The Modulation of Nrf-2/HO-1 Signaling Axis by Carthamus tinctorius L. Alleviates Vascular Inflammation in Human Umbilical Vein Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ECT
2.2. Chemicals and Reagents for HPLC Analysis
2.3. HPLC Analysis of Six Marker Analytes in Flowers of C. tinctorius
2.4. Cell Cultures
2.5. Enzyme-Linked Immunsorbent Assay
2.6. Western Blot Analysis
2.7. Total mRNA Preparation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.8. HL-60 Monocyte and HUVEC Adhesion Analysis
2.9. Intracellular ROS Production Assay
2.10. NF-κB Activation Assay
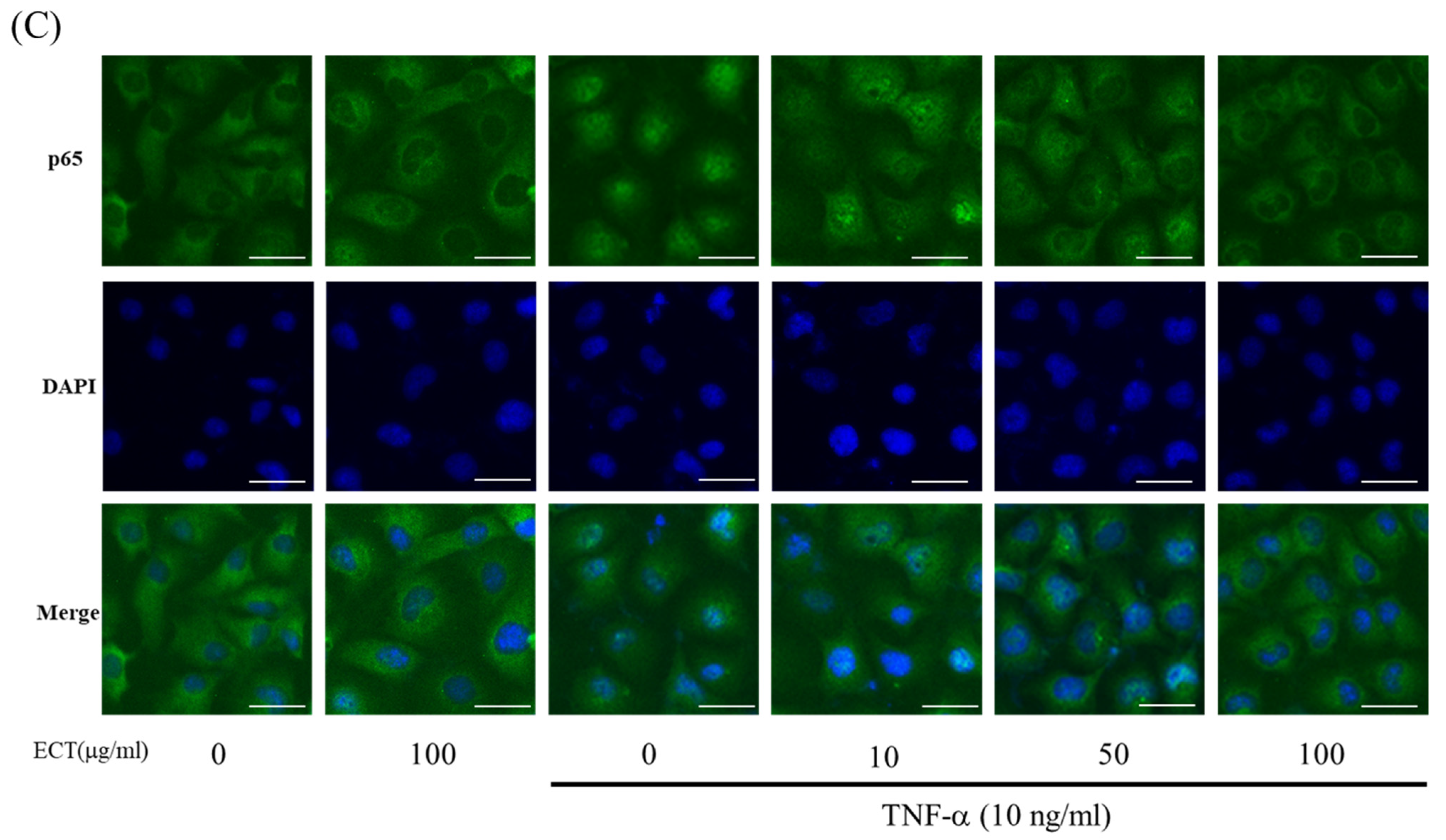
2.11. Immunofluorescence Microscopy
2.12. Statistical Analysis
3. Results
3.1. Quantification of Six Marker Compounds in ECT
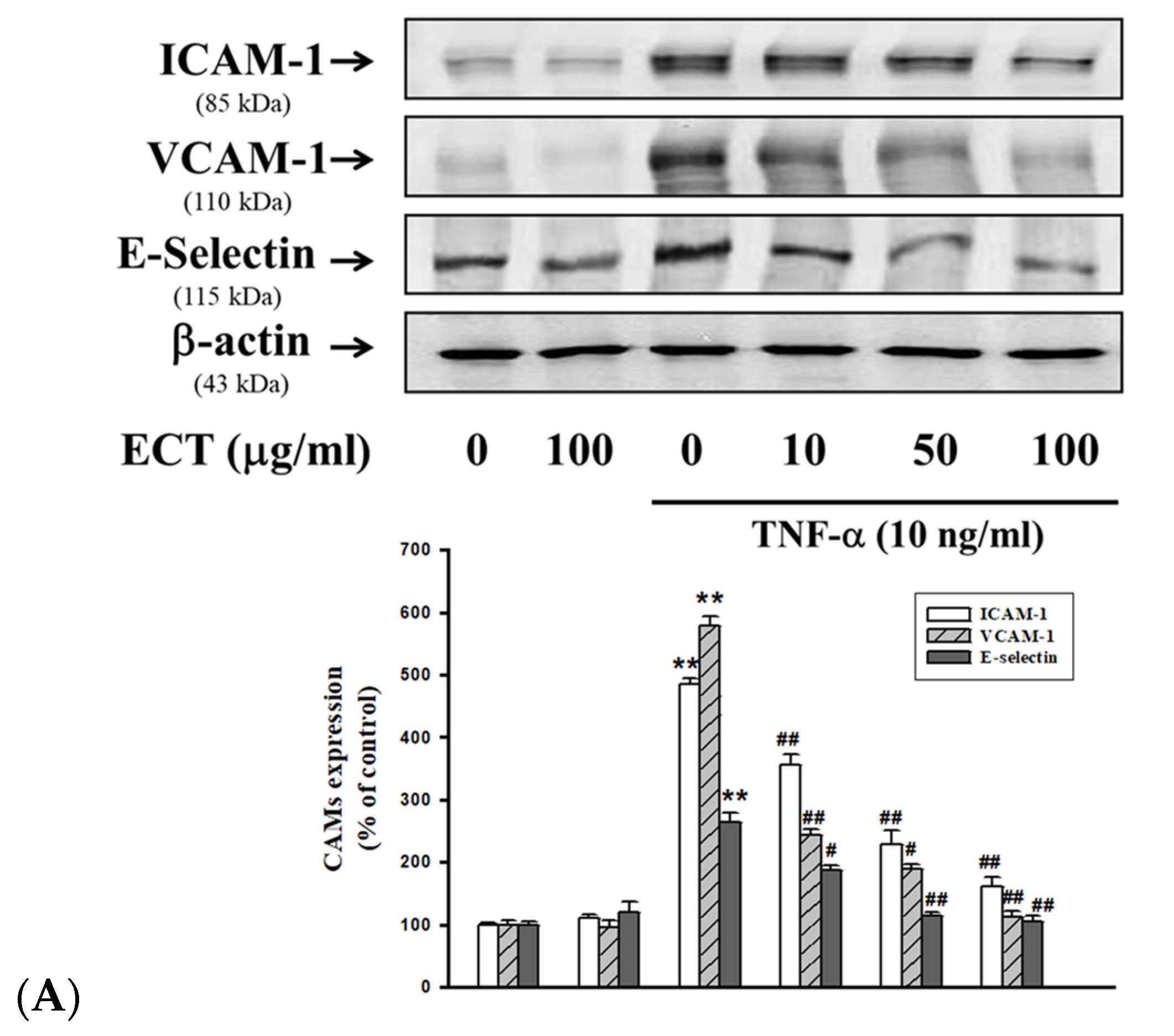
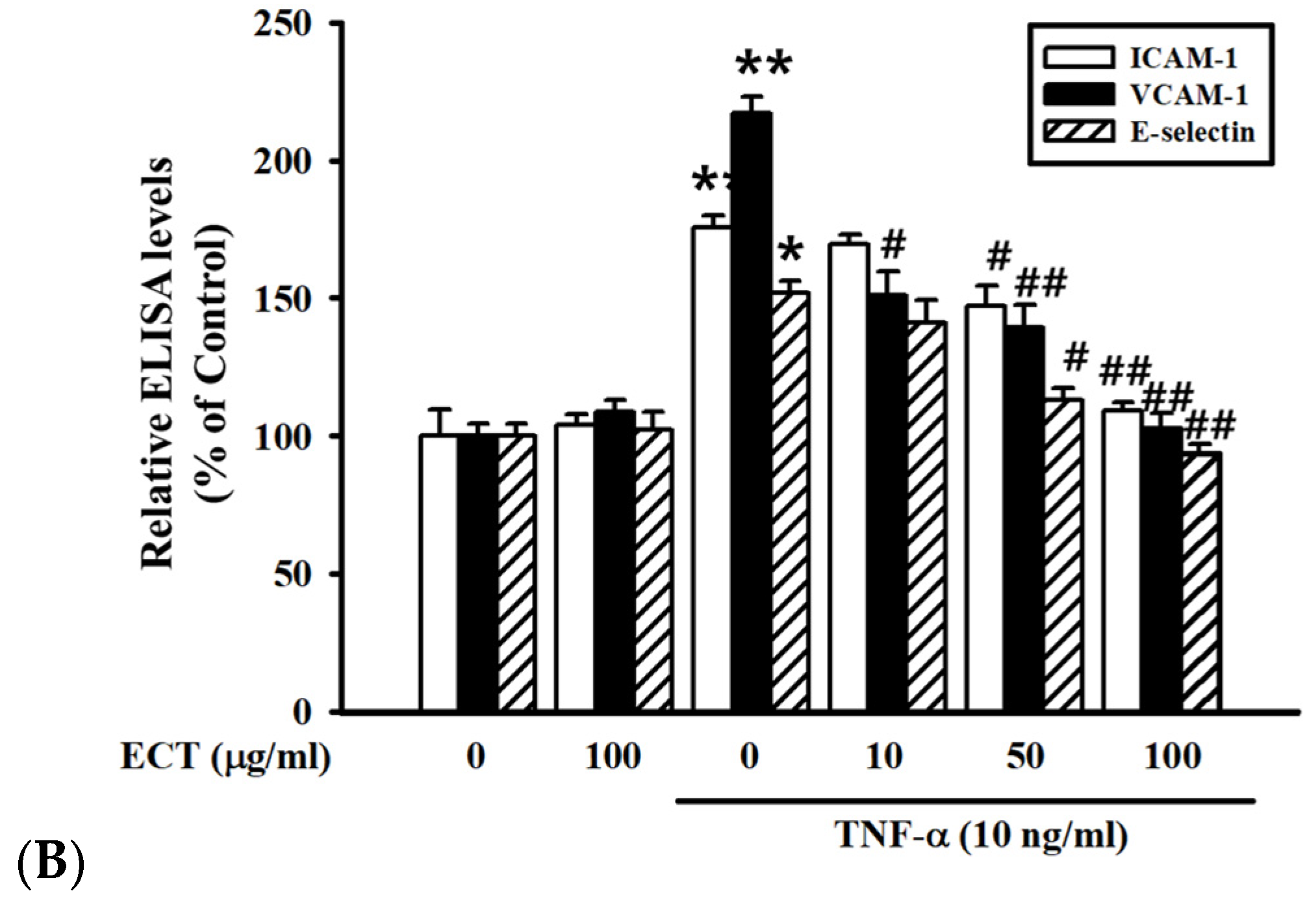
3.2. Effect of ECT on TNF-α-Induced CAMs Expression in HUVEC
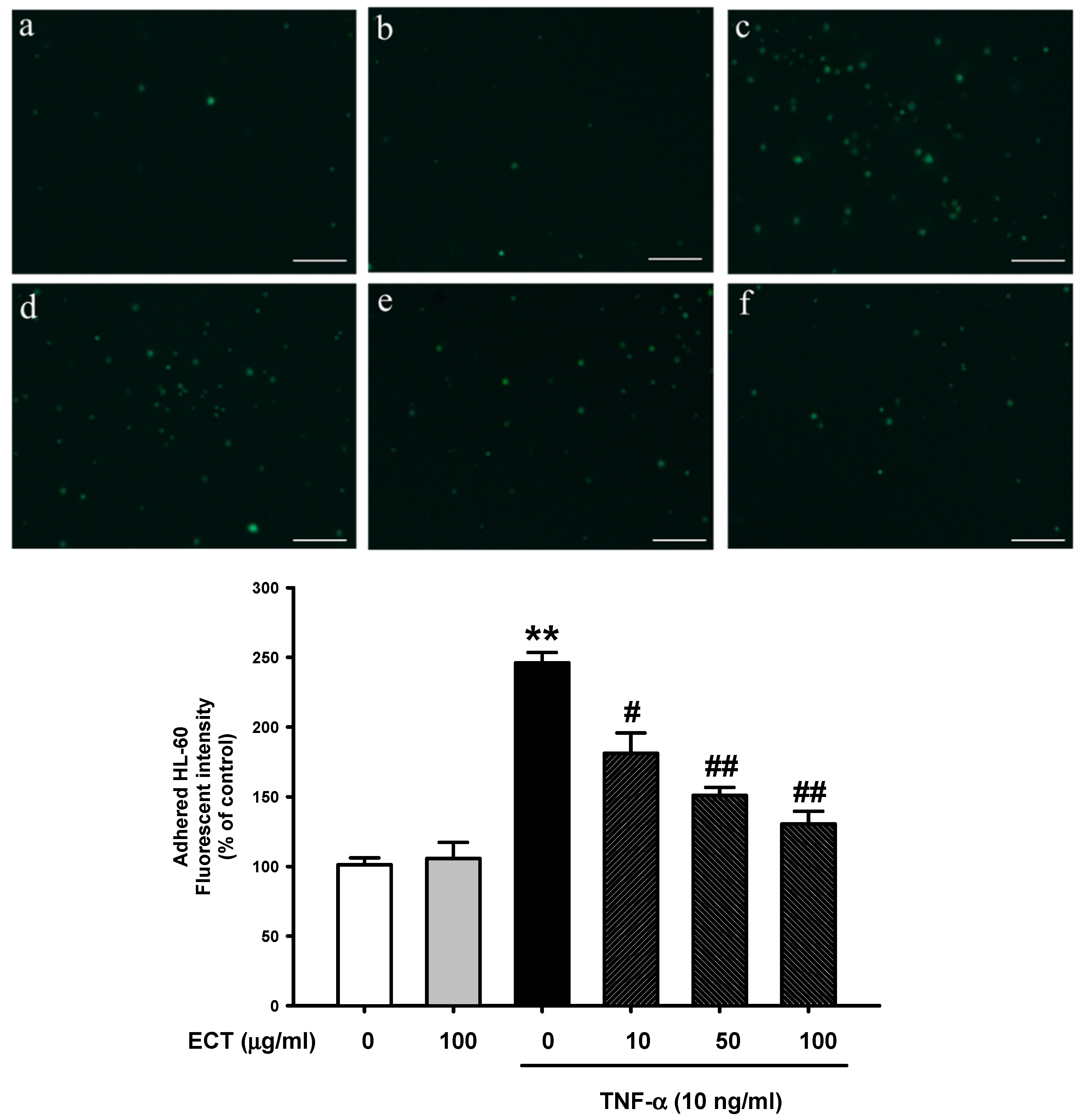
3.3. Effect of ECT on Leukocyte Adhesion to Vascular Endothelial Cells
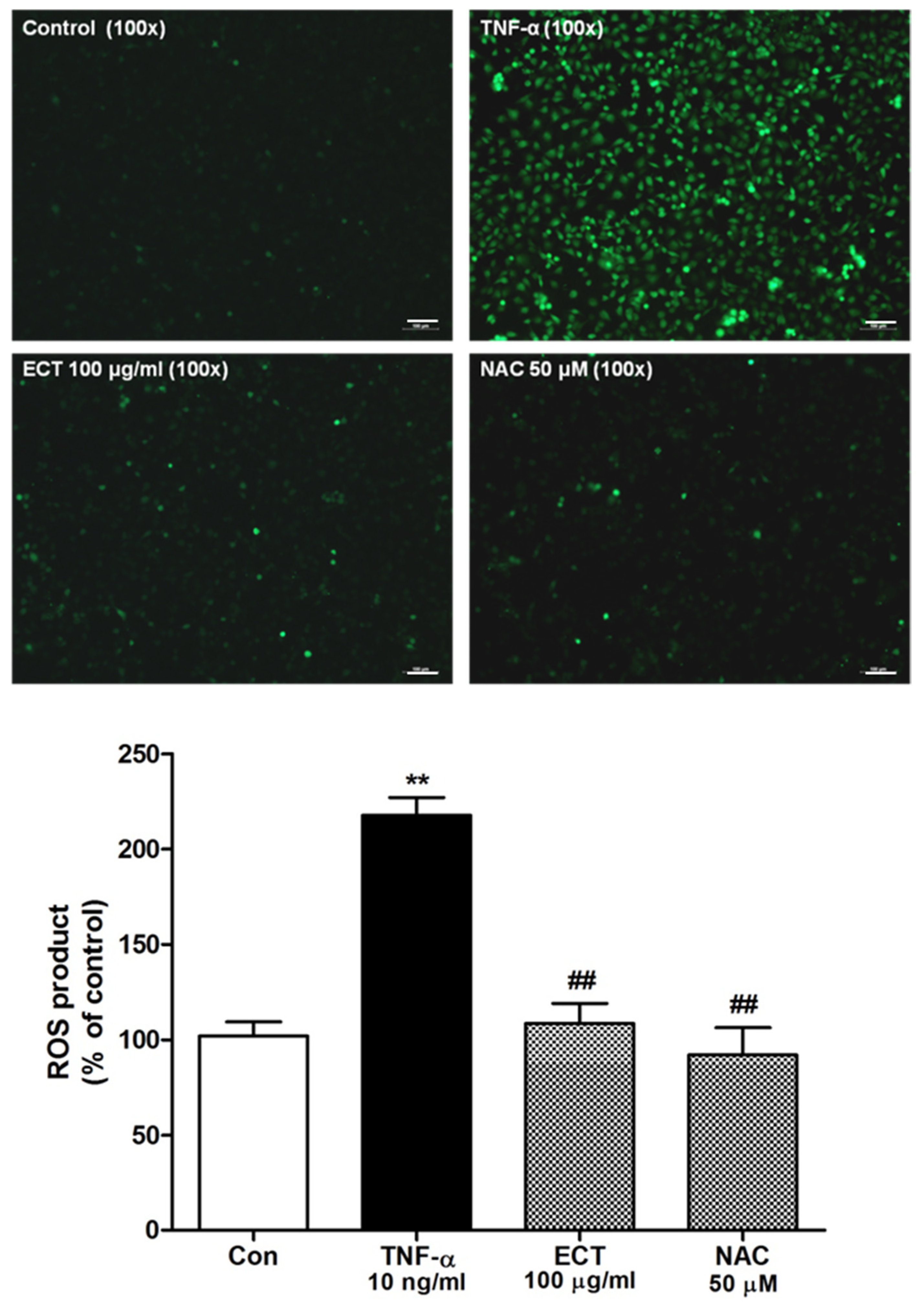
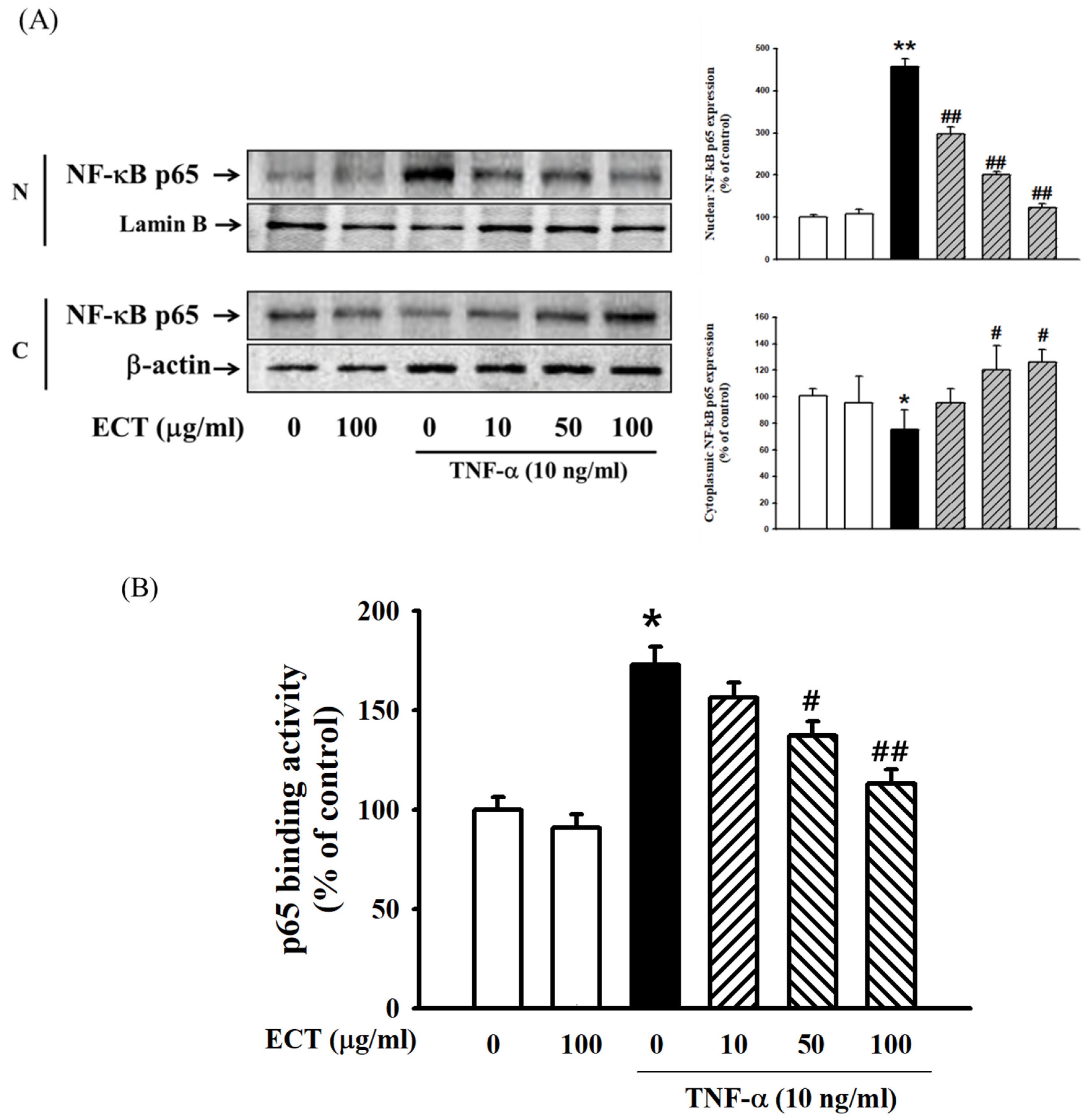
3.4. Effect of ECT on TNF-α-Induced ROS/NF-κB Pathway
