The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review
Abstract
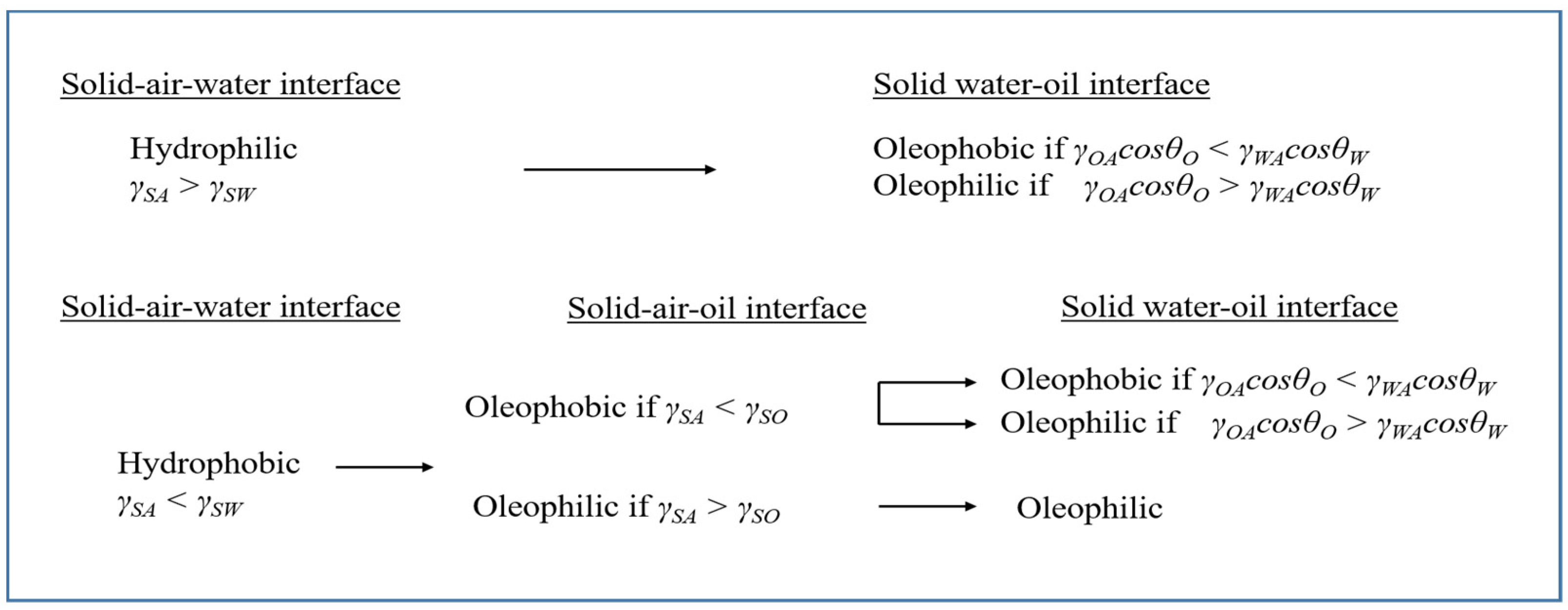
1. Introduction
2. Characterizations of the Foams
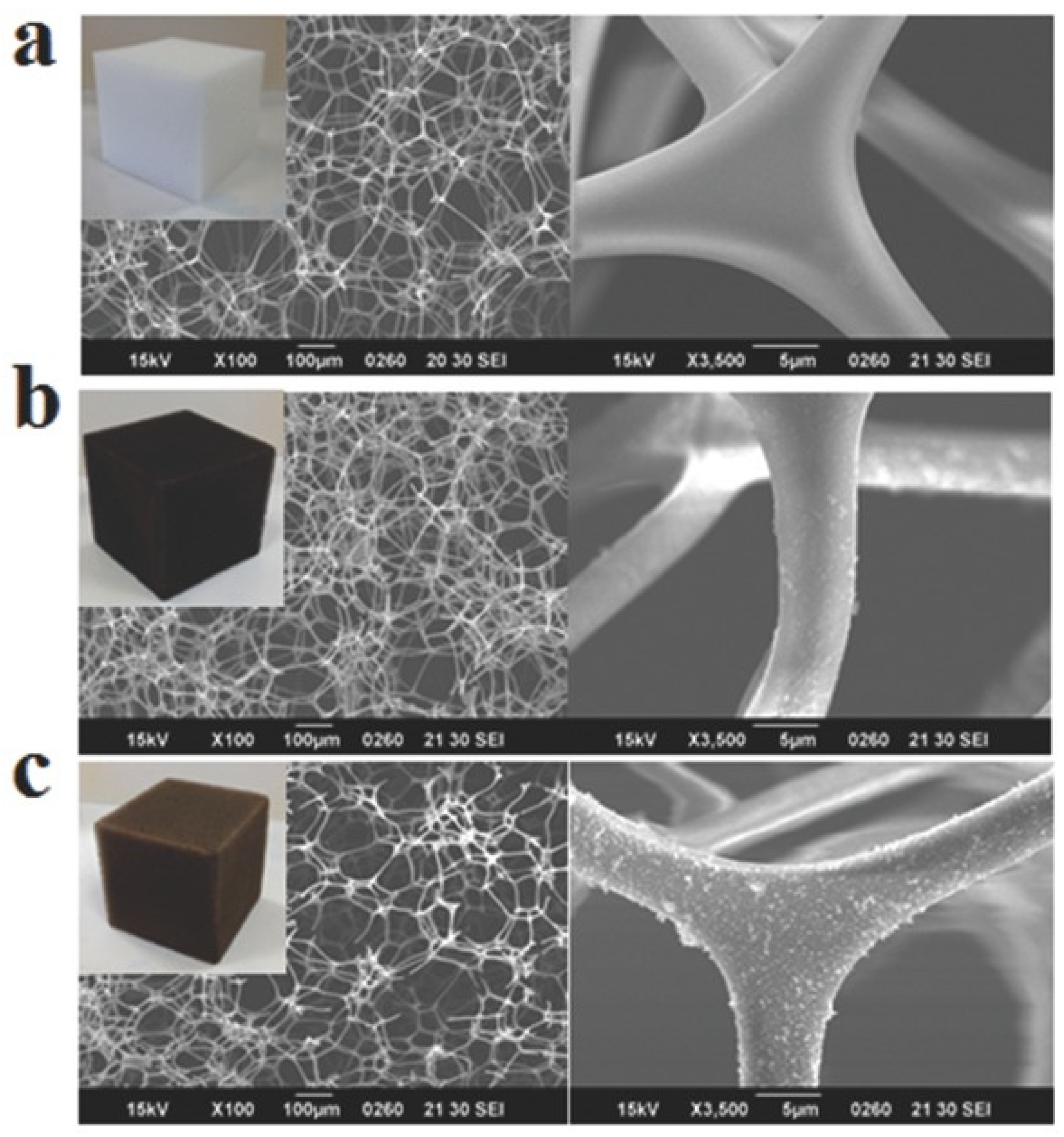
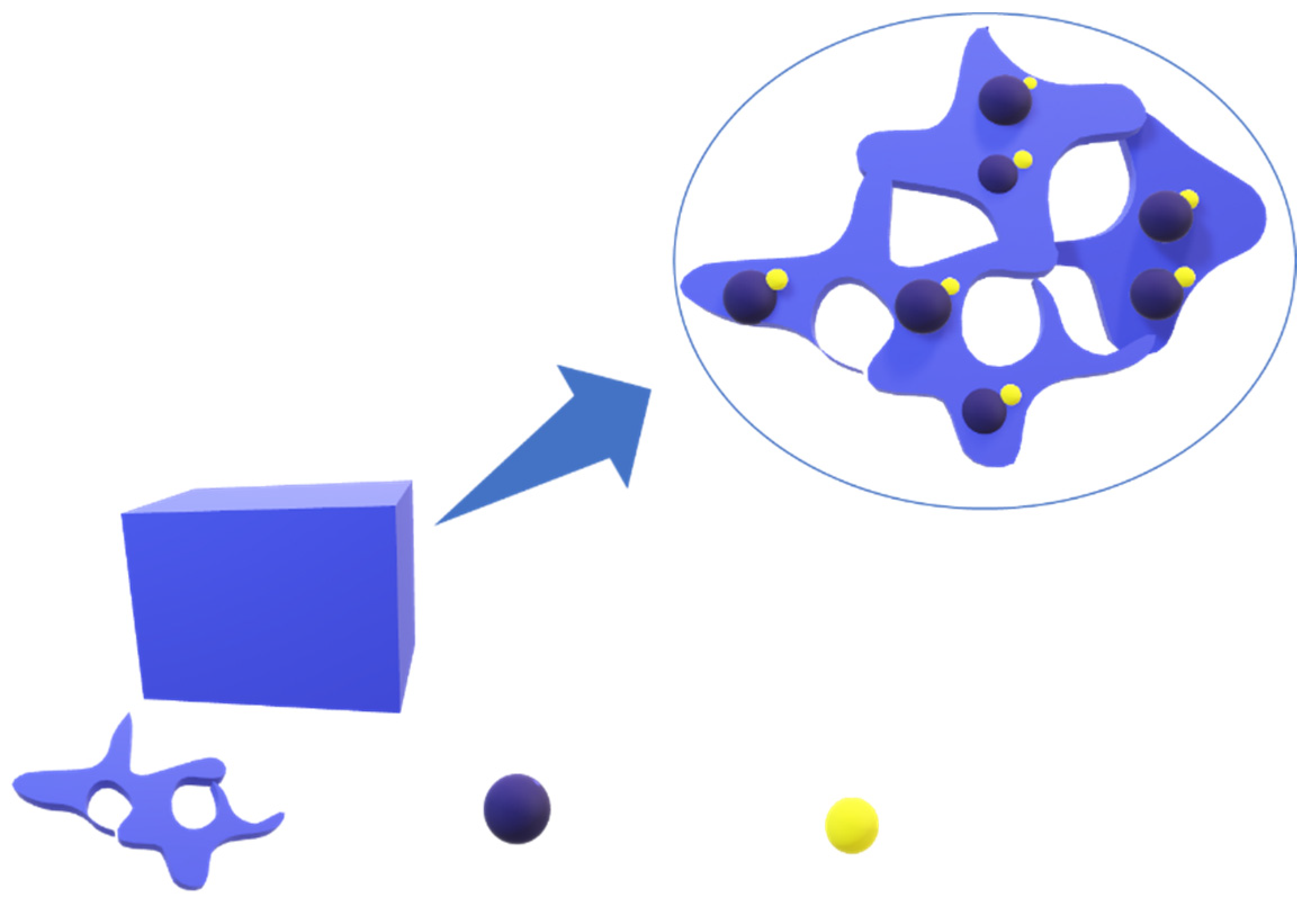
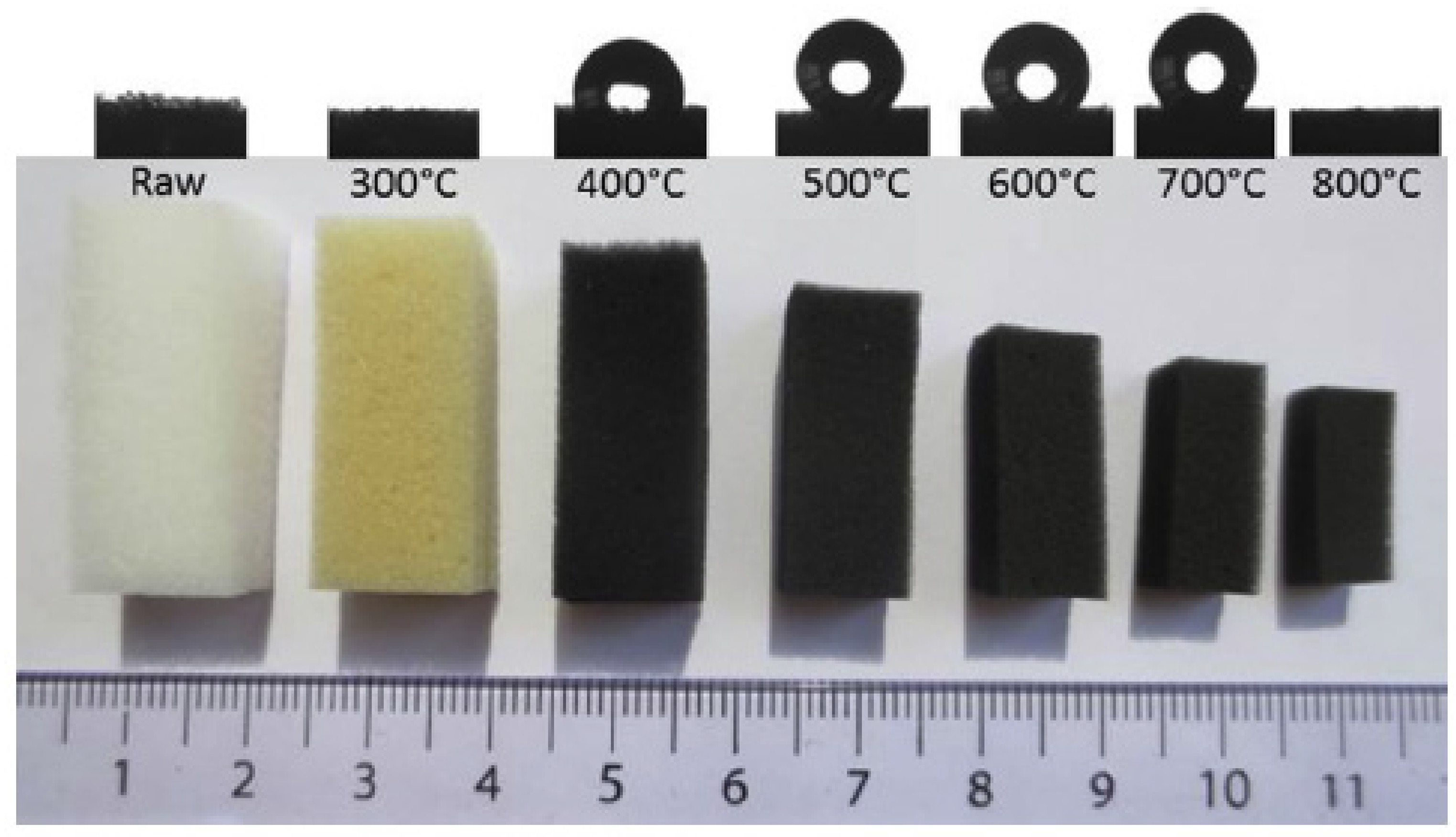
2.1. MA Based Foams
2.2. PU Based Foams
3. Oil/Water Emulsion Separation by MA and PU Foams
3.1. Separating Oil in Water Emulsions
3.2. A Separation of Water in Oil Emulsions
3.3. Recyclability and Durability
4. Challenges and Possibilities of Commercialization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- El-Samak, A.A.; Ponnamma, D.; Hassan, M.K.; Ammar, A.; Adham, S.; Al-Maadeed, M.A.A.; Karim, A. Designing Flexible and Porous Fibrous Membranes for Oil Water Separation—A Review of Recent Developments. Polym. Rev. 2020, 60, 671–716. [Google Scholar] [CrossRef]
- Samanta, A.; Bera, A.; Ojha, K.; Mandal, A. Comparative studies on enhanced oil recovery by alkali–surfactant and polymer flooding. J. Pet. Explor. Prod. Technol. 2012, 2, 67–74. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Eibling, R. Saltstone Vault Classification Samples Modular Caustic Side Solvent Extraction Unit/Actinide Removal Process Waste Stream; Health & Environmental Research Online (HERO): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Coonrod, C.L.; Ben Yin, Y.; Hanna, T.; Atkinson, A.; Alvarez, P.J.; Tekavec, T.N.; Reynolds, M.A.; Wong, M.S. Fit-for-purpose treatment goals for produced waters in shale oil and gas fields. Water Res. 2020, 173, 115467. [Google Scholar] [CrossRef]
- Li, F.; Bhushan, B.; Pan, Y.; Zhao, X. Bioinspired superoleophobic/superhydrophilic functionalized cotton for efficient separation of immiscible oil-water mixtures and oil-water emulsions. J. Colloid Interface Sci. 2019, 548, 123–130. [Google Scholar] [CrossRef]
- Takahashi, M.; Sakamoto, K. Regulations on Cosmetics. Cosmet. Sci. Technol. Theor. Princ. Appl. 2017, 137–146. [Google Scholar] [CrossRef]
- Sobolciak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Materials and Technologies for the Tertiary Treatment of Produced Water Contaminated by Oil Impurities through Nonfibrous Deep-Bed Media: A Review. Water 2020, 12, 3419. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Some Theoretical Aspects of Tertiary Treatment of Water/Oil Emulsions by Adsorption and Coalescence Mechanisms: A Review. Water 2021, 13, 652. [Google Scholar] [CrossRef]
- Sun, J.; Bi, H.; Jia, H.; Su, S.; Dong, H.; Xie, X.; Sun, L. A low cost paper tissue-based PDMS/SiO2 composite for both high efficient oil absorption and water-in-oil emulsion separation. J. Clean. Prod. 2020, 244, 118814. [Google Scholar] [CrossRef]
- El-Samak, A.A.; Ponnamma, D.; Hassan, M.K.; Adham, S.; Karim, A.; Ammar, A.; Alser, M.; Shurbaji, S.; Eltai, N.O.; Al-Maadeed, M.A.A. Multifunctional Oil Absorption with Macroporous Polystyrene Fibers Incorporating Silver-Doped ZnO. ACS Omega 2021, 6, 8081–8093. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.Y. Separating oil from oil-water emulsions by electroflotation technique. Sep. Technol. 1996, 6, 9–17. [Google Scholar] [CrossRef]
- Al-Shamrani, A.A.; James, A.; Xiao, H. Destabilisation of oil–water emulsions and separation by dissolved air flotation. Water Res. 2002, 36, 1503–1512. [Google Scholar] [CrossRef]
- Chen, N.; Pan, Q. Versatile Fabrication of Ultralight Magnetic Foams and Application for Oil–Water Separation. ACS Nano 2013, 7, 6875–6883. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Synthesis of a Novel Highly Oleophilic and Highly Hydrophobic Sponge for Rapid Oil Spill Cleanup. ACS Appl. Mater. Interfaces 2015, 7, 25326–25333. [Google Scholar] [CrossRef] [PubMed]
- Ponnamma, D.; Nair, S.S.; Parangusan, H.; Hassan, M.K.; Adham, S.; Karim, A.; Al-Maadeed, M.A.A. White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption. Polymers 2019, 12, 4. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Zhang, R.; Singh, M.; Ammar, A.; Cousins, D.; Hassan, M.K.; Ponnamma, D.; Adham, S.; Al-Maadeed, M.A.A.; et al. Vertically oriented nanoporous block copolymer membranes for oil/water separation and filtration. Soft Matter 2020, 16, 9648–9654. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al-Deyab, S.S.; Zhang, K.Q.; Chen, T.; et al. Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Pan, S.; Guo, R.; Xu, W. Durable superoleophobic fabric surfaces with counterintuitive superwettability for polar solvents. AIChE J. 2014, 60, 2752–2756. [Google Scholar] [CrossRef]
- Solomon, B.R.; Hyder, M.N.; Varanasi, K.K. Separating Oil-Water Nanoemulsions using Flux-Enhanced Hierarchical Membranes. Sci. Rep. 2014, 4, 5504. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Huang, F.M.; Cheng, L.P. Effect of salt additive on the formation of microporous poly(vinylidene fluoride) membranes by phase inversion from LiC1o4/water/DMF/PVDF system. Polymer 2002, 44, 413–422. [Google Scholar] [CrossRef]
- Elgawady, Y.; Ponnamma, D.; Adham, S.; Al-Maas, M.; Ammar, A.; Alamgir, K.; Al-Maadeed, M.A.A.; Hassan, M.K. Mesoporous silica filled smart super oleophilic fibers of triblock copolymer nanocomposites for oil absorption applications. Emergent Mater. 2020, 3, 279–290. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Ma, Y.; Nie, J.; Sui, G. Super-hydrophobic graphene coated polyurethane (GN@PU) sponge with great oil-water separation performance. Appl. Surf. Sci. 2017, 422, 116–124. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Continuous Oil–Water Separation Using Polydimethylsiloxane-Functionalized Melamine Sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chemie Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, H.-Y.; Zhu, H.-W.; Huang, J.; Shi, L.-A.; Yu, S.-H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.K.; Jessop, G. CCL—Angles of contact and polarity of solid surfaces. J. Chem. Soc. Trans. 1925, 127, 1863–1868. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact angles and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies. BioResources 2015, 10, 8657–8749. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, M.; Jiang, L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1209–1224. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Niu, H.; Lin, T. Recent Progress in Durable and Self-Healing Super-Nonwettable Fabrics. Adv. Mater. Interfaces 2018, 5, 1800461. [Google Scholar] [CrossRef]
