - | - | - |
| % at. | 42.00 | 20.00 | 26.50 | 5.00 | 0.00 | 1.00 | 5.50 |
| % wg. | 48.75 | 22.00 | 5.64 | 2.77 | 0.00 | 1.25 | 19.60 |
| % at. | 99.99 | 99.97 | 99.9 | 99.999 | 99.95 | 99.999 | 99.995 |
| Temperature [°C] | Tg | Tx | Tx2 | Tx3 | ΔTx | Tm | Tl |
|---|---|---|---|---|---|---|---|
| Co47.6B21.9Fe20.4Si5.1Nb5 | 598 | 656 | 782 | - | 58 | 1074 | 1144 |
| Co42B26.5Fe20.0Ta5.5Si5Cu1 | - | 595 | 768 | 928 | - | 1069 | 1116 |
| As-Atomized | Annealed at Tg | Annealed at Tx | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HIT | HVIT | EIT | HIT | HVIT | EIT | HIT | HVIT | EIT |
| Unit | (MPa) | (HV) | (GPa) | (MPa) | (HV) | (GPa) | (MPa) | (HV) | (GPa) |
| Mean | 16,768.6 | 1553.0 | 139.5 | 18,592.3 | 1721.9 | 173.0 | 19,463.8 | 1802.6 | 182.5 |
| Standard deviation | 1085.4 | 100.5 | 8.9 | 703.6 | 65.2 | 8.1 | 1221.5 | 113.1 | 5.0 |
| Coefficient of variation [%] | 6.5 | 6.5 | 6.4 | 3.8 | 3.8 | 4.7 | 6.3 | 6.3 | 2.7 |
| Component | IS (mm/s) | QS (mm/s) | B (T) | FWHM (mm/s) | A (%) | Compound |
|---|---|---|---|---|---|---|
| Co47.6B21.9Fe20.4Si5.1Nb5 as-atomized | ||||||
| D1 | 0.16 | 0.79 | - | 0.42 | 7 | Fe-based |
| S1 | 0.16 | −0.02 | 23.4 | 0.32 | 5 | Fe2B |
| S2 | 0.16 | −0.01 | 21.5 | 0.32 | 8 | |
| S3 | 0.18 | −0.06 | 19.4 | 0.54 | 23 | Fe-B-Nb |
| S4 | 0.15 | −0.01 | 16.9 | 0.54 | 20 | |
| S5 | 0.11 | 0.07 | 14.5 | 0.54 | 17 | |
| S6 | 0.10 | 0.01 | 11.3 | 0.54 | 14 | |
| S7 | 0.17 | 0.03 | 8.3 | 0.54 | 6 | |
| Co47.6B21.9Fe20.4Si5.1Nb5 annealed at T = 597 °C for 20 min. | ||||||
| D1 | 0.19 | 0.76 | - | 0.36 | 5 | Fe-based |
| S1 | 0.15 | −0.02 | 23.5 | 0.32 | 7 | Fe2B |
| S2 | 0.15 | −0.01 | 21.6 | 0.32 | 9 | |
| S3 | 0.16 | −0.06 | 19.7 | 0.52 | 20 | Fe-B-Nb |
| S4 | 0.15 | −0.02 | 17.3 | 0.52 | 20 | |
| S5 | 0.10 | 0.07 | 14.7 | 0.52 | 16 | |
| S6 | 0.07 | 0.04 | 11.2 | 0.52 | 12 | |
| S7 | 0.17 | 0.05 | 8.1 | 0.52 | 11 | |
| Co47.6B21.9Fe20.4Si5.1Nb5 annealed at T = 656 °C for 20 min. | ||||||
| D1 | 0.15 | 0.78 | - | 0.41 | 4 | Fe-based |
| S1 | 0.17 | −0.02 | 23.3 | 0.32 | 9 | Fe2B |
| S2 | 0.16 | −0.01 | 21.5 | 0.32 | 9 | |
| S3 | 0.19 | −0.06 | 19.5 | 0.55 | 22 | Fe-B-Nb |
| S4 | 0.15 | −0.01 | 16.8 | 0.55 | 20 | |
| S5 | 0.11 | 0.07 | 14.5 | 0.55 | 15 | |
| S6 | 0.09 | 0.01 | 11.3 | 0.55 | 15 | |
| S7 | 0.17 | 0.03 | 8.3 | 0.55 | 6 | |
| Co42 B26.5Fe20Ta5.5Si5Cu1 as- atomized | ||||||
| D1 | 0.22 | 0.44 | - | 0.43 | 24 | Fe-Cu |
| D2 | 0.27 | 0.68 | - | 0.43 | 47 | |
| S1 | 0.12 | 0.00 | 23.8 | 0.45 | 14 | Fe2B |
| S2 | 0.13 | 0.00 | 21.7 | 0.45 | 8 | |
| S3 | 0.21 | 0.00 | 19.1 | 0.59 | 7 | Fe-B-Nb |
| Co42 B26.5Fe20Ta5.5Si5Cu1annealed at T = 545 °C for 20 min. | ||||||
| D1 | 0.21 | 0.41 | - | 0.41 | 18 | Fe-Cu |
| D2 | 0.28 | 0.66 | - | 0.41 | 51 | |
| S1 | 0.11 | 0.00 | 23.5 | 0.52 | 12 | Fe2B |
| S2 | 0.09 | 0.00 | 21.5 | 0.52 | 12 | |
| S3 | 0.21 | 0.00 | 13.9 | 0.52 | 6 | Fe-B-Nb |
| Co42 B26.5Fe20Ta5.5Si5Cu1annealed at T = 597 °C for 20 min. | ||||||
| D1 | 0.24 | 0.46 | - | 0.41 | 26 | Fe-Cu |
| D2 | 0.28 | 0.68 | - | 0.41 | 40 | |
| S1 | 0.11 | 0.00 | 23.8 | 0.39 | 10 | Fe2B |
| S2 | 0.12 | 0.00 | 21.7 | 0.39 | 11 | |
| S3 | 0.08 | 0.00 | 19.1 | 0.39 | 5 | Fe-B-Nb |
| S4 | 0.20 | 0.00 | 13.7 | 0.58 | 8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuś, A.; Pilarczyk, W.; Małachowska, A.; Ambroziak, A.; Gębara, P. Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization. Materials 2021, 14, 7357. https://doi.org/10.3390/ma14237357
Kuś A, Pilarczyk W, Małachowska A, Ambroziak A, Gębara P. Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization. Materials. 2021; 14(23):7357. https://doi.org/10.3390/ma14237357
Chicago/Turabian StyleKuś, Anna, Wirginia Pilarczyk, Aleksandra Małachowska, Andrzej Ambroziak, and Piotr Gębara. 2021. "Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization" Materials 14, no. 23: 7357. https://doi.org/10.3390/ma14237357
APA StyleKuś, A., Pilarczyk, W., Małachowska, A., Ambroziak, A., & Gębara, P. (2021). Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization. Materials, 14(23), 7357. https://doi.org/10.3390/ma14237357