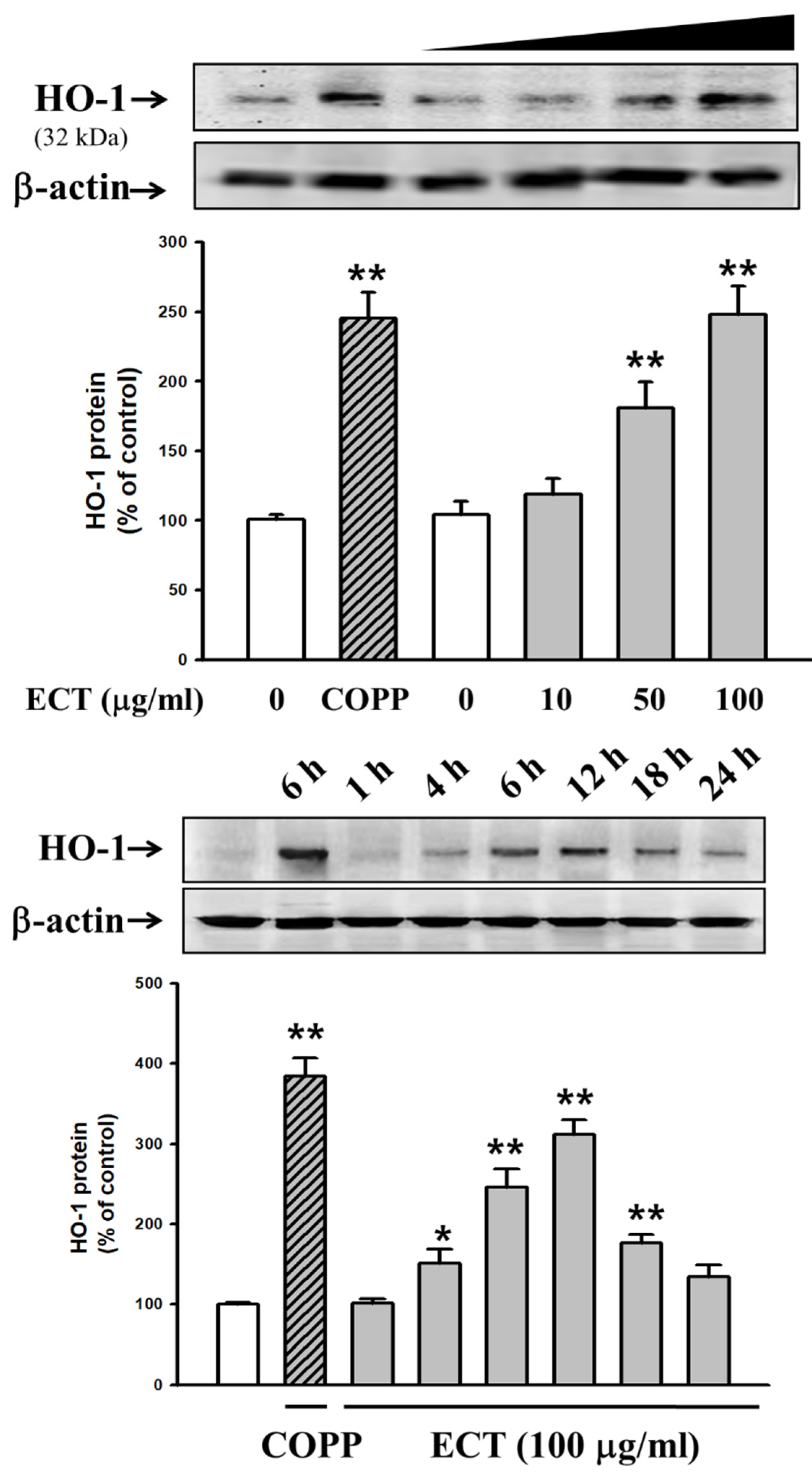
3.5. Involvement of HO-1/Nrf2 Pathway in ECT Protective Effect against Vascular Inflammation
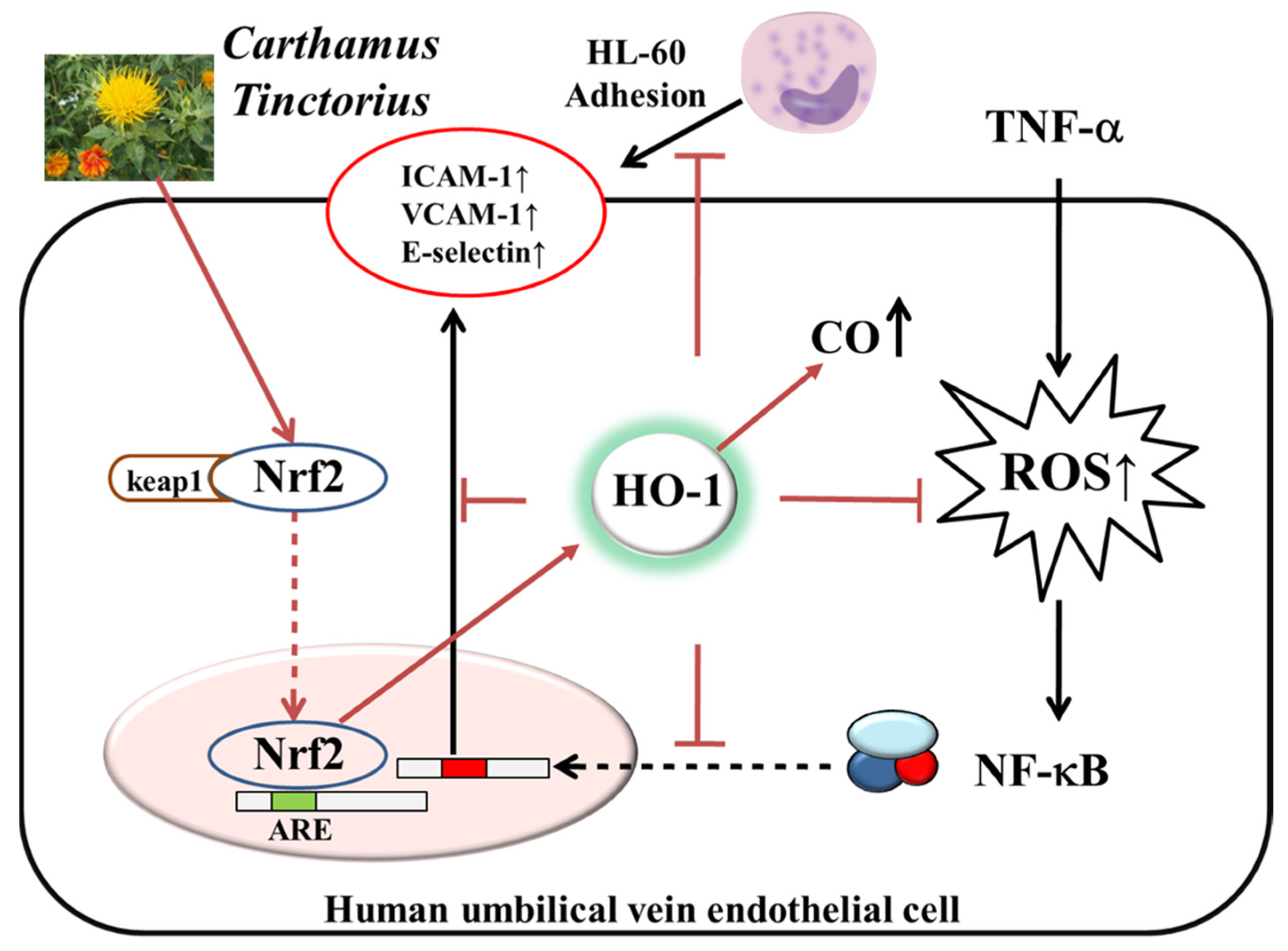
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.; Kim, S.; Han, S.; Rane, P.P.; Fox, K.M.; Qian, Y.; Suh, H.S. Prevalence and incidence of atherosclerotic cardiovascular disease and its risk factors in Korea: A nationwide population-based study. BMC Public Health. 2019, 19, 1112. [Google Scholar] [CrossRef] [PubMed]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Li, S.; Lu, J.; Chen, Y.; Xiong, N.; Li, L.; Zhang, J.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. MCP-1-induced ERK/GSK-3β/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell. Mol. Immunol. 2017, 14, 621–630. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef]
- Javan, H.; Szucsik, A.M.; Li, L.; Schaaf, C.L.; Salama, M.E.; Selzman, C.H. Cardiomyocyte p65 nuclear factor-κB is necessary for compensatory adaptation to pressure overload. Circ. Heart Fail. 2015, 8, 109–118. [Google Scholar] [CrossRef][Green Version]
- Jarmi, T.; Agarwal, A. Heme oxygenase and renal disease. Curr. Hypertens. Rep. 2009, 11, 56–62. [Google Scholar] [CrossRef]