- Gupta, P.; Kandasubramanian, B. Directional Fluid Gating by Janus Membranes with Heterogeneous Wetting Properties for Selective Oil-Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 19102–19113. [Google Scholar] [CrossRef]
- Arora, R.; Balasubramanian, K. Hierarchically porous PVDF/nano-SiC foam for distant oil-spill cleanups. RSC Adv. 2014, 4, 53761–53767. [Google Scholar] [CrossRef]
- Udayakumar, K.V.; Gore, P.M.; Kandasubramanian, B. Foamed materials for oil-water separation. Chem. Eng. J. Adv. 2021, 5, 100076. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Effect of the porous structure of polymer foams on the remediation of oil spills. J. Phys. D. Appl. Phys. 2016, 49, 145601. [Google Scholar] [CrossRef]
- Pinto, J.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Reusable nanocomposite-coated polyurethane foams for the remediation of oil spills. Int. J. Environ. Sci. Technol. 2017, 14, 2055–2066. [Google Scholar] [CrossRef]
- Shimizu, K.; Hashimoto, T.; Wang, N.; Watanabe, K.; Ohata, Y.; Kikuchi, A.; Amagai, M.; Nishikawa, T. A Case of Herpetiform Pemphigus Associated with Autoimmune Hemolytic Anemia: Detection of Autoantibodies against Multiple Epidermal Antigens. Dermatology 1996, 192, 179–182. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Adebayo, A.R.; Hossain, M.E. A sustainable approach to controlling oil spills. J. Environ. Manag. 2012, 113, 213–227. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef]
- Xu, Z.; Miyazaki, K.; Hori, T. Dopamine-Induced Superhydrophobic Melamine Foam for Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1500255. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Xiang, Y.; Pang, Y.; Jiang, X.; Huang, J.; Xi, F.; Liu, J. One-step fabrication of novel superhydrophobic and superoleophilic sponge with outstanding absorbency and flame-retardancy for the selective removal of oily organic solvent from water. Appl. Surf. Sci. 2018, 428, 338–347. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Yuan, K.; Xi, F.; Liu, J.; Dong, X. Mussel-inspired fabrication of novel superhydrophobic and superoleophilic sponge modified using a high density of nanoaggregates at low concentration of dopamine. RSC Adv. 2016, 6, 71905–71912. [Google Scholar] [CrossRef]
- Ruan, C.; Ai, K.; Li, X.; Lu, L. A Superhydrophobic Sponge with Excellent Absorbency and Flame Retardancy. Angew. Chemie Int. Ed. 2014, 53, 5556–5560. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Wang, Y.; Peng, B.; Deng, Z. Bioinspired polydopamine particles-assisted construction of superhydrophobic surfaces for oil/water separation. J. Colloid Interface Sci. 2016, 482, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Shang, B.; Wen, P.; Peng, B.; Deng, Z. Bioinspired one-step construction of hierarchical superhydrophobic surfaces for oil/water separation. J. Colloid Interface Sci. 2018, 531, 300–310. [Google Scholar] [CrossRef]
- Gao, H.; Sun, P.; Zhang, Y.; Zeng, X.; Wang, D.; Zhang, Y.; Wang, W.; Wu, J. A two-step hydrophobic fabrication of melamine sponge for oil absorption and oil/water separation. Surf. Coatings Technol. 2018, 339, 147–154. [Google Scholar] [CrossRef]
- Ge, B.; Zhu, X.; Li, Y.; Men, X.; Li, P.; Zhang, Z. Versatile fabrication of magnetic superhydrophobic foams and application for oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 687–692. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Su, C.; Jiang, Z.; Lu, Z. Ultrasonic-microwave assisted synthesis of stable reduced graphene oxide modified melamine foam with superhydrophobicity and high oil adsorption capacities. Chem. Eng. J. 2016, 306, 504–511. [Google Scholar] [CrossRef]
- Stolz, A.; Le Floch, S.; Reinert, L.; Ramos, S.M.M.; Tuaillon-Combes, J.; Soneda, Y.; Chaudet, P.; Baillis, D.; Blanchard, N.; Duclaux, L.; et al. Melamine-derived carbon sponges for oil-water separation. Carbon N. Y. 2016, 107, 198–208. [Google Scholar] [CrossRef]
- Chen, S.; He, G.; Hu, H.; Jin, S.; Zhou, Y.; He, Y.; He, S.; Zhao, F.; Hou, H. Elastic carbon foam via direct carbonization of polymer foam for flexible electrodes and organic chemical absorption. Energy Environ. Sci. 2013, 6, 2435–2439. [Google Scholar] [CrossRef]
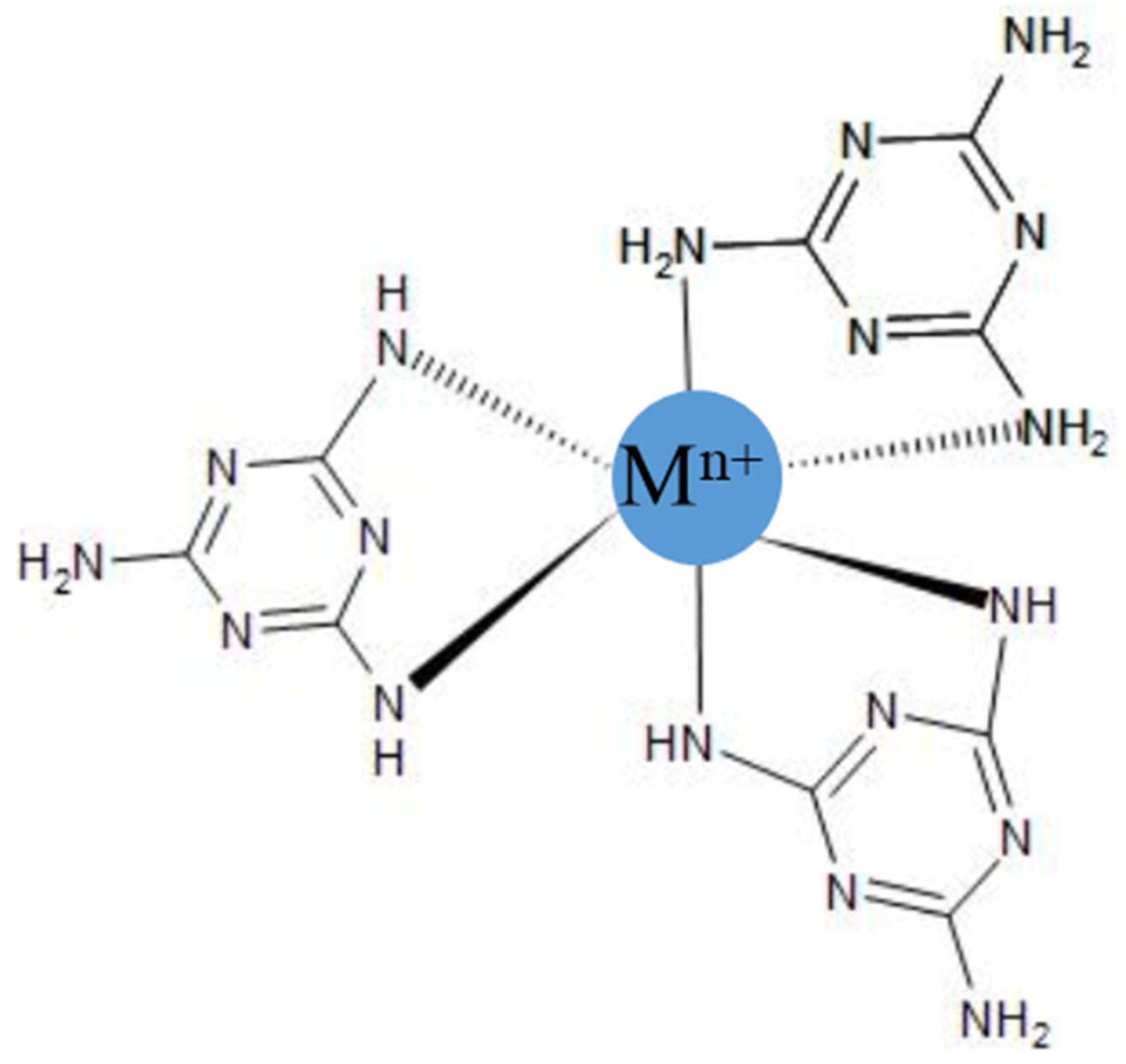
- Ding, Y.; Xu, W.; Yu, Y.; Hou, H.; Zhu, Z. One-Step Preparation of Highly Hydrophobic and Oleophilic Melamine Sponges via Metal-Ion-Induced Wettability Transition. ACS Appl. Mater. Interfaces 2018, 10, 6652–6660. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Xu, L.; Li, T.; Jiang, X.; Li, C.M. Facile synthesis of a two-tier hierarchical structured superhydrophobic-superoleophilic melamine sponge for rapid and efficient oil/water separation. J. Colloid Interface Sci. 2017, 506, 659–668. [Google Scholar] [CrossRef]
- Pham, V.H.; Dickerson, J.H. Superhydrophobic silanized melamine sponges as high efficiency oil absorbent materials. ACS Appl. Mater. Interfaces 2014, 6, 14181–14188. [Google Scholar] [CrossRef]
- Liu, Y.H. Sponge for Oil Seperation and Compositon for Making the Same. U.S. Patent 16039970, 19 July 2018. [Google Scholar]
- Guo, G.; Liu, L.; Dang, Z.; Fang, W. Recent Progress of Polyurethane-Based Materials for Oil/Water Separation. Nano 2017, 12. [Google Scholar] [CrossRef]
