Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic and Total Flavonoid Contents and Antioxidant Activities of H. cordata Extracts
2.2. Active Compound Determination of H. cordata Extracts Using HPLC
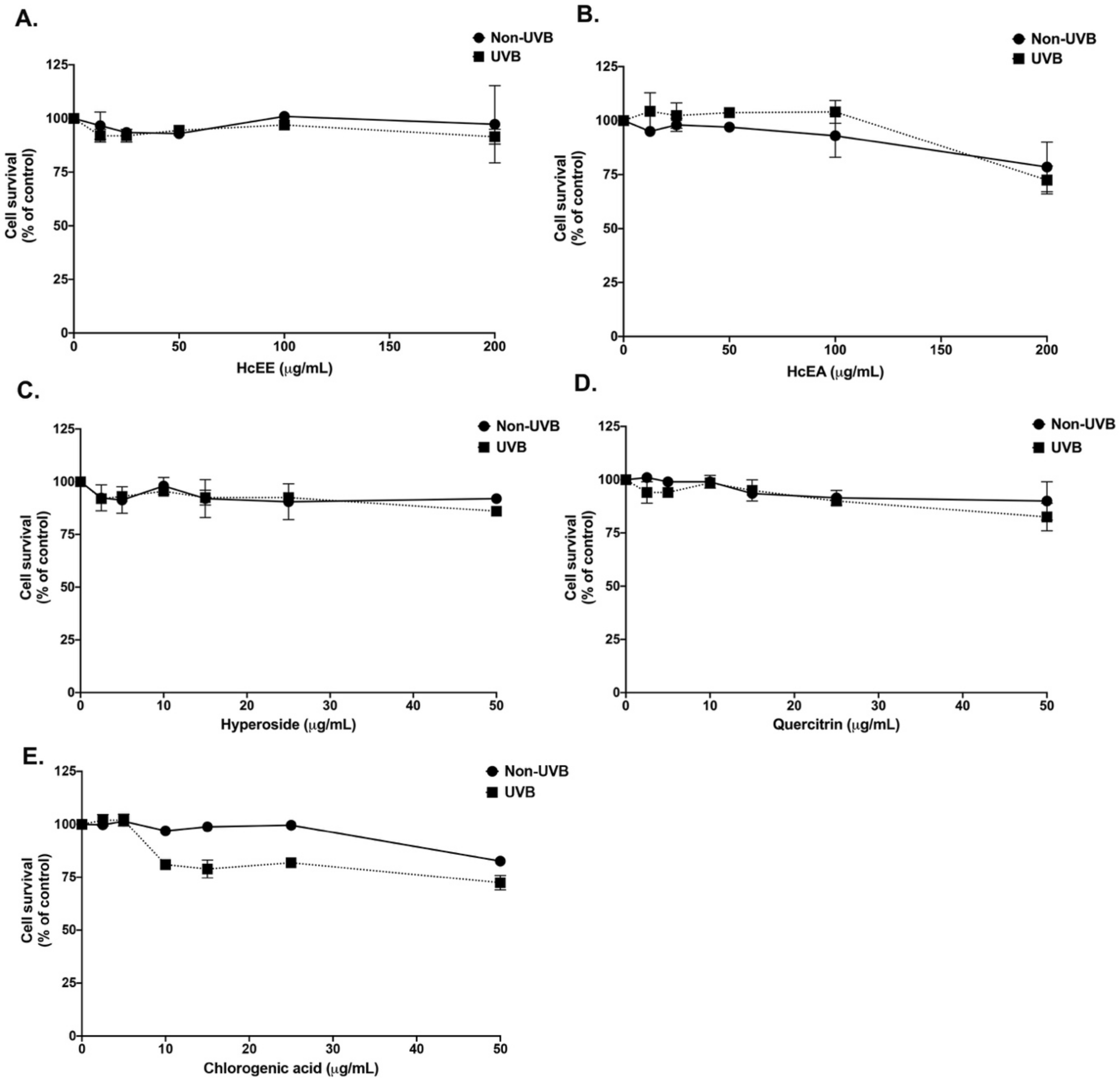
2.3. H. cordata Thunb. Extracts Displayed No Cytotoxicity on Human Dermal Fibroblasts
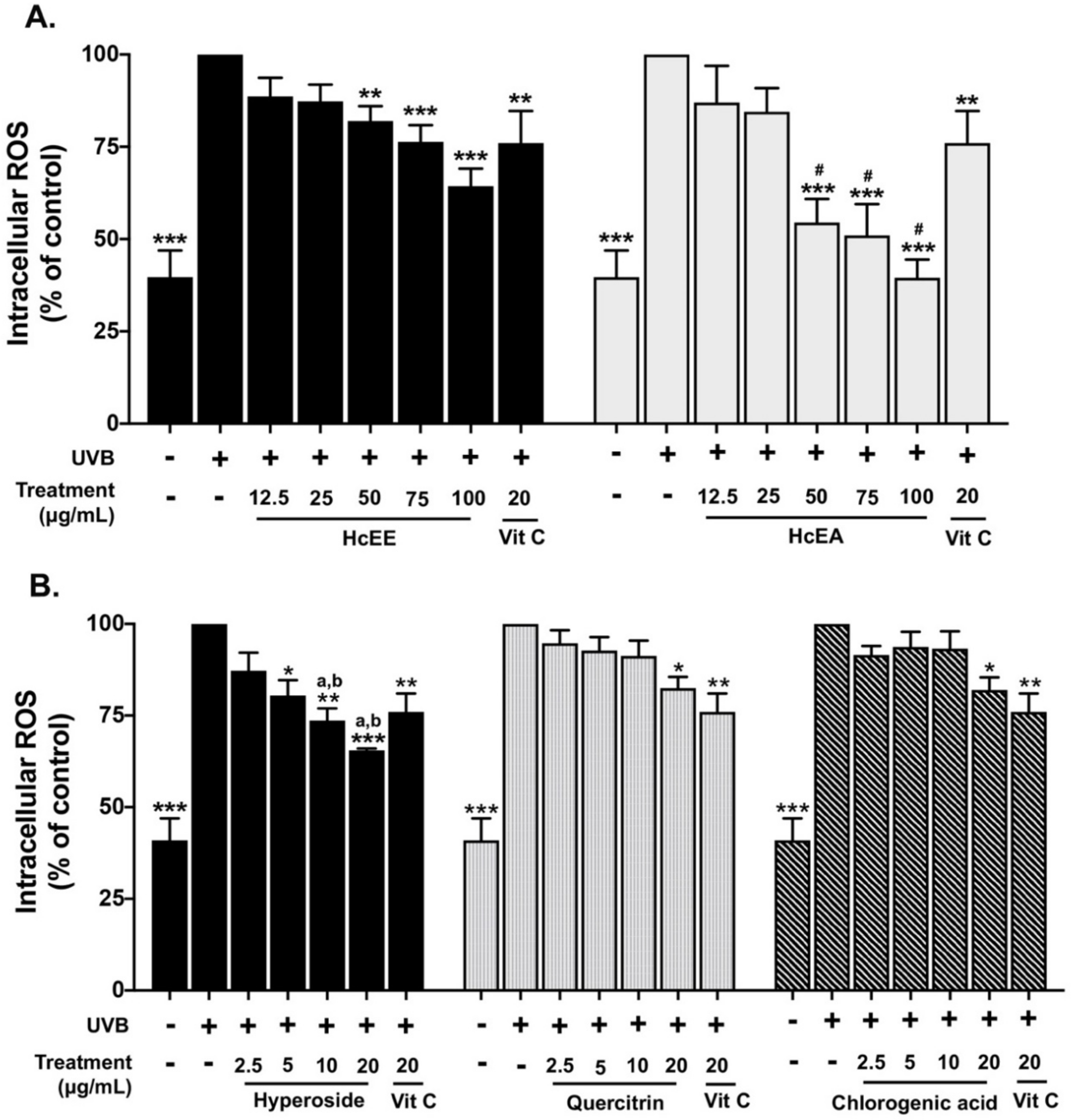
2.4. H. cordata Extracts Inhibited Intracellular ROS in UVB-Irradiated Fibroblasts
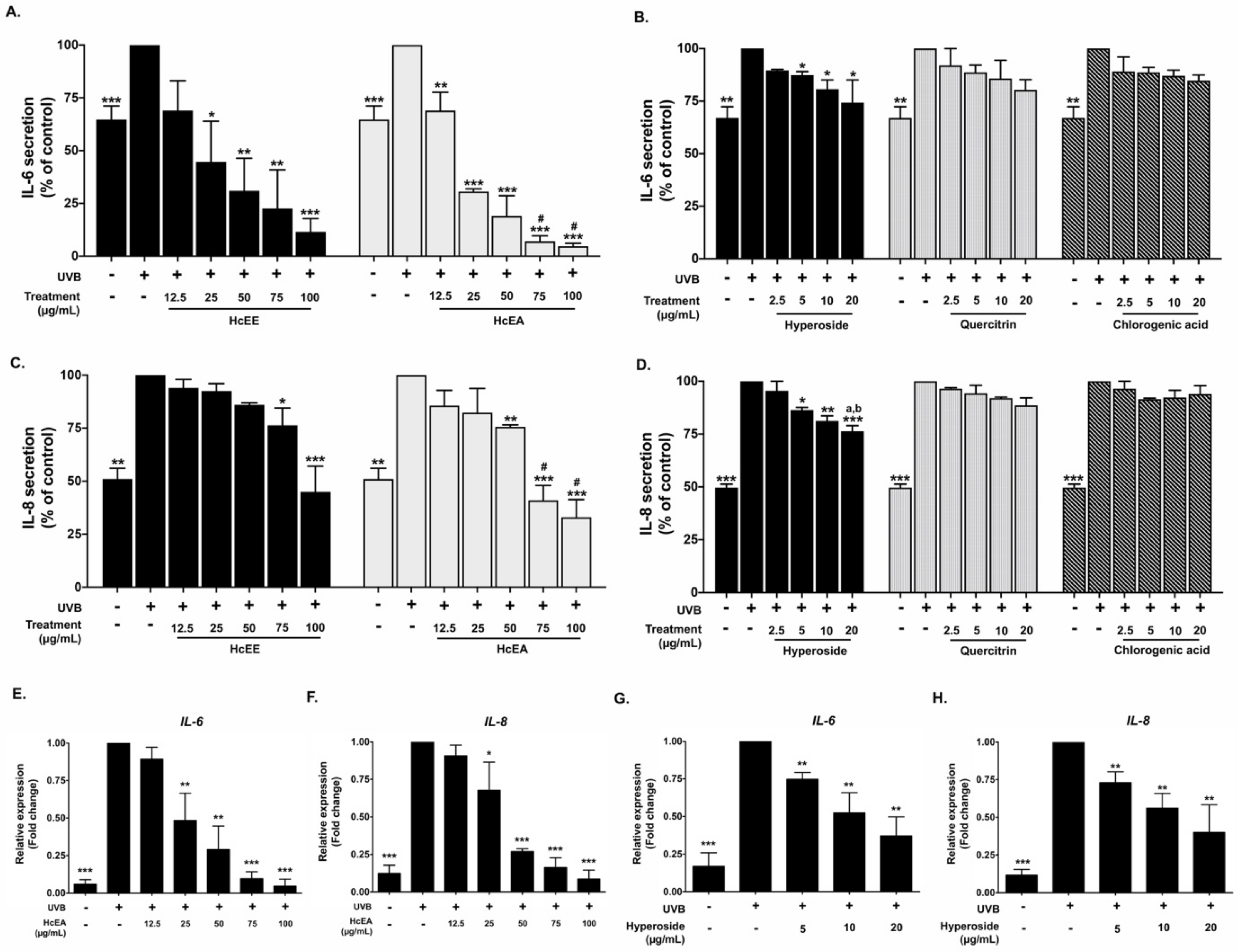
2.5. Inhibitory Effects of HcEE, HcEA, and Its Bioactive Compounds on the Pro-Inflammatory Cytokine in UVB-Irradiated Human Dermal Fibroblasts
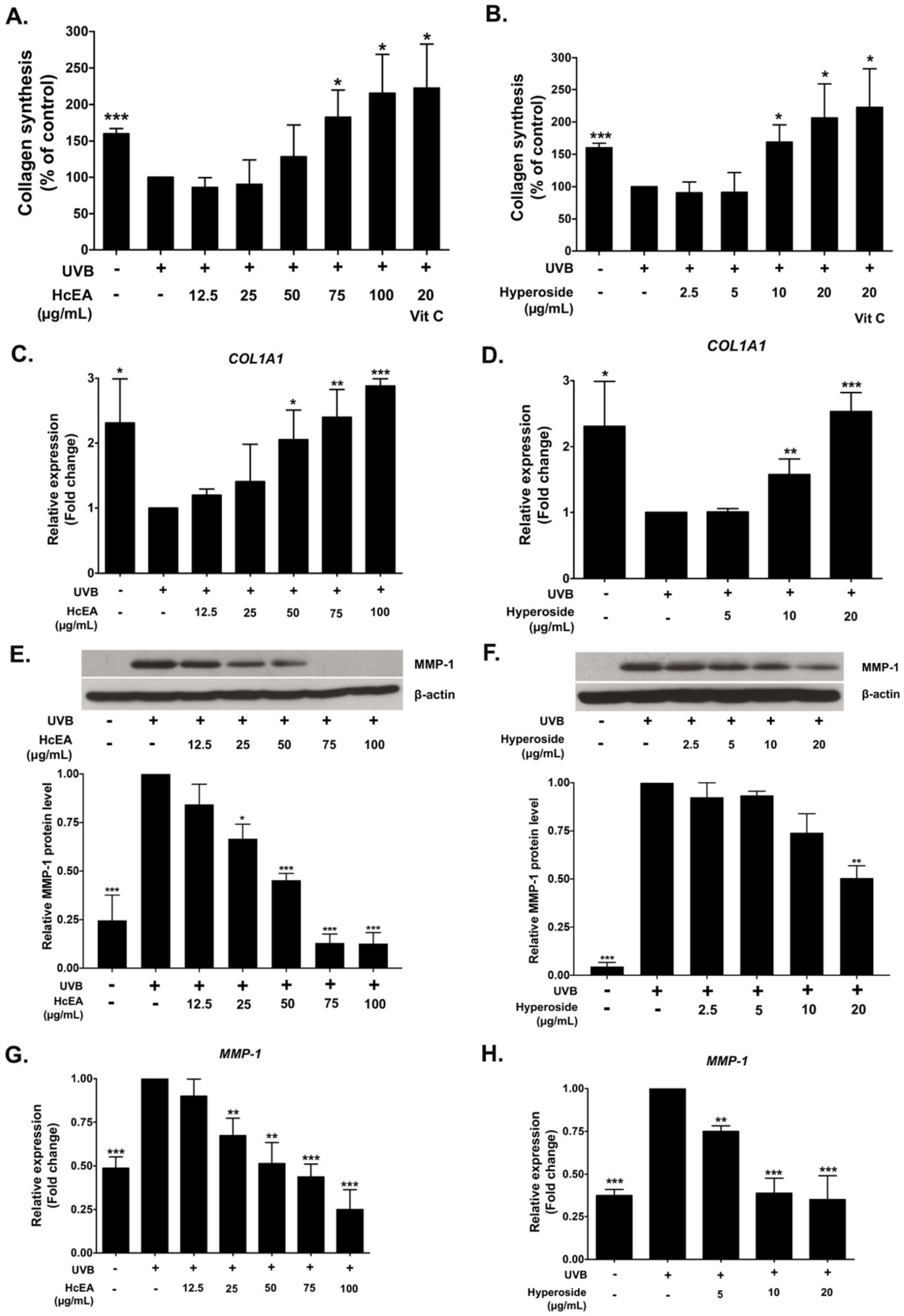
2.6. HcEA and Hyperoside Increased Collagen Synthesis and Decreased MMP-1 Expression in UVB-Irradiated Dermal Fibroblasts
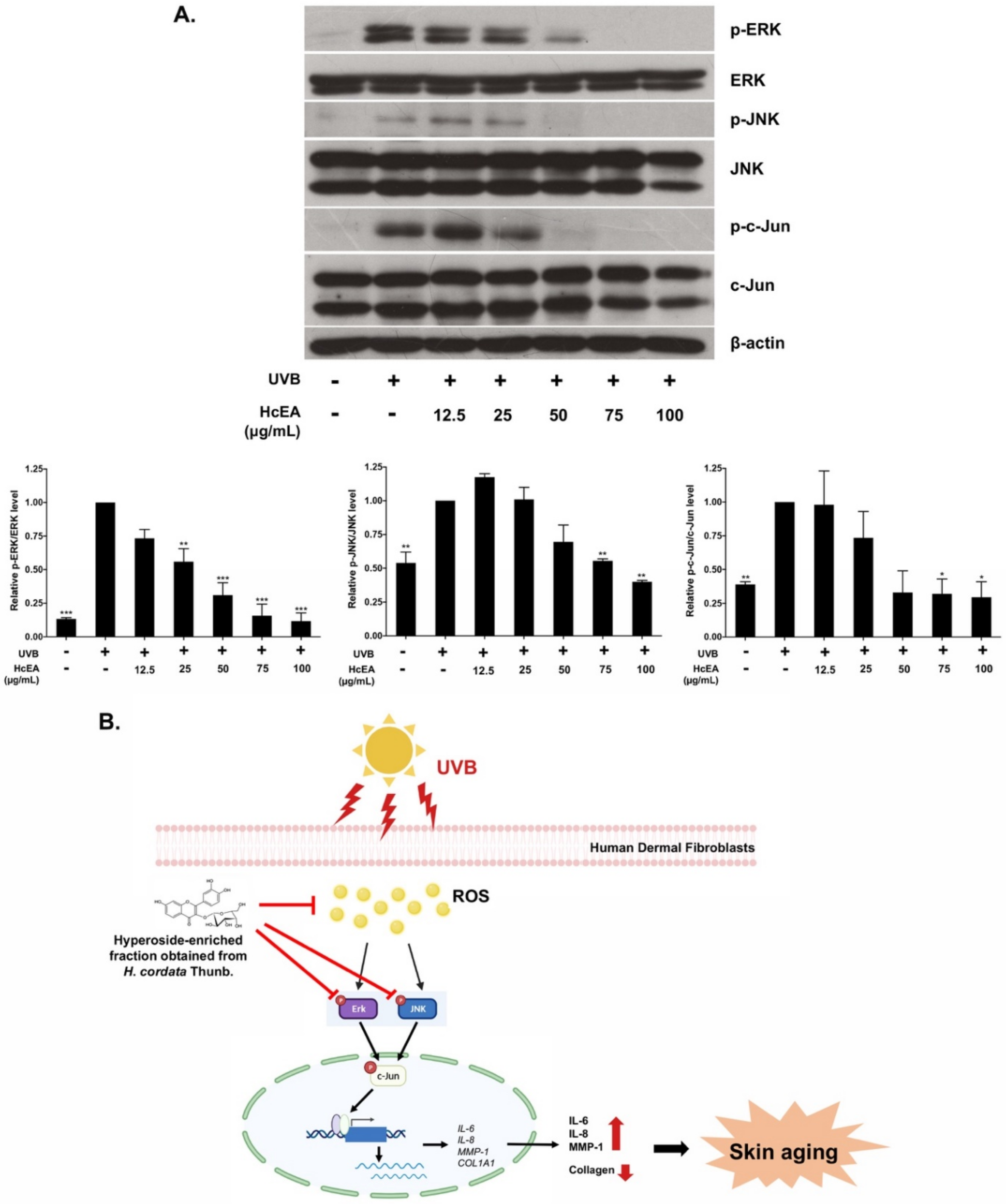
2.7. HcEA Exhibited Photoprotective Skin-Aging Properties through the Inhibition of MAPK Signaling Pathway in UVB-Irradiated Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Herb Materials
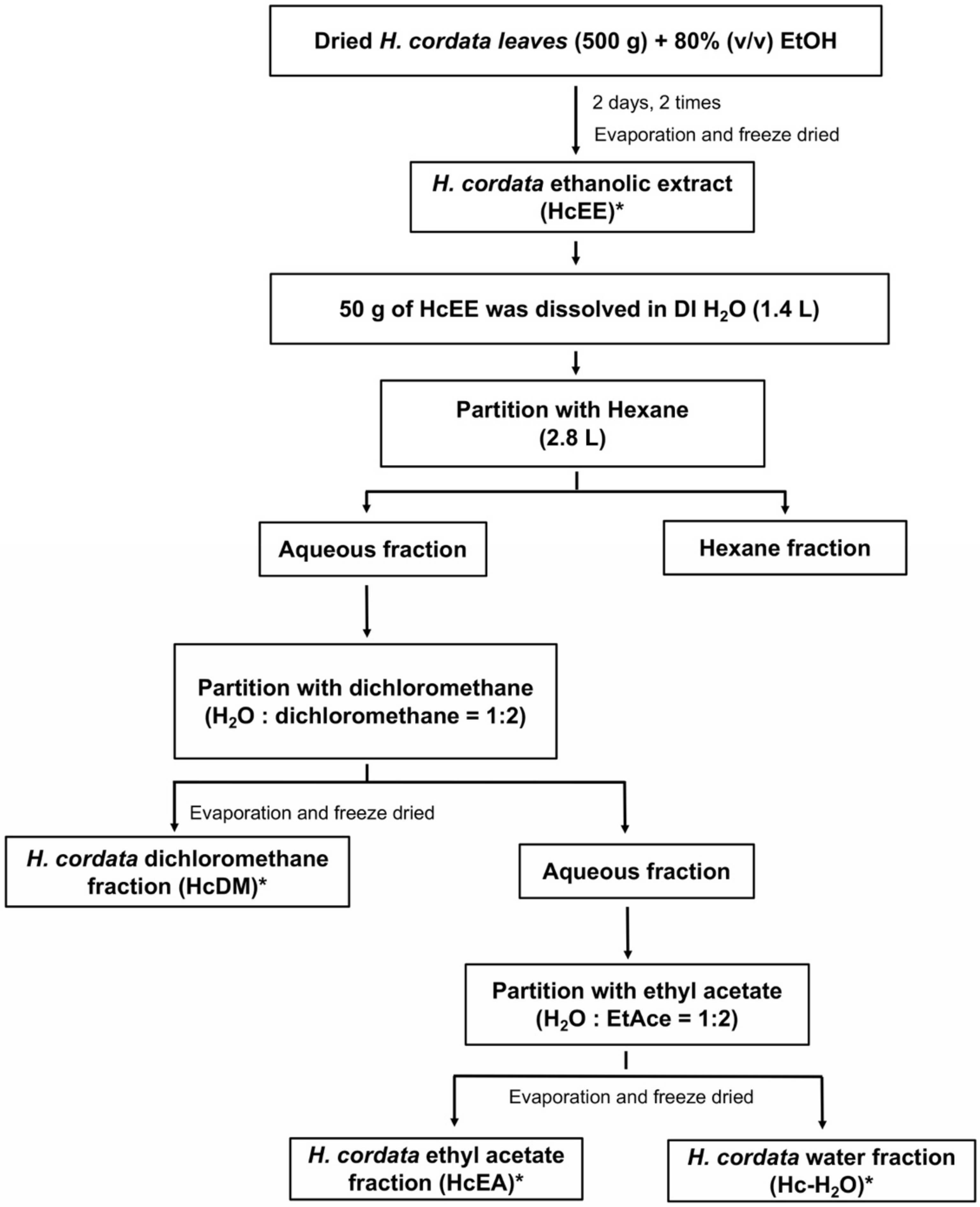
4.3. Preparation of Herbal Extracts and Solvent-Partitioned Extraction Technique
4.4. Total Phenolic Content
4.5. Total Flavonoid Content
4.6. Identification of Active Compounds Using HPLC
4.7. ABTS and DPPH Assays for Antioxidant Properties
4.8. Primary Cell Cultures