- Origassa, C.S.; Câmara, N.O. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J. Hepatol. 2013, 5, 541–549. [Google Scholar] [CrossRef]
- Abraham, N.G.; Cao, J.; Sacerdoti, D.; Li, X.; Drummond, G. Heme oxygenase: The key to renal function regulation. Am. J. Physiol. Renal. Physiol. 2009, 297, F1137–F1152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sun, G.; Dong, X.; Wang, M.; Qin, M.; Yu, Y.; Sun, X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS ONE 2015, 10, e0120259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Huang, E.; Gao, S.; Li, H.; Lu, J.; Tian, K.; Little, P.J.; Shen, X.; Xu, S.; et al. Tanshinone IIA suppresses cholesterol accumulation in human macrophages: Role of heme oxygenase-1. J. Lipid Res. 2014, 55, 201–213. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.T.; Ye, L.; Tang, J.J.; Zhang, Z.Z.; He, X.H. Dexmedetomidine Ameliorates Lung Injury Induced by Intestinal Ischemia/Reperfusion by Upregulating Cannabinoid Receptor 2, Followed by the Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6120194. [Google Scholar] [CrossRef]
- Shopit, A.; Niu, M.; Wang, H.; Tang, Z.; Li, X.; Tesfaldet, T.; Ai, J.; Ahmad, N.; Al-Azab, M.; Tang, Z. Protection of diabetes-induced kidney injury by phosphocreatine via the regulation of ERK/Nrf2/HO-1 signaling pathway. Life Sci. 2020, 242, 117248. [Google Scholar] [CrossRef]
- Che, C.T.; Wong, M.S.; Lam, C.W. Natural Products from Chinese Medicines with Potential Benefits to Bone Health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef]
- Delshad, E.; Yousefi, M.; Sasannezhad, P.; Rakhshandeh, H.; Ayati, Z. Medical uses of Carthamus tinctorius L. (Safflower): A comprehensive review from Traditional Medicine to Modern Medicine. Electron. Physician. 2018, 10, 6672–6681. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef]
- Zhou, F.R.; Zhao, M.B.; Tu, P.F. Simultaneous determination of four nucleosides in Carthamus tinctorius L. and Safflower injection using highperformance liquid chromatography. J. Chin. Pharm. Sci. 2009, 18, 326–330. [Google Scholar]
- Kim, D.H.; Lee, S.M.; Lee, Y.J.; Yoon, J.J.; Tan, R.; Yu, Y.C.; Kang, D.G.; Lee, H.S. Effect of Paeotang on tumor necrosis factor α-induced vascular inflammation in human umbilical vein endothelial cells. Chin. J. Integr. Med. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Mani, V.; Lee, S.K.; Yeo, Y.; Hahn, B.S. A Metabolic Perspective and Opportunities in Pharmacologically Important Safflower. Metabolites 2020, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Shoelson, S.E. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J. Clin. Investig. 2017, 127, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Gáll, T.; Balla, G.; Balla, J. Heme, Heme Oxygenase, and Endoplasmic Reticulum Stress-A New Insight into the Pathophysiology of Vascular Diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef]
- Schumacher, A.; Zenclussen, A.C. Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance. Front. Pharmacol. 2015, 5, 288. [Google Scholar] [CrossRef]
- Durante, W. Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease. Antioxidants 2020, 9, 829. [Google Scholar] [CrossRef]
- Ho, Y.C.; Wu, M.L.; Gung, P.Y.; Chen, C.H.; Kuo, C.C.; Yet, S.F. Heme oxygenase-1 deficiency exacerbates angiotensin II-induced aortic aneurysm in mice. Oncotarget 2016, 7, 67760–67776. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, N.; Yan, L.; Cao, X.; Wang, J.; Wang, Y. Correlation between genetic polymorphisms within the MAPK1/HIF-1/HO-1 signaling pathway and risk or prognosis of perimenopausal coronary artery disease. Clin. Cardiol. 2017, 40, 597–604. [Google Scholar] [CrossRef]
- Villeneuve, N.F.; Lau, A.; Zhang, D.D. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: An insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 2010, 13, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol. Pharmacol. 2009, 76, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Nucleotide Sequence |
|---|---|
| ICAM-1 | Forward: 5′-CTCACCCGTGTACTGGACTC-3′ Reverse: 5′-CGCCGG AAAGCTGTAGATGG-3′ |
| VCAM-1 | Forward: 5′-ATGCCTGGG AAGATGGTCGTGA-3′ Reverse: 5′-TGGAGCTGGTAGACCCTCGCTG-3′ |
| E-selectin | Forward: 5′-ATCATCCTGCAACTTCACC-3′ Reverse: 5′-ACACCTCACCAAACCCTTC-3′ |
| MCP-1 | Forward: 5’-CAGCCAGATGCAATCAATGC-3’ Reverse: 5’-GTGGTCCATGGAATCCTGAA-3’ |
| β-actin | Forward: 5′-AGGGAGGCGT TCACCTCAGG-3′ Reverse 5′-AACTCCATCACCAGGCG TGGG-3′ |
| Compound | Linear Range (μg/mL) | Regression Equation (y = ax + b) a | r2 | LOD (μg/mL) b | LOQ (μg/mL) c |
|---|---|---|---|---|---|