- Huang, S. Mussel-inspired one-step copolymerization to engineer hierarchically structured surface with superhydrophobic properties for removing oil from water. ACS Appl. Mater. Interfaces 2014, 6, 17144–17150. [Google Scholar] [CrossRef]
- Cao, N.; Yang, B.; Barras, A.; Szunerits, S.; Boukherroub, R. Polyurethane sponge functionalized with superhydrophobic nanodiamond particles for efficient oil/water separation. Chem. Eng. J. 2017, 307, 319–325. [Google Scholar] [CrossRef]
- Xiong, S.; Zhong, Z.; Wang, Y. Direct silanization of polyurethane foams for efficient selective absorption of oil from water. AIChE J. 2017, 63, 2232–2240. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Hu, W.; Li, J.; Yang, Y.; Wu, Y. Stable superhydrophobic and superoleophilic silica coated polyurethane sponges for the continuous capture and removal of oils from the water surface. New J. Chem. 2015, 39, 9958–9962. [Google Scholar] [CrossRef]
- Shi, H.; Shi, D.; Yin, L.; Yang, Z.; Luan, S.; Gao, J.; Zha, J.; Yin, J.; Li, R.K.Y. Ultrasonication assisted preparation of carbonaceous nanoparticles modified polyurethane foam with good conductivity and high oil absorption properties. Nanoscale 2014, 6, 13748–13753. [Google Scholar] [CrossRef] [PubMed]
- Anju, M.; Renuka, N.K. Magnetically actuated graphene coated polyurethane foam as potential sorbent for oils and organics. Arab. J. Chem. 2020, 13, 1752–1762. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Chen, F.; Liu, J.; Chen, Y.; Zhang, F.; Guan, N. An environmentally friendly and cost-effective method to fabricate superhydrophobic PU sponge for oil/water separation. J. Dispers. Sci. Technol. 2020, 41, 1136–1144. [Google Scholar] [CrossRef]
- Kozlowski, Z.J. Liquid Sorbent. U.S. Patent 5,239,040, 24 August 1993. [Google Scholar]
- Benachenou, A.; Parent, J.-P. Polyurethane Oil De-Emulsification Unit. U.S. Patent 8,721,895 B2, 13 May 2014. [Google Scholar]
- De Lemos Chernicharo, C.A.; SertÓrio De Almeida, P.G. Polyurethane Foam-Based Supporting Medium Designed for Use in Waste Water Treatment Systems. Patent WO2014063219A1, 1 May 2014. [Google Scholar]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nat. Cell Biol. 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Fu, Q.; Wang, X.; Zhu, J.; Yu, J.; Sun, G.; Ding, B. Superelastic and Superhydrophobic Nanofiber-Assembled Cellular Aerogels for Effective Separation of Oil/Water Emulsions. ACS Nano. 2015, 9, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.A.; Bartels, C.R. Removal of organics from offshore produced waters using nanofiltration membrane technology. Environ. Prog. 1990, 9, 183–186. [Google Scholar] [CrossRef]
- Han, L.; Bi, H.; Xie, X.; Su, S.; Mao, P.; Sun, L. Superhydrophobic graphene-coated sponge with microcavities for high efficiency oil-in-water emulsion separation. Nanoscale 2020, 12, 17812–17820. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Federici, F.; Ingo, G.M.; Gigli, G. Superhydrophobic fabrics for oil–water separation through a diamond like carbon (DLC) coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z. Superhydrophobic copper mesh films with rapid oil/water separation properties by electrochemical deposition inspired from butterfly wing. Appl. Phys. Lett. 2013, 103, 063704. [Google Scholar] [CrossRef]
- Chen, Q.; de Leon, A.; Advincula, R.C. Inorganic–Organic Thiol–Ene Coated Mesh for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 18566–18573. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, J.; Lu, X.; Zhu, Y.; Jiang, L.; Berlin, S.-V. In situ fastening graphene sheets into a polyurethane sponge for the highly efficient continuous cleanup of oil spills. Nano Res. 2017, 10, 1756–1766. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Zhang, Y.; Wang, R.; Zha, F.; She, H. Robust superhydrophobic attapulgite coated polyurethane sponge for efficient immiscible oil/water mixture and emulsion separation. J. Mater. Chem. A 2016, 4, 15546–15553. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Shao, L.; Xia, Y.; Zeng, T.; Wang, Y. Cost-effective one-pot surface modified method to engineer a green superhydrophobic sponge for efficient oil/water mixtures as well as emulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 43–54. [Google Scholar] [CrossRef]
- Wang, C.F.; Huang, H.C.; Chen, L.T. Protonated Melamine Sponge for Effective Oil/Water Separation. Sci. Rep. 2015, 5, 14294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Geng, G. Flame-retardant superhydrophobic coating derived from fly ash on polymeric foam for efficient oil/corrosive water and emulsion separation. J. Colloid Interface Sci. 2018, 525, 11–20. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yang, Y.; Chen, Z.; Jia, G.; Zhang, L. Silk fibroin-graphene oxide functionalized melamine sponge for efficient oil absorption and oil/water separation. Appl. Surf. Sci. 2019, 497, 143762. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, L.; Zhu, X.; Li, H.; Ma, C.; Yu, S.; Sun, D.; Xia, F.; Xue, Q. Tetradecylamine-MXene functionalized melamine sponge for effective oil/water separation and selective oil adsorption. Sep. Purif. Technol. 2021, 259, 118106. [Google Scholar] [CrossRef]
- Guselnikova, O.; Barras, A.; Addad, A.; Sviridova, E.; Szunerits, S.; Postnikov, P.; Boukherroub, R. Magnetic polyurethane sponge for efficient oil adsorption and separation of oil from oil-in-water emulsions. Sep. Purif. Technol. 2020, 240, 116627. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Tao, Z.; Liu, X.; Wang, Z.; Yue, R.; Cui, Z. 3D superhydrophobic sponge with a novel compression strategy for effective water-in-oil emulsion separation and its separation mechanism. Chem. Eng. J. 2019, 359, 149–158. [Google Scholar] [CrossRef]