4.9. Cell Viability Assay
4.10. Intracellular ROS Determination
4.11. Determination of Cytokine Secretion
4.12. Collagen Synthesis Assay
4.13. Expression of IL-6, IL-8, COL1A1 and MMP1 Genes by RT- qPCR Analysis
4.14. Western Blot Analysis
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Gegotek, A.; Domingues, P.; Skrzydlewska, E. Proteins involved in the antioxidant and inflammatory response in rutin-treated human skin fibroblasts exposed to UVA or UVB irradiation. J. Dermatol. Sci. 2018, 90, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn Polyphenol Extract Inhibits UVB-Induced Skin Photoaging by Regulating MMP Expression and Type I Procollagen Production in Mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Choi, S.; Youn, J.; Kim, K.; Joo da, H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domaszewska-Szostek, A.; Puzianowska-Kuznicka, M.; Kurylowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, H.S.; Kim, G.J.; Jeon, C.Y.; Park, J.H.; Jang, B.H.; Park, S.J.; Shin, Y.C.; Ko, S.G. Houttuynia cordata Thunb inhibits the production of pro-inflammatory cytokines through inhibition of the NFkappaB signaling pathway in HMC-1 human mast cells. Mol. Med. Rep. 2013, 8, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, L.; Pils, V.; Bobbili, M.R.; Lammermann, I.; Perrotta, I.; Grillenberger, T.; Schwestka, J.; Weiss, K.; Pum, D.; Arcalis, E.; et al. Extracellular Vesicles in Human Skin: Cross-Talk from Senescent Fibroblasts to Keratinocytes by miRNAs. J. Invest. Dermatol. 