| Quercetin 3-rutinoside | 0.31–20.00 | y = 28,539.15x + 2768.62 | 1.0000 | 0.06 | 0.18 |
| Quercetin 3-glycoside | 0.31–20.00 | y = 28,930.08x + 1776.94 | 1.0000 | 0.04 | 0.12 |
| Kaempferol 3-rutinoside | 0.78–50.00 | y = 21,078.39x + 3828.68 | 1.0000 | 0.14 | 0.43 |
| Hydroxysafflor yellow A | 1.56–100.00 | y = 33,781.29x + 3563.25 | 0.9999 | 0.19 | 0.57 |
| Kaempferol 3-glycoside | 0.31–20.00 | y = 38,389.02x + 1978.30 | 1.0000 | 0.01 | 0.02 |
| Bidenoside C | 0.31–20.00 | y = 18,983.02x + 1748.57 | 1.0000 | 0.01 | 0.04 |
| Compound | Leaves | ||
|---|---|---|---|
| Mean (mg/g) | SD (×10−2) | RSD (%) | |
| Quercetin 3-rutinoside | 0.02 | 0.04 | 2.60 |
| Quercetin 3-glycoside | 0.05 | 0.06 | 1.25 |
| Kaempferol 3-rutinoside | 0.10 | 0.19 | 1.81 |
| Hydroxysafflor yellow A | 1.59 | 0.12 | 0.07 |
| Kaempferol 3-glycoside | 0.02 | 0.06 | 2.44 |
| Bidenoside C | 0.03 | 0.06 | 2.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Lee, Y.P.; Seo, C.S.; Choi, E.S.; Han, B.H.; Yoon, J.J.; Jang, S.H.; Jeong, C.G.; Mun, Y.J.; Kang, D.G.; et al. The Modulation of Nrf-2/HO-1 Signaling Axis by Carthamus tinctorius L. Alleviates Vascular Inflammation in Human Umbilical Vein Endothelial Cells. Plants 2021, 10, 2795. https://doi.org/10.3390/plants10122795
