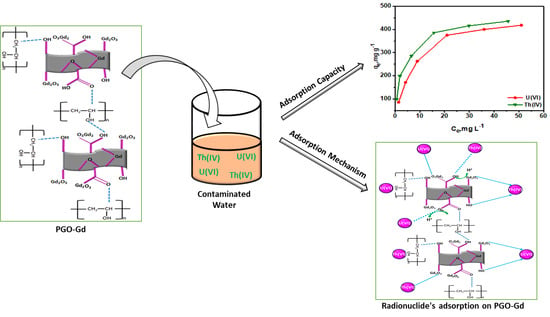
Polyvinyl Alcohol Polymer Functionalized Graphene Oxide Decorated with Gadolinium Oxide for Sequestration of Radionuclides from Aqueous Medium: Characterization, Mechanism, and Environmental Feasibility Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. PGO–Gd Preparation
2.2. Removal Studies
2.3. PGO–Gd Regeneration and Application to Surface Water Samples
3. Results and Discussions
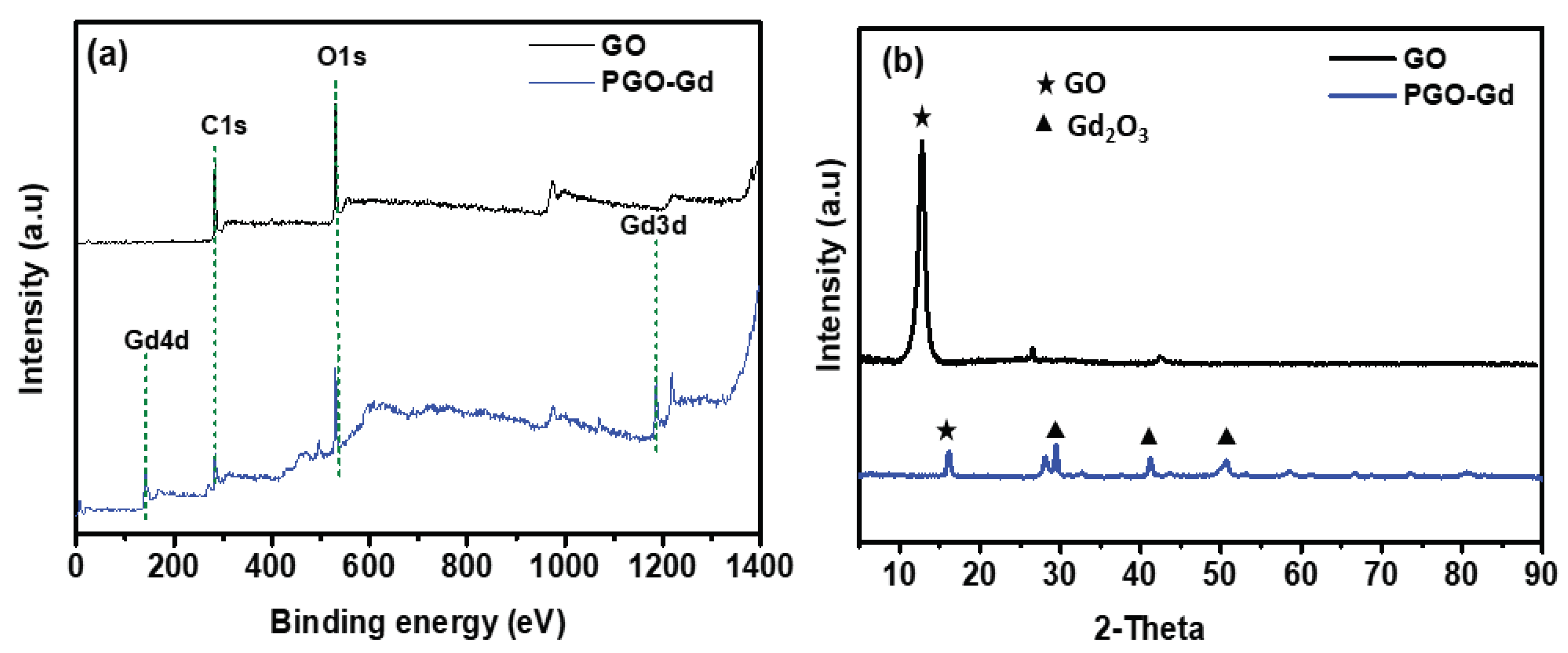
3.1. PGO–Gd Structural Chracterization
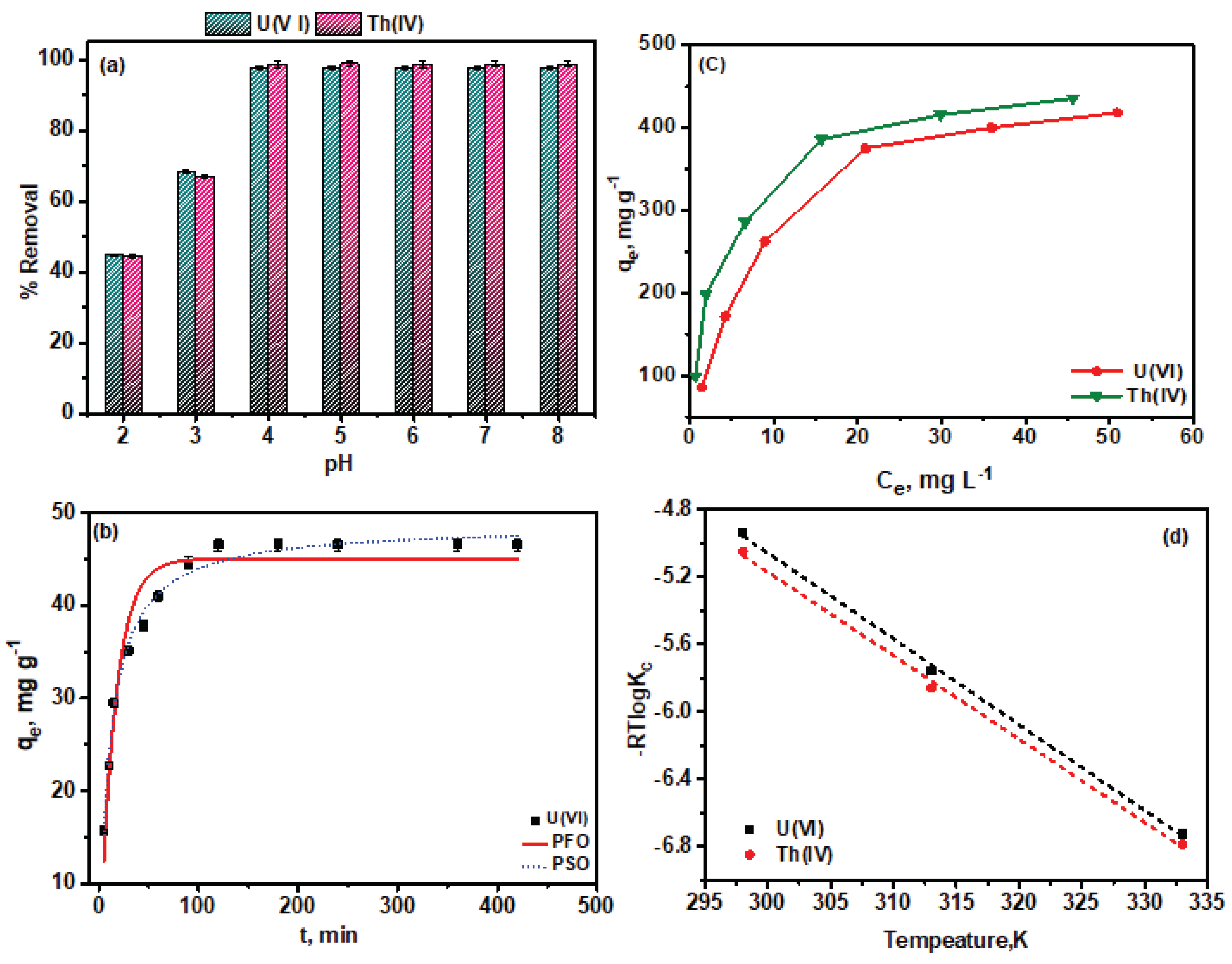
3.2. pH Impact on Adsorption