2019, 139, 2425–2436.e5. [Google Scholar] [CrossRef] [Green Version]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharm. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am. J. Chin. Med. 2005, 33, 415–424. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.E.; Lee, Y.S.; Lee, Y.J.; Park, Y.M.; Park, S.N. Antimicrobial, antioxidant and cellular protective effects of Houttuynia cordata extract and fraction. Appl. Chem. Eng. 2018, 29, 452–460. [Google Scholar]
- Ling-Shang, W.U.; Jin-Ping, S.I.; Xiao-Qing, Y.U.A.N.; Xue-Rong, S.H.I. Quantitive variation of flavonoids in Houttuynia cordata from different geographic origins in China. Chin. J. Nat. Med. 2009, 7, 40–46. [Google Scholar] [CrossRef]
- Nguyen, V.; Le, V.; Vo, T.; Bui, L.; Anh, H.; Danh, V. Preliminary Phytochemical Screening and Determination of Total Polyphenols and Flavonoids Content in the Leaves of Houttuynia cordata Thunb, Proceedings of the IOP Conference Series: Materials Science and Engineering, Penang, Malaysia, 17–19 July 2019; IOP Publishing: Bristol, UK, 2020; p. 062013. [Google Scholar]
- Tian, L.; Shi, X.; Yu, L.; Zhu, J.; Ma, R.; Yang, X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J. Agric. Food Chem. 2012, 60, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.J.; Lu, Y.; Zhang, Y.Y.; Zhu, H.Y.; Tu, P.; Li, H.; Chen, D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective effects of unsaponifiable matter from perilla seed meal on UVB-induced damages and the underlying mechanisms in human skin fibroblasts. Antioxidants 2019, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef]
- Cho, Y.H.; Bahuguna, A.; Kim, H.H.; Kim, D.I.; Kim, H.J.; Yu, J.M.; Jung, H.G.; Jang, J.Y.; Kwak, J.H.; Park, G.H.; et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B 2017, 174, 323–332. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Lee, W.; Jeon, Y.J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-kappaB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective effects of Spirulina platensis extract against UVB irradiated human skin fibroblasts. South Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Ruszova, E.; Cheel, J.; Pavek, S.; Moravcova, M.; Hermannova, M.; Matejkova, I.; Spilkova, J.; Velebny, V.; Kubala, L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protection in vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.M.; Nho, K.J.; Kim, H.S.; Lee, A.Y.; Moon, B.C.; Kim, H.K. An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-small ka, CyrillicB and MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Compl. Altern. Med. 2014, 14, 234. [Google Scholar] [CrossRef] [Green Version]