- Li, M.; Bian, C.; Yang, G.; Qiang, X. Facile fabrication of water-based and non-fluorinated superhydrophobic sponge for efficient separation of immiscible oil/water mixture and water-in-oil emulsion. Chem. Eng. J. 2019, 368, 350–358. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust Superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef]
- Ejeta, D.D.; Wang, C.F.; Lin, C.H.; Kuo, S.W.; Chen, J.K.; Tsai, H.C.; Hung, W.S.; Hu, C.C.; Lai, J.Y. Preparation of a main-chain-type polybenzoxazine-modified melamine sponge via non-solvent-induced phase inversion for oil absorption and very-high-flux separation of water-in-oil emulsions. Sep. Purif. Technol. 2021, 263, 118387. [Google Scholar] [CrossRef]
- Ahmed, R.M.G.; Anis, B.; Khalil, A.S.G. Facile surface treatment and decoration of graphene-based 3D polymeric sponges for high performance separation of heavy oil-in-water emulsions. J. Environ. Chem. Eng. 2021, 9, 105087. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Ke, L.; Ye, X.; Huang, X.; Shi, B. Plant polyphenols as multifunctional platforms to fabricate three-dimensional superhydrophobic foams for oil/water and emulsion separation. Ind. Eng. Chem. Res. 2018, 57, 16442–16450. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Anbazhagan, R.; Tsai, H.C.; Wang, C.F.; Lai, J.Y. Preparation of caffeic acid-polyethyleneimine modified sponge for emulsion separation and dye adsorption. J. Taiwan Inst. Chem. Eng. 2021, 118, 325–333. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Peng, K.; Xiong, Y.; Zhang, J.; Lu, L.; Liu, J.; Li, S. PDA-PEI copolymerized highly hydrophobic sponge for oil-in-water emulsion separation via oil adsorption and water filtration. Surf. Coat. Technol. 2021, 406, 126743. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Fan, L.; Wang, R.; Yang, M.; Zhou, Y. 3D Superhydrophobic Sponge Coated with Magnesium Hydroxide for Effective Oil/Water Mixture and Emulsion Separation. Ind. Eng. Chem. Res. 2020, 59, 11713–11722. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Chen, Q.; Zhang, P.; Guo, S.; Yao, J. Flexible Co-ZIF-L@melamine sponge with underwater superoleophobicity for water/oil separation. Mater. Chem. Phys. 2020, 241, 122385. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Geng, G. Highly efficient oil-in-water emulsion and oil layer/water mixture separation based on durably superhydrophobic sponge prepared via a facile route. Mar. Pollut. Bull. 2018, 127, 108–116. [Google Scholar] [CrossRef]
- Li, J.; Tenjimbayashi, M.; Zacharia, N.S.; Shiratori, S. One-Step Dipping Fabrication of Fe3O4/PVDF-HFP Composite 3D Porous Sponge for Magnetically Controllable Oil-Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 10706–10713. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y. Oil/water mixtures and emulsions separation of stearic acid-functionalized sponge fabricated via a facile one-step coating method. Sep. Purif. Technol. 2017, 181, 183–191. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, Z.; Wu, X.; Ren, T.; Han, J.; Xue, Q. Three-dimensional structured sponge with high oil wettability for the clean-up of oil contaminations and separation of oil–water mixtures. Polym. Chem. 2014, 5, 5942–5948. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Fabrication of an Oil Spill Collector Package by Using Polyurethane Foam Wrapped with Superhydrophobic ZnO Microrods/Carbon Cloth. Chempluschem 2018, 83, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xue, Y.; Wu, Q.; Dong, Y.; Meng, X. Self-assembly modification of polyurethane sponge for application in oil/water separation. RSC Adv. 2019, 9, 40378–40387. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, S.; Zhou, Y.; Wan, H.; Zhang, C.; Yao, B.; Chen, T. Bio-inspired magnetic superhydrophobic PU-PDA-Fe3O4-Ag for effective oil-water separation and its antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126122. [Google Scholar] [CrossRef]
- Jamsaz, A.; Goharshadi, E.K.; Barras, A.; Ifires, M.; Szunerits, S.; Boukherroub, R. Magnetically driven superhydrophobic/superoleophilic graphene-based polyurethane sponge for highly efficient oil/water separation and demulsification. Sep. Purif. Technol. 2021, 274, 118931. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem. Eng. J. 2020, 384, 123339. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Men, X.; Zhu, X. Facile fabrication of superhydrophobic sponge with selective absorption and collection of oil from water. Ind. Eng. Chem. Res. 2013, 52, 9411–9416. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z. Synthesis of magnetic, durable and superhydrophobic carbon sponges for oil/water separation. Mater. Res. Bull. 2019, 115, 19–26. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; An, W.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Robust and Cost-Effective Superhydrophobic Graphene Foam for Efficient Oil and Organic Solvent Recovery. Small 2015, 11, 5222–5229. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zheng, P.; Niu, L.; Yang, Y.; Shen, J.; Zhang, W.; Wang, C. Ultralight, robustly compressible and super-hydrophobic biomass-decorated carbonaceous melamine sponge for oil/water separation with high oil retention. Appl. Surf. Sci. 2019, 489, 922–929. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Zhang, Y.; Su, J.; Yu, P.; Luo, Y. A superhydrophobic 3D porous material for oil spill cleanup. RSC Adv. 2014, 4, 46470–46475. [Google Scholar] [CrossRef]
- Lytle, J.C.; Wallace, J.M.; Sassin, M.B.; Barrow, A.J.; Long, J.W.; Dysart, J.L.; Renninger, C.H.; Saunders, M.P.; Brandell, N.L.; Rolison, D.R. The right kind of interior for multifunctional electrode architectures: Carbon nanofoam papers with aperiodic submicrometre pore networks interconnected in 3D. Energy Environ. Sci. 2011, 4, 1913–1925. [Google Scholar] [CrossRef]
- Instron. Materials Testing Machines for Tensile, Fatigue, Impact, Rheology and Structural Testing—Instron. Available online: https://www.instron.com/en/ (accessed on 12 September 2021).