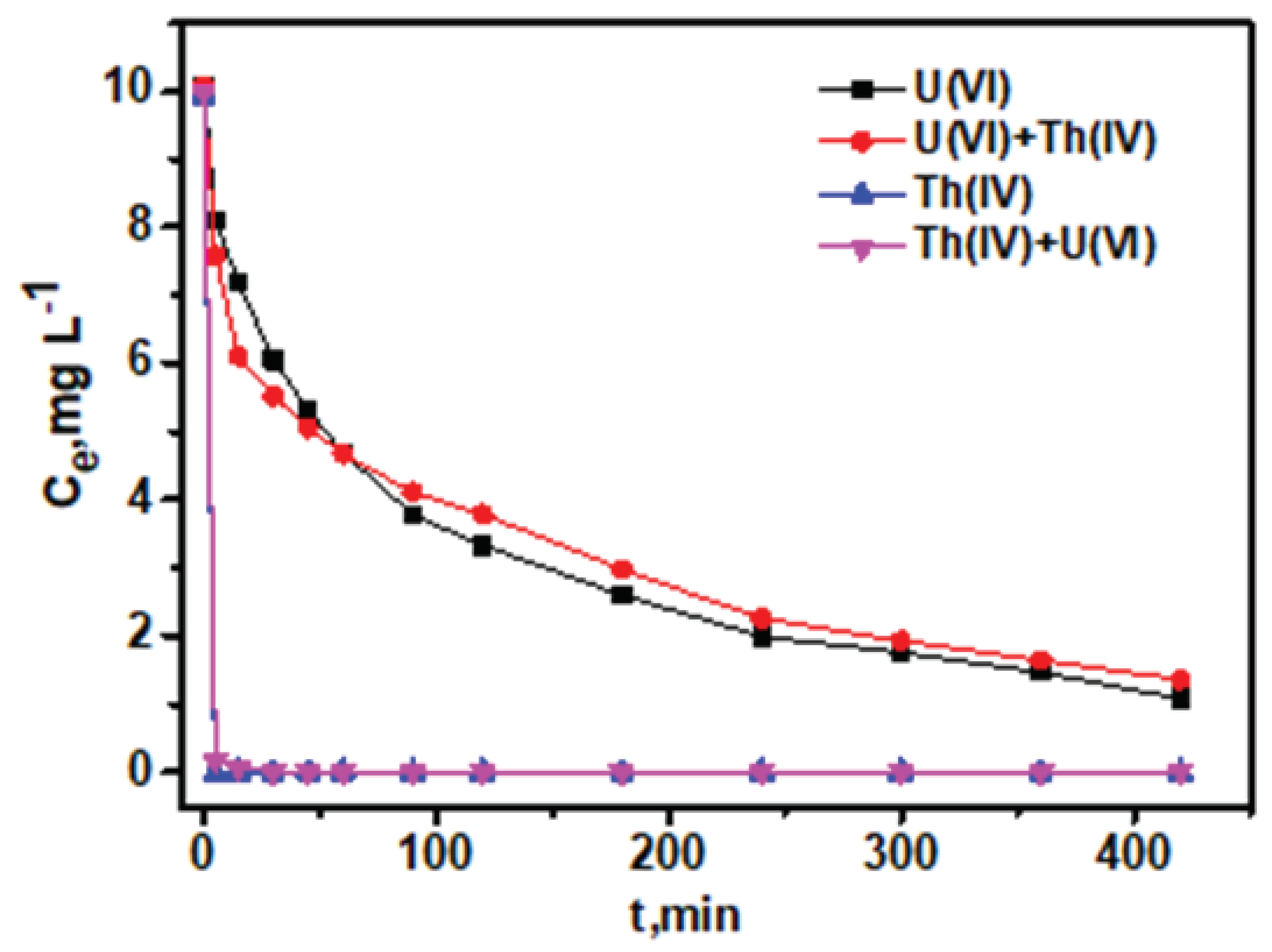
3.3. Kinetics of Adsorption
3.4. Equilibrium Isotherms
3.5. Comparison of Adsorption Capacities and Cost
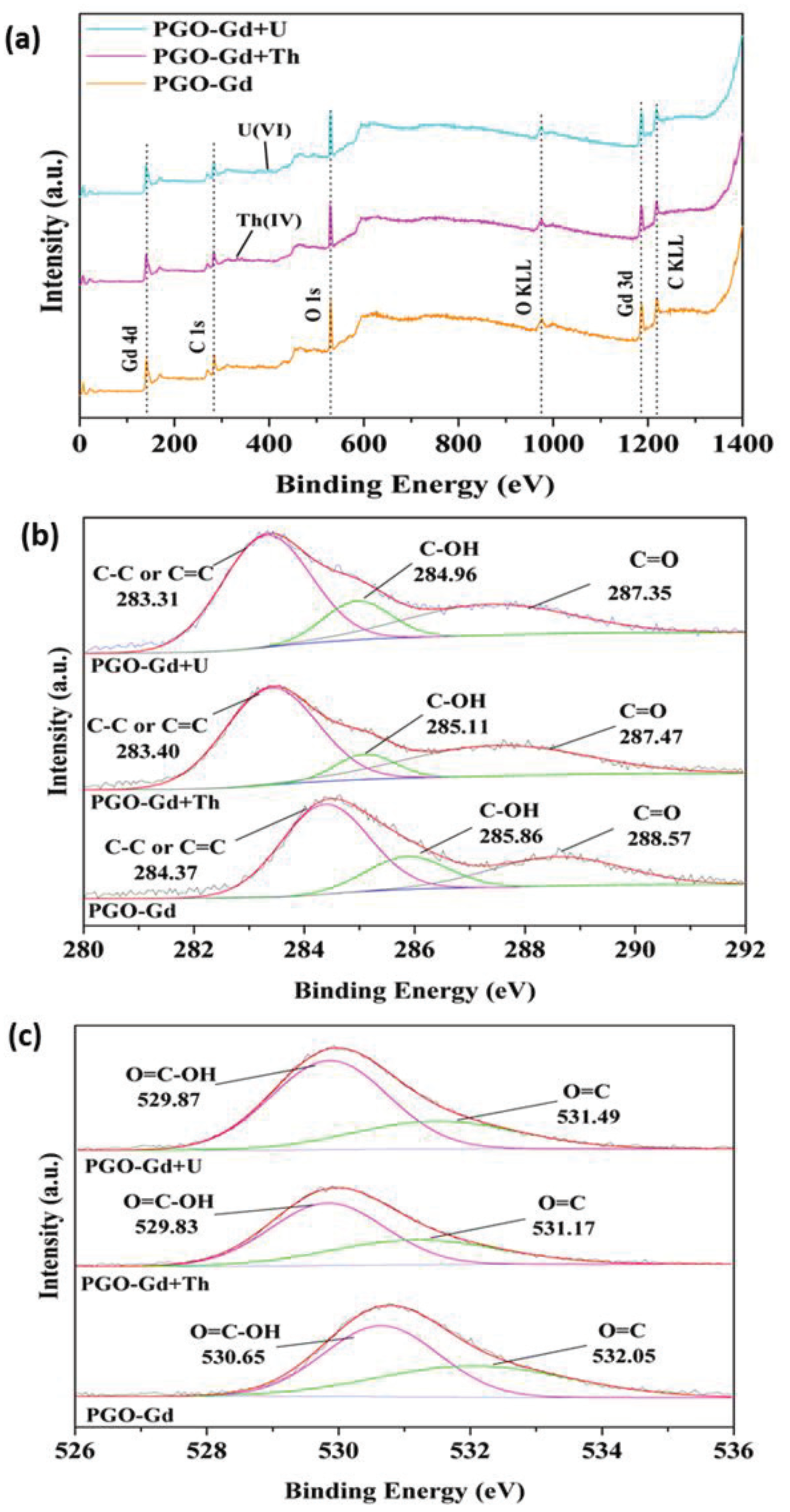