- Chiow, K.; Phoon, M.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, D.-S.; Kim, J.-M.; Lee, Y.-M.; Yoo, J.-L.; Kim, Y.-S.; Kim, J.-H.; Kim, J.-S. Flavonols from Houttuynia cordata with protein glycation and aldose reductase inhibitory activity. Nat. Prod. Sci. 2006, 12, 210–213. [Google Scholar]
- Fuse, J.-i.; Kanamori, H.; Sakamoto, I.; Yahara, S. Studies on flavonol glycosides in Houttuynia cordata. Nat. Med. 1994, 48, 307–311. [Google Scholar]
- Kawamura, T.; Hisata, Y.; Okuda, K.; Noro, Y.; Tanaka, T.; Yoshida, M.; Sakai, E. Pharmacognostical studies of Houttuyniae Herba (1). Flavonoid glycosides contents of Houttuynia cordata Thunb. Nat. Med. 1994, 48, 208–212. [Google Scholar]
- Shukla, R.; Pandey, V.; Vadnere, G.; Lodhi, S. Chapter 18-Role of Flavonoids in Management of Inflammatory Disorders. Bioact. Food Diet. Interv. Arthritis Relat. Inflamm. Dis. 2019, 293–322. [Google Scholar]
- Zakaria, N.; Okello, E.; Howes, M.J.; Birch-Machin, M.; Bowman, A. In vitro protective effects of an aqueous extract of Clitoria ternatea L. flower against hydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother. Res. 2018, 32, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Im, A.