- Liu, Y.; Liu, N.; Jing, Y.; Jiang, X.; Yu, L.; Yan, X. Surface design of durable and recyclable superhydrophobic materials for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 128–138. [Google Scholar] [CrossRef]
- Ren, Q.; Dai, T.; Jin, X.; Wu, D.; Wang, C.; Li, J.; Zhu, S. Solution Processed Coating of Polyolefin on Melamine Foams to Fabricate Tough Oil Superabsorbents. Macromol. Mater. Eng. 2018, 303, 1800436. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Wang, H.; Wang, E.; Liu, Z.; Gao, D.; Yuan, R.; Sun, L.; Zhu, Y. A novel carbon nanotubes reinforced superhydrophobic and superoleophilic polyurethane sponge for selective oil–water separation through a chemical fabrication. J. Mater. Chem. A 2014, 3, 266–273. [Google Scholar] [CrossRef]
- Peng, L.; Yuan, S.; Yan, G.; Yu, P.; Luo, Y. Hydrophobic sponge for spilled oil absorption. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-Effective Reduced Graphene Oxide-Coated Polyurethane Sponge as a Highly Efficient and Reusable Oil-Absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Z.; Ge, W.; Lu, Y.; Liu, T.; Yang, W.; Dai, J. Robust, fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–Water separation. Chinese J. Chem. Eng. 2021, 33, 50–60. [Google Scholar] [CrossRef]
- Ong, C.; Shi, Y.; Chang, J.; Alduraiei, F.; Ahmed, Z.; Wang, P. Polydopamine as a Versatile Adhesive Layer for Robust Fabrication of Smart Surface with Switchable Wettability for Effective Oil/Water Separation. Ind. Eng. Chem. Res. 2019, 58, 4838–4843. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, S.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Robust Absorbent Material Based on Light-Responsive Superhydrophobic Melamine Sponge for Oil Recovery. Adv. Mater. Interfaces 2016, 3, 1500683. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, Y.; Chen, S.; Song, K.; Peng, Y.; Zhao, N.; Ouyang, X.; Wang, X. Facile fabrication of a highly durable and flexible MoS2@RTV sponge for efficient oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 237–243. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Zhang, X.; Guo, Z.; Hu, Y. Durable multifunctional superhydrophobic sponge for oil/water separation and adsorption of volatile organic compounds. Res. Chem. Intermed. 2020, 46, 4297–4309. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, Y.; Su, J.; Mo, Y.; Yu, S.; Wang, Z. Durable superhydrophobic melamine sponge based on polybenzoxazine and Fe3O4 for oil/water separation. Sep. Purif. Technol. 2021, 275, 119130. [Google Scholar] [CrossRef]