Lee YJ, Lee YP, Seo CS, Choi ES, Han BH, Yoon JJ, Jang SH, Jeong CG, Mun YJ, Kang DG, et al. The Modulation of Nrf-2/HO-1 Signaling Axis by Carthamus tinctorius L. Alleviates Vascular Inflammation in Human Umbilical Vein Endothelial Cells. Plants. 2021; 10(12):2795. https://doi.org/10.3390/plants10122795
Chicago/Turabian StyleLee, Yun Jung, Yong Pyo Lee, Chang Seob Seo, Eun Sik Choi, Byung Hyuk Han, Jung Joo Yoon, Se Hoon Jang, Chae Ghang Jeong, Yeun Ja Mun, Dae Gill Kang, and et al. 2021. "The Modulation of Nrf-2/HO-1 Signaling Axis by Carthamus tinctorius L. Alleviates Vascular Inflammation in Human Umbilical Vein Endothelial Cells" Plants 10, no. 12: 2795. https://doi.org/10.3390/plants10122795
APA StyleLee, Y. J., Lee, Y. P., Seo, C. S., Choi, E. S., Han, B. H., Yoon, J. J., Jang, S. H., Jeong, C. G., Mun, Y. J., Kang, D. G., & Lee, H. S. (2021). The Modulation of Nrf-2/HO-1 Signaling Axis by Carthamus tinctorius L. Alleviates Vascular Inflammation in Human Umbilical Vein Endothelial Cells. Plants, 10(12), 2795. https://doi.org/10.3390/plants10122795