-R.; Kim, S.-M.; Kang, H.-S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Compl. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sun, W.; Wu, P. Hyperoside exerts potent anticancer activity in skin cancer. Front. Biosci. 2020, 25, 463–479. [Google Scholar]
- Zheng, Y.-M.; Xu, X.-Y.; Fu, S.-Q.; Yang, Y.-H. Quantitative Determination of Hyperoside and Quercitrin in Houttuynia cordata by HPLC. Res. Pract. Chin. Med. 2005, 3, 27–28. [Google Scholar]
- Kim, S.-J.; Um, J.-Y.; Hong, S.-H.; Lee, J.-Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef]
- Jin, X.N.; Yan, E.Z.; Wang, H.M.; Sui, H.J.; Liu, Z.; Gao, W.; Jin, Y. Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis. Acta Pharm. Sin. 2016, 37, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Limtrakul Dejkriengkraikul, P. Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Arjsri, P.; Phetcharaburanin, J.; Suksawat, M.; Mapoung, S.; Subkamkaew, C.; Semmarath, W.; Yodkeeree, S.; Limtrakul, P. Spirogyra neglecta (Hassall) Kützing attenuates metastasis of castration-resistant human prostate cancer via the blockage of AKT signaling pathway. South Afr. J. Bot. 2021, 139, 26–37. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin Anti-aging Assays of Proanthocyanidin Rich Red Rice Extract, Oryzanol and Other Phenolic Compounds. Nat. Prod. Commun. 