- Toro, R.G.; Calandra, P.; Federici, F.; de Caro, T.; Mezzi, A.; Cortese, B.; Pellegrino, A.L.; Malandrino, G.; Caschera, D. Development of superhydrophobic, self-cleaning, and flame-resistant DLC/TiO2 melamine sponge for application in oil–water separation. J. Mater. Sci. 2019, 55, 2846–2859. [Google Scholar] [CrossRef]
- Peng, M.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Shi, J.; Li, H.; Yuan, L.; et al. Superhydrophobic kaolinite modified graphene oxide-melamine sponge with excellent properties for oil-water separation. Appl. Clay Sci. 2018, 163, 63–71. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun porous structure fibrous film with high oil adsorption capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q.; Liu, F. Facile Removal and Collection of Oils from Water Surfaces through Superhydrophobic and Superoleophilic Sponges. J. Phys. Chem. C 2011, 115, 17464–17470. [Google Scholar] [CrossRef]
- Chowdhury, S.R. Advancement of oil/water separating materials: Merits and demerits in real-time applications. MOJ Polym. Sci. 2017, 1. [Google Scholar] [CrossRef]
- Guan, Y.; Cheng, F.; Pan, Z. Superwetting Polymeric Three Dimensional (3D) Porous Materials for Oil/Water Separation: A Review. Polymers 2019, 11, 806. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Wu, L.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic polyurethane sponges inspired by mussel and lotus leaf for the selective removal of organic pollutants from water. Chempluschem 2014, 79, 850–856. [Google Scholar] [CrossRef]
- Liu, C.; Huang, A. One-step synthesis of the superhydrophobic zeolitic imidazolate framework F-ZIF-90 for efficient removal of oil. New J. Chem. 2018, 42, 2372–2375. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Yang, X.B.; Wang, Z.X.; Long, J.; Shao, L. Designing multifunctional 3D magnetic foam for effective insoluble oil separation and rapid selective dye removal for use in wastewater remediation. J. Mater. Chem. A 2017, 5, 7316–7325. [Google Scholar] [CrossRef]
- Salehabadi, S.; Seyfi, J.; Hejazi, I.; Davachi, S.M.; Naeini, A.H.; Khakbaz, M. Nanosilica-decorated sponges for efficient oil/water separation: Role of nanoparticle’s type and concentration. J. Mater. Sci. 2017, 52, 7017–7027. [Google Scholar] [CrossRef]

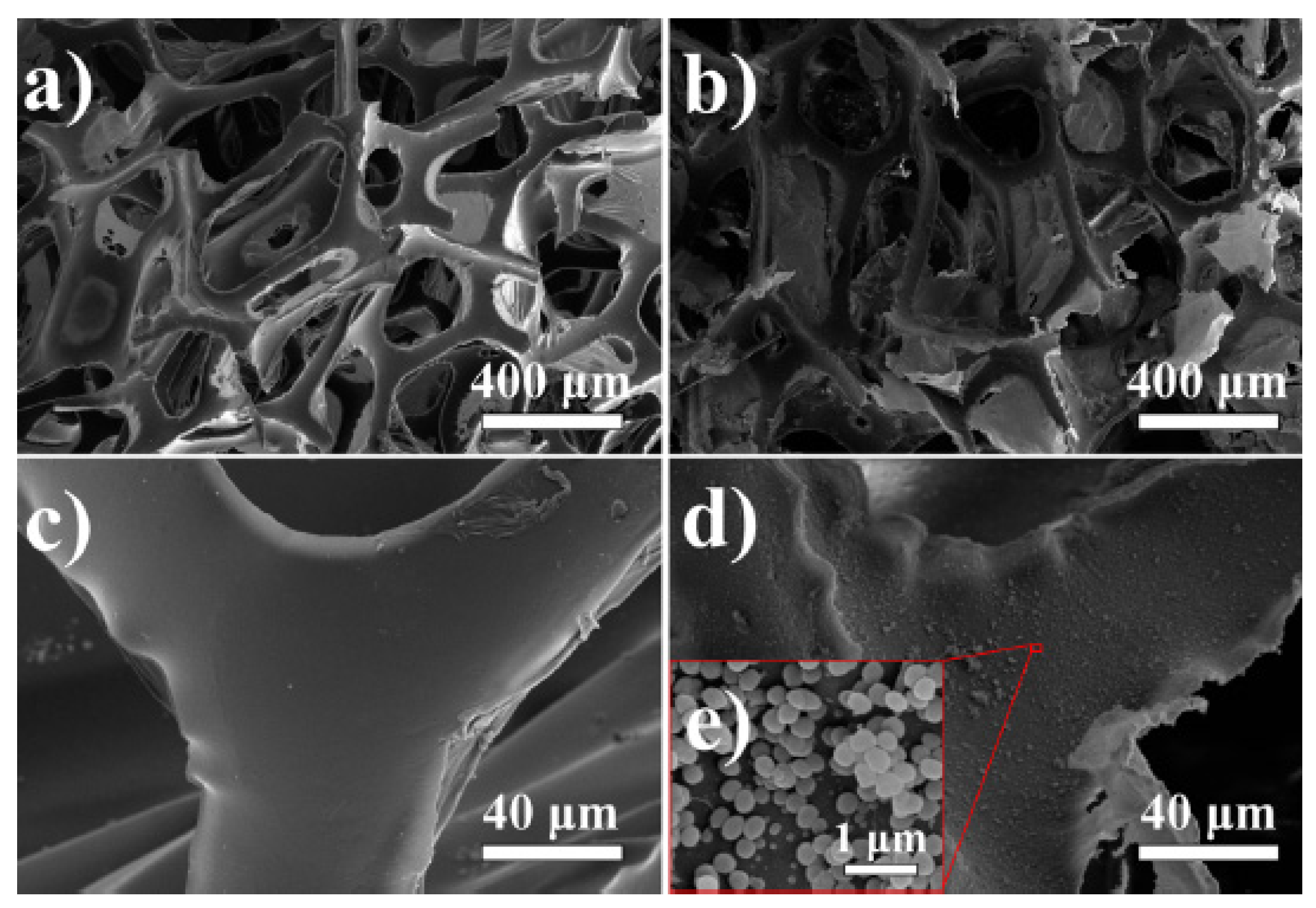

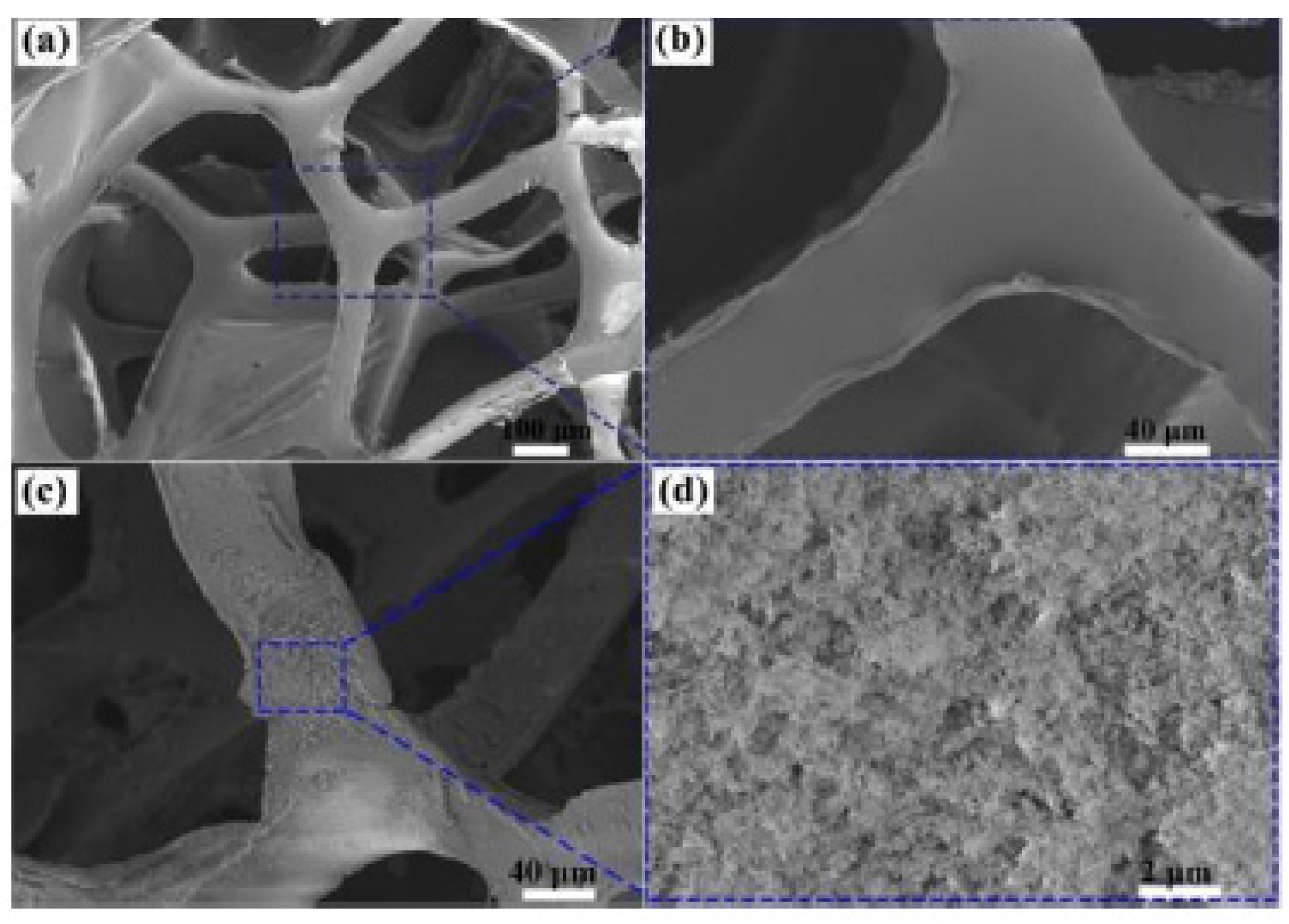
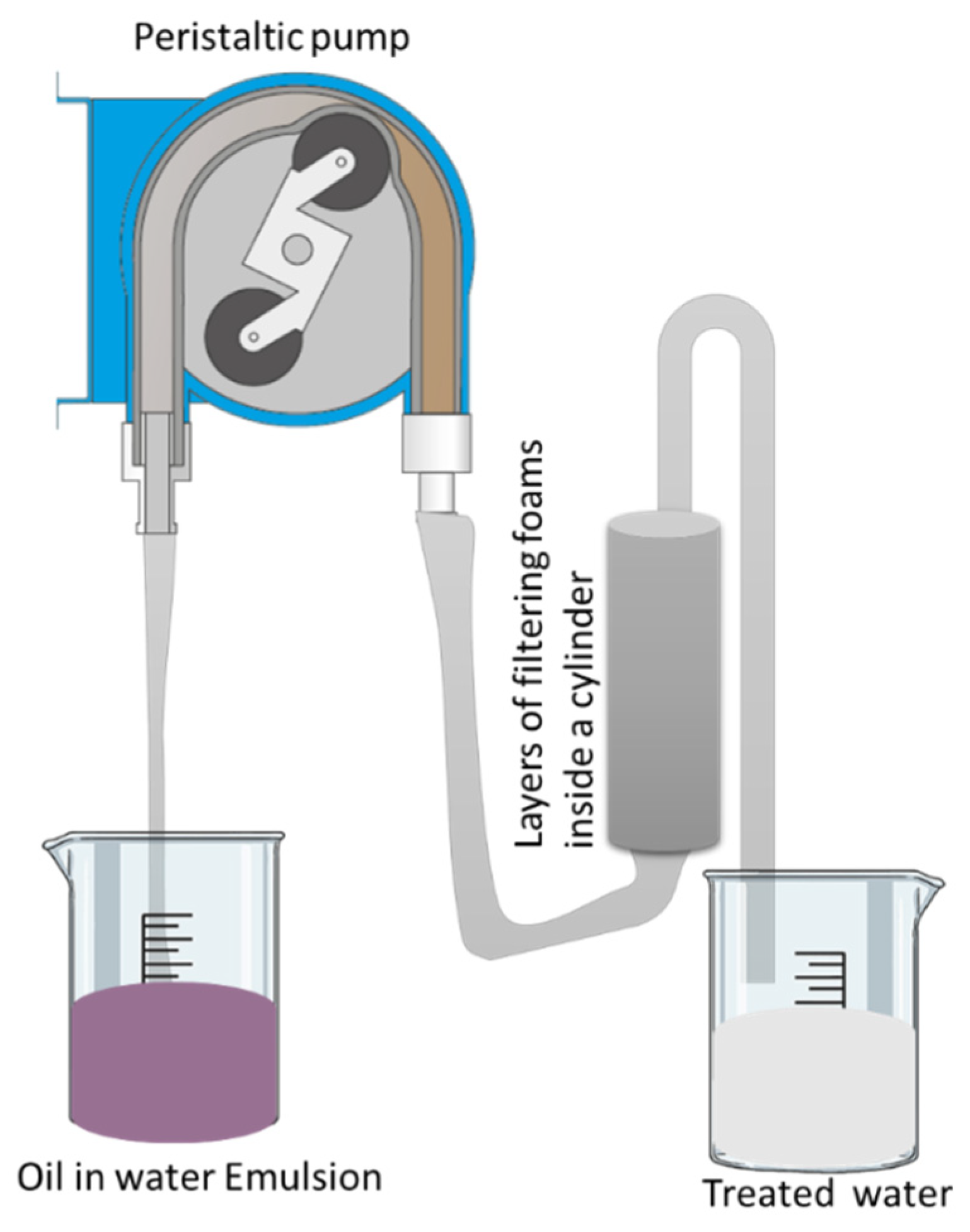


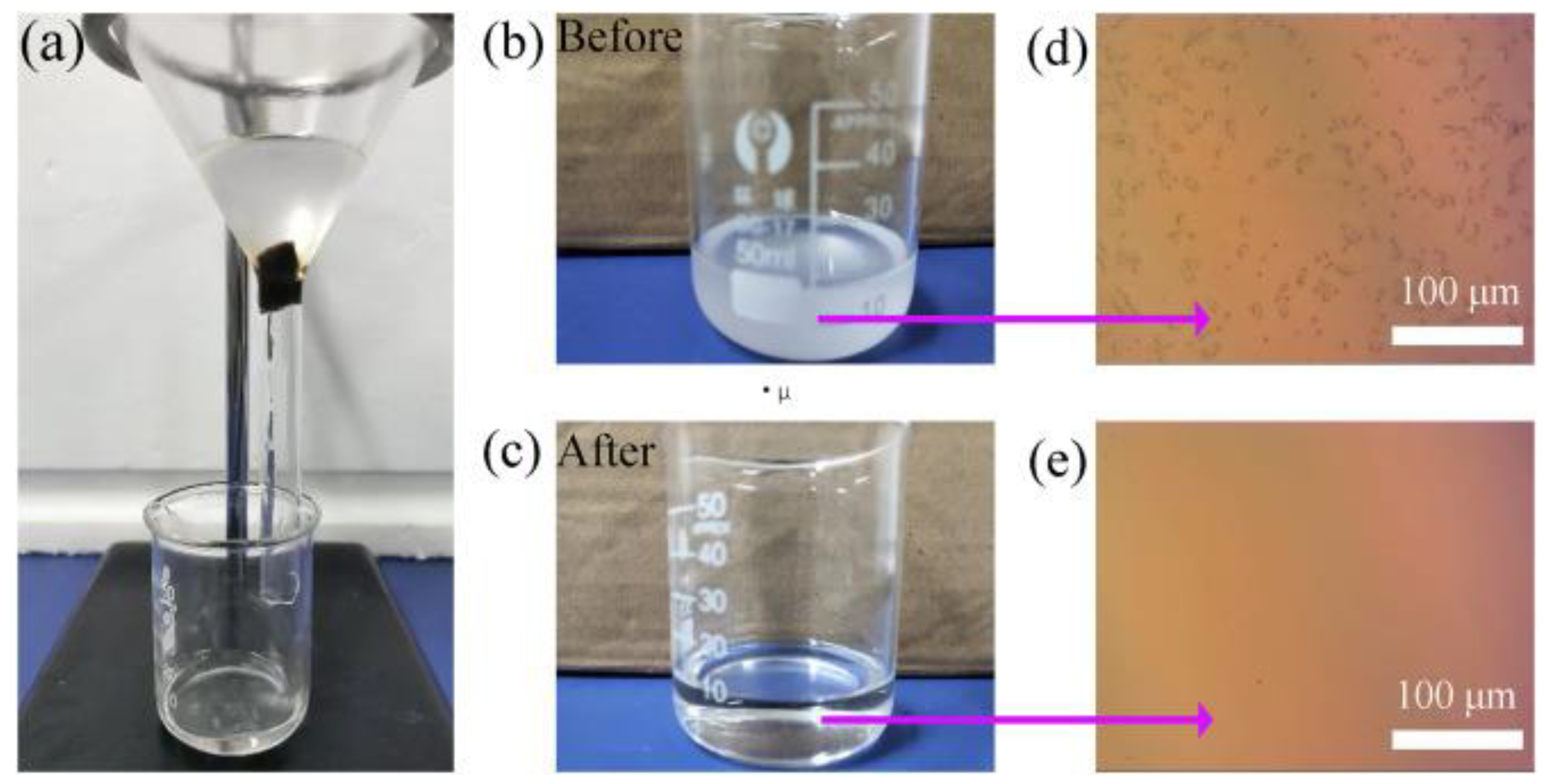
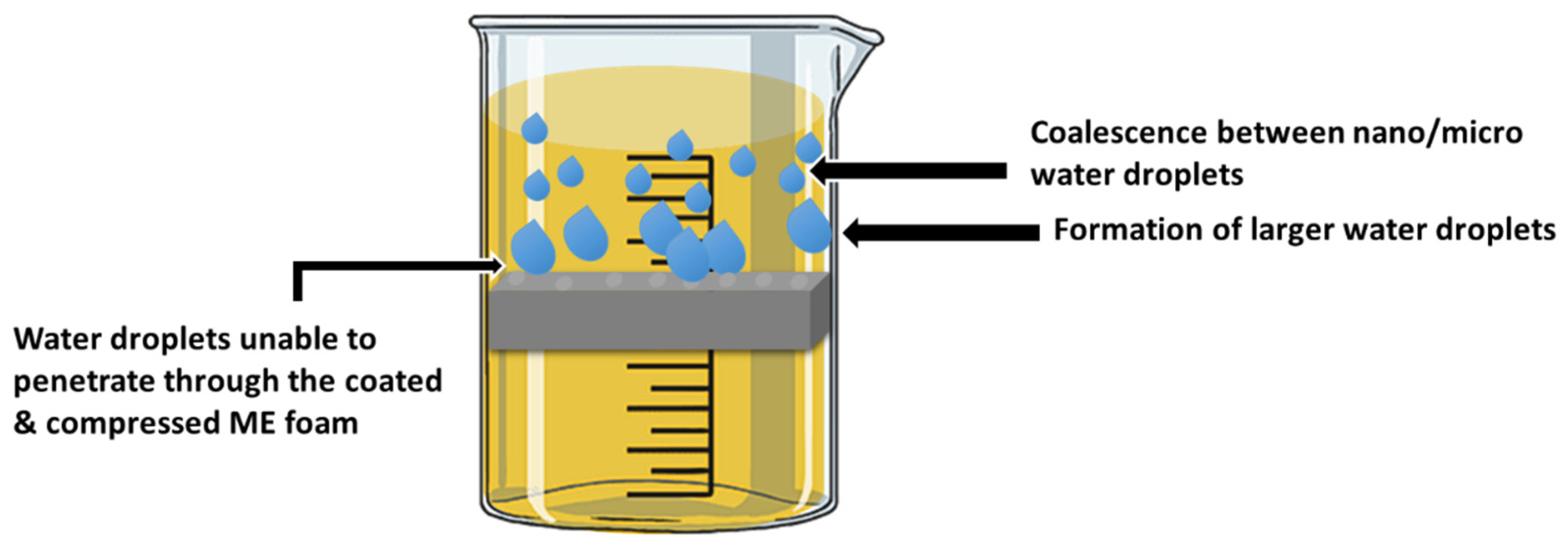

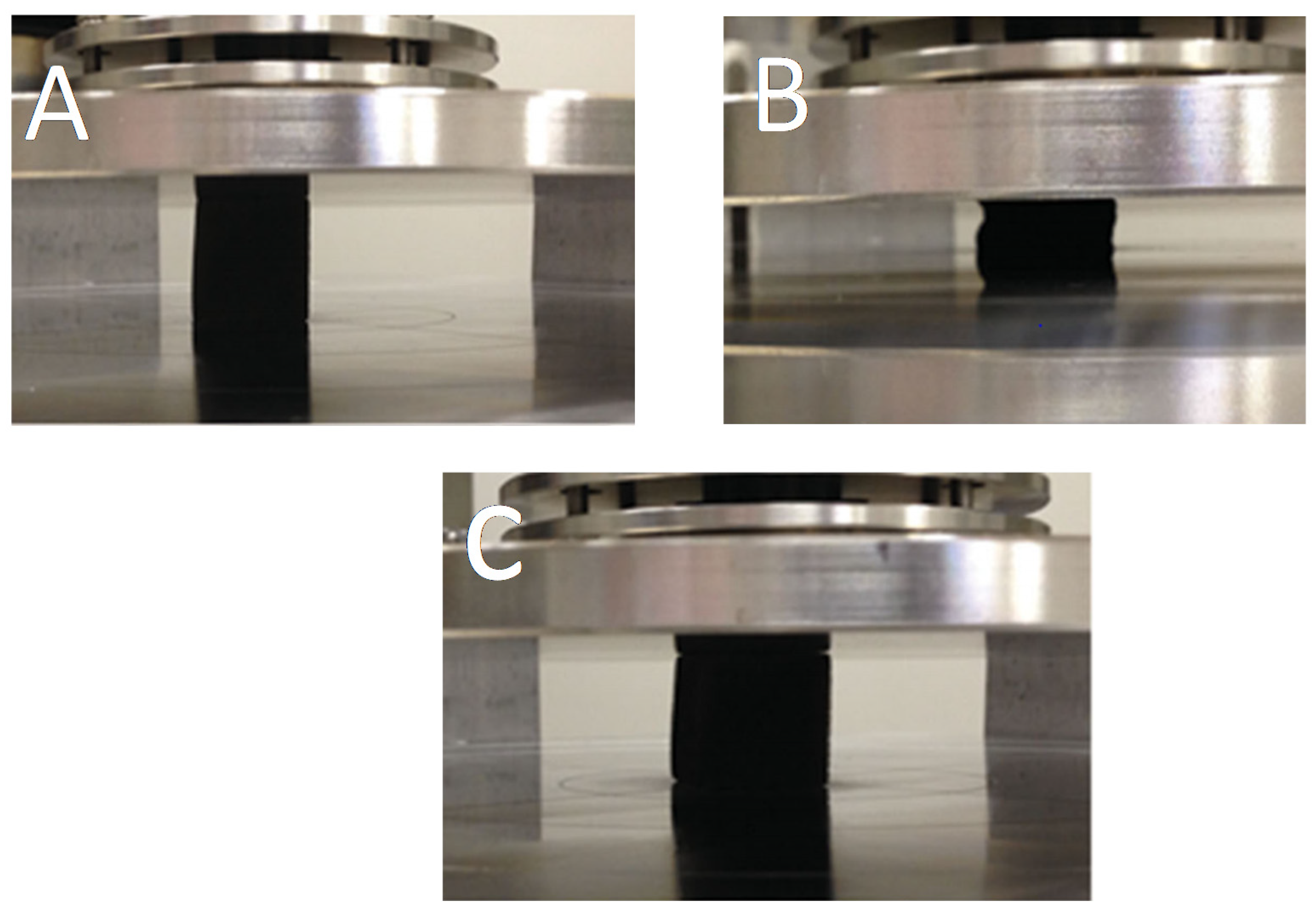

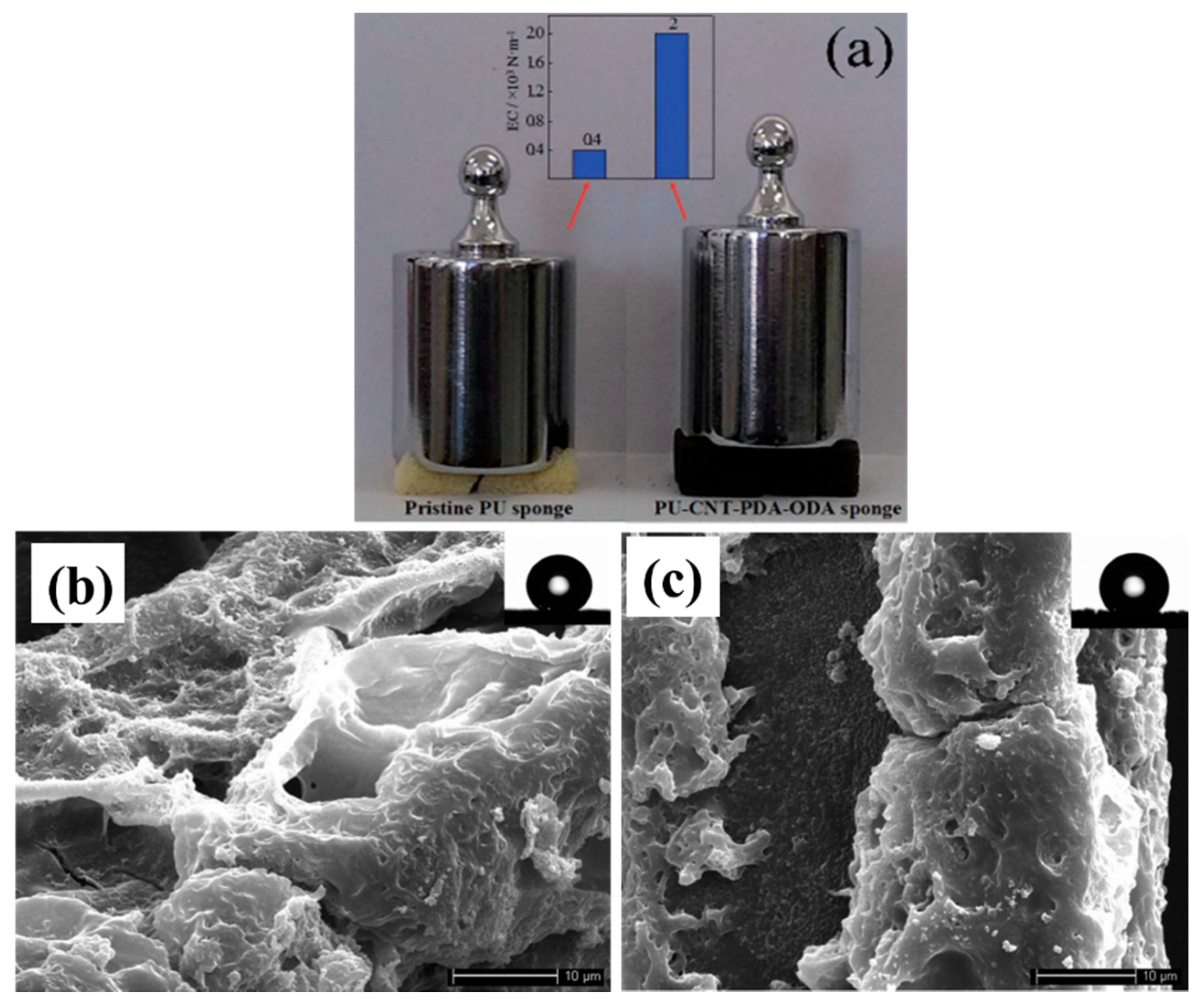