2018, 13, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Temerdashev, Z.; Milevskaya, V.; Vinitskaya, E. The method of establishing the authenticity and quality of Hypericum perforatum L. and Salvia officinalis L. MethodsX 2021, 8, 101487. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Tsimidou, M. Assessing the activity of natural food antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: Amsterdam, The Netherlands, 2010; pp. 332–367. [Google Scholar]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid. Based Compl. Alternat. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [Green Version]
- Lorrio, S.; Rodríguez-Luna, A.; Delgado-Wicke, P.; Mascaraque, M.; Gallego, M.; Pérez-Davó, A.; González, S.; Juarranz, Á. Protective effect of the aqueous extract of Deschampsia antarctica (EDAFENCE®) on skin cells against blue light emitted from digital devices. Int. J. Mol. Sci. 2020, 21, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Choi, S.-I.; Kim, E.K. Uptake of cell debris and enhanced expression of inflammatory factors in response to dead cells in corneal fibroblast cells. Exp. Eye Res. 2020, 194, 108017. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef] [PubMed]
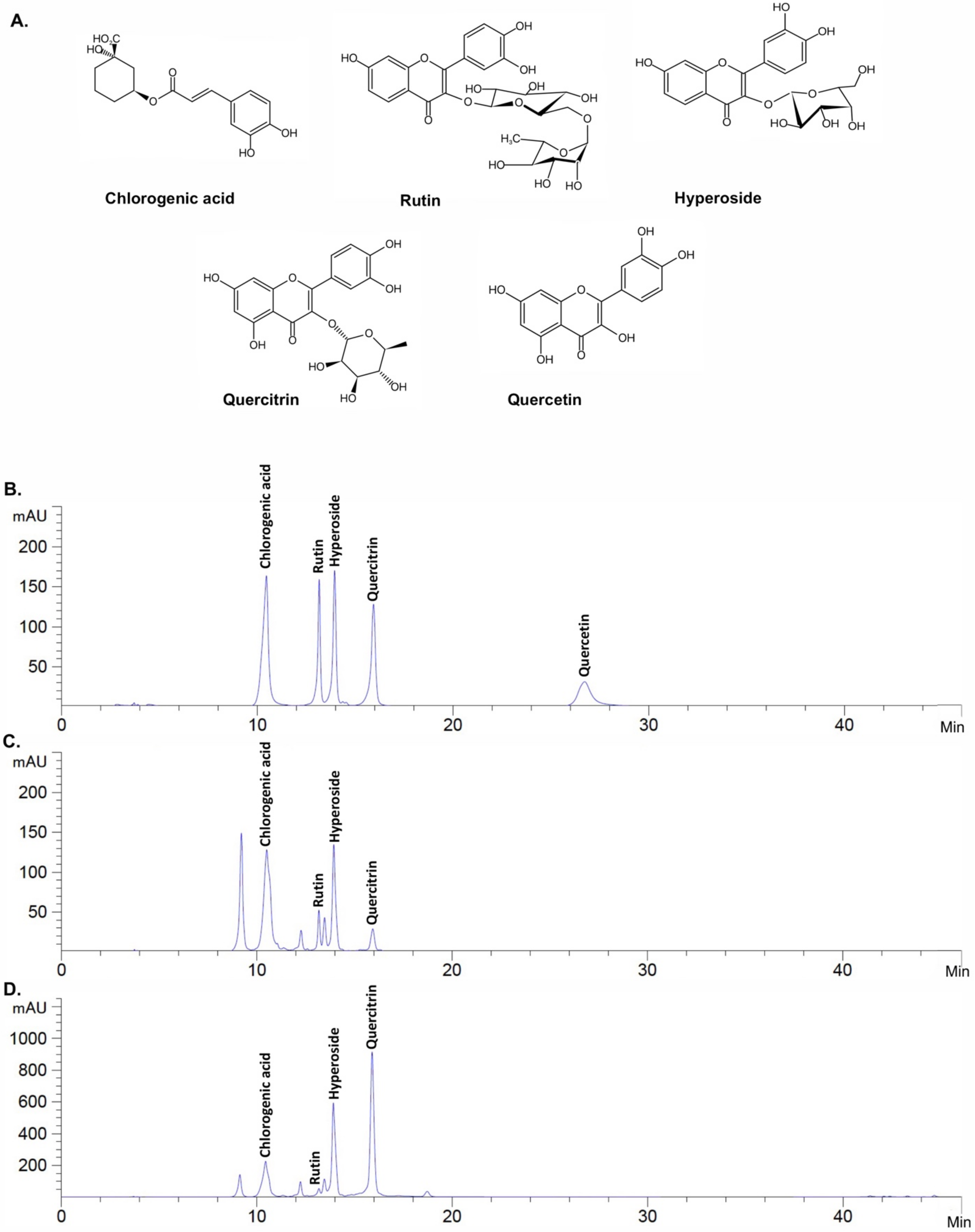
| H. cordata Extracts | Polyphenols | Antioxidant Capacity | ||
|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg CE/g Extract) | IC50 DPPH Assay (μg/mL) | IC50 ABTS Assay (μg/mL) | |
| HcEE | 172.57 ± 6.73 | 126.97 ± 7.44 | 41.95 ± 2.29 | 17.36 ± 0.84 |
| HcDM | 113.08 ± 9.24 | 97.23 ± 10.16 | 113.94 ± 10.70 | 26.69 ± 1.40 |
| HcEA | 591.01 ± 7.11 ** | 433.86 ± 10.16 ** | 17.64 ± 2.25 ** | 4.96 ± 0.24 ** |
| Hc-H2O | 128.07 ± 4.40 | 90.09 ± 6.78 | 66.35 ± 3.84 | 19.72 ± 1.75 |
| Vitamin E | 33.97 ± 1.62 | 14.95 ± 0.41 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. https://doi.org/10.3390/plants10122628
Mapoung S, Umsumarng S, Semmarath W, Arjsri P, Srisawad K, Thippraphan P, Yodkeeree S, Dejkriengkraikul P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants. 2021; 10(12):2628. https://doi.org/10.3390/plants10122628
Chicago/Turabian StyleMapoung, Sariya, Sonthaya Umsumarng, Warathit Semmarath, Punnida Arjsri, Kamonwan Srisawad, Pilaiporn Thippraphan, Supachai Yodkeeree, and Pornngarm Dejkriengkraikul. 2021. "Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway" Plants 10, no. 12: 2628. https://doi.org/10.3390/plants10122628
APA StyleMapoung, S., Umsumarng, S., Semmarath, W., Arjsri, P., Srisawad, K., Thippraphan, P., Yodkeeree, S., & Dejkriengkraikul, P. (2021). Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants, 10(12), 2628. https://doi.org/10.3390/plants10122628