| Foam Type | Treatment | WCA | AC | Effeiciny (%) | Emulsion Type: Oil in Water (OWE) or Water in Oil (WOE) | Additional Information | Ref. |
|---|---|---|---|---|---|---|---|
| MA | Polybenzoxazine | 162° | 170 | 99.96% | Surfactant stabilized WOE | - | [89] |
| MA | rGO | 164° | 2010/5647 (mg/g) | 97 ± 6% &95 ± 3% | OWE (crude oil) | Ratio: 10,000/30,000 (mg/l) | [90] |
| MA | β-FeOOH nanoparticles | 155° | 65–136 | 99.5% | OWE (pump oil) | 1:25 (Vwater/Voil) | [91] |
| MA | caffeic acid-polyethyleneimine | 150° | - | 97% | SOWE | - | [92] |
| MA | dopamine (DA) and polyethyleneimine (PEI) | 141. 9° | 67.2–178.6 | 93.5% | OWE | - | [93] |
| MA | Mg(OH)2 | 160° | 70–190 | 99.7% | OWE (toluene) | - | [94] |
| MA | Co-ZIF-L | - | 26–61 | 97.7% | OWE (dodcane) | Ratio:1: 1000 w/w | [95] |
| MA | PDA/dodecanethiol (DDT) | 158° | 45.2–98.6 | 76.6–93.8% | OWE | - | [96] |
| MA | PVDF-HFP+Fe3O4 NPs | ~130° | 29.2–43.73 | - | WOE | Ratio: (1:10 w/w) | [97] |
| PU | Stearic acid | 151° | 17.4–41.6 | 80% | OWE (toluene) | Oil concentration of 10:1 (Vwater/Voil) | [98] |
| PU | 3,5-bis(trifluoromethyl) benzenediazonium tosylate [ADT-(CF3)2] | 168 | 40–75 | 99% | OWE | - | [85] |
| PU | 1,3-oxazolidin/1,4-dioxane, stearoyl chloride, and NaHCO3 | 152 | 23 | - | WOE (crude) | - | [99] |
| PU | carbon cloth (CC)/ZnO/SA | 160 | 40–70 | - | OWE (toluene) | - | [100] |
| PU | octadecyltrichlorosilane (OTS) | 156 | 25 | ~97.7% | WOE (toluene) | - | [101] |
| PU | PDA/Fe3O4/Ag | 156 | 22.9–52.0 | 96.4% | OWE (toluene) | - | [102] |
| PU | Graphene | 151.8 | - | 99.91% | SOWE | - | [78] |
| PU | MCFO/RGO nanocomposite | 165 | 39.8–131.4 | 99.8–99.9% | WOE+SOWE | - | [103] |
| PU | Dopamine+ fly ash, and dodecanethiol | 161 | 34–47 | 93% | OWE | - | [82] |
| PU | HDPE/Fe3O4 | 155 | 40–75 | 98.2% | WOE+SOWE (toluene) | (1:9 v:v) | [104] |
| PU | thermoplastic PU (TPU) /SiO2 | 149 | 30 | 95% | WOE | 10 to 80 v.v%. | [105] |
| MA | Carbonization followed by 1H, 1H, 2H, 2H-perfluorodecanethiol coating | 158 | 17–43 | - | OWE+SOWE (tetrachloromethane) | - | [106] |
| Foam | Treatment | Recycle (Cycles) | WCA (°) | Durability | Compressibility | Additional Information | Ref. |
|---|---|---|---|---|---|---|---|
| PU | rGO | 1000 | - | - | 1000 cycles/80% | Foam lost 10% of its absorption capacity after cycles 1000 | [58] |
| MA | PDA | 100 | 158.3 | - | 1000 cycles/60% | Foam absorb flammable oil and organic solvents | [47] |
| PU | methyltrichlorsilane/ hexane solution | 300 | <150 | Stabile at different pH and temperatures | - | - | [114] |
| MA | dopamine and 1H,1H,2H,2H-perfluorodecanethiol | 100 | 158.3 | Flame Retardancy and chemical and mechanical robustness. | 1000 cycles/60% | Tested using cyclohexane oil | [48] |
| PU | CNTs,dopamine, and octadecylamine | 150 | ~143 | The foam is stable at different pH and temperatures. | - | Oil absorption capacity Decreased after 150 cycles by 14% using lubricating oil | [115] |
| PU | chromic acid,TMCS and TEOS | 200 | - | - | 200 cycles/80% | The capacity lowers after 200 cycles | [116] |
| PU | rGO | 50 | - | - | - | Cost of rGO/PU foam ˂ Cost of graphene and carbon nanotube aerogels | [117] |
| MA | Dual Silanized SiO2 | 20 | - | - | 25–50 cycles/80% | 5% deformation for the foam. Efficiency 98.3% after 20 cycles. | [118] |
| MA | PDA and P2VP-b-PDMS- copolymer | 10 | - | Foam kept in dichloromethane for 24 h and WCA of 146°–150° | - | The foam has switchable pH hydrophobicity, and it is robust in corrosive media. | [119] |
| MA | Spiropyran methacrylate (SPMA) derivative | - | - | - | 100 cycles/50% | The wettability of the foam is light responsive. | [120] |
| MA | MoS2 | 50 | 150 | Stable at abrasion test of 20 cycles. | 20 cycles/90% | Recyclability tested using cyclohexane and silicon oil. | [121] |
| MA | PDA/PDVB | 20 | - | Immersed in ethanol for 3 days and ramined unchanged. | 250 cycles | Tested using toluene. | [122] |
| MA | PC-O/Fe3O4 | 50 | 149 | Stable at different pH. | - | 92% oil retention after 100 cycles | [123] |
| MA | diamond-like carbon/TiO2 | 35 | - | Foam is flame retardant | - | Tested using DMF. | [124] |
| MA | Kaolinite/ GO | 30 | - | Stable at different pH and temperatures. | - | 94% adsorption capacity retention for diesel oil after 30 cycles. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hailan, S.M.; Ponnamma, D.; Krupa, I. The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review. Polymers 2021, 13, 4142. https://doi.org/10.3390/polym13234142
Hailan SM, Ponnamma D, Krupa I. The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review. Polymers. 2021; 13(23):4142. https://doi.org/10.3390/polym13234142
Chicago/Turabian StyleHailan, Sarah Mohammed, Deepalekshmi Ponnamma, and Igor Krupa. 2021. "The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review" Polymers 13, no. 23: 4142. https://doi.org/10.3390/polym13234142
APA StyleHailan, S. M., Ponnamma, D., & Krupa, I. (2021). The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review. Polymers, 13(23), 4142. https://doi.org/10.3390/polym13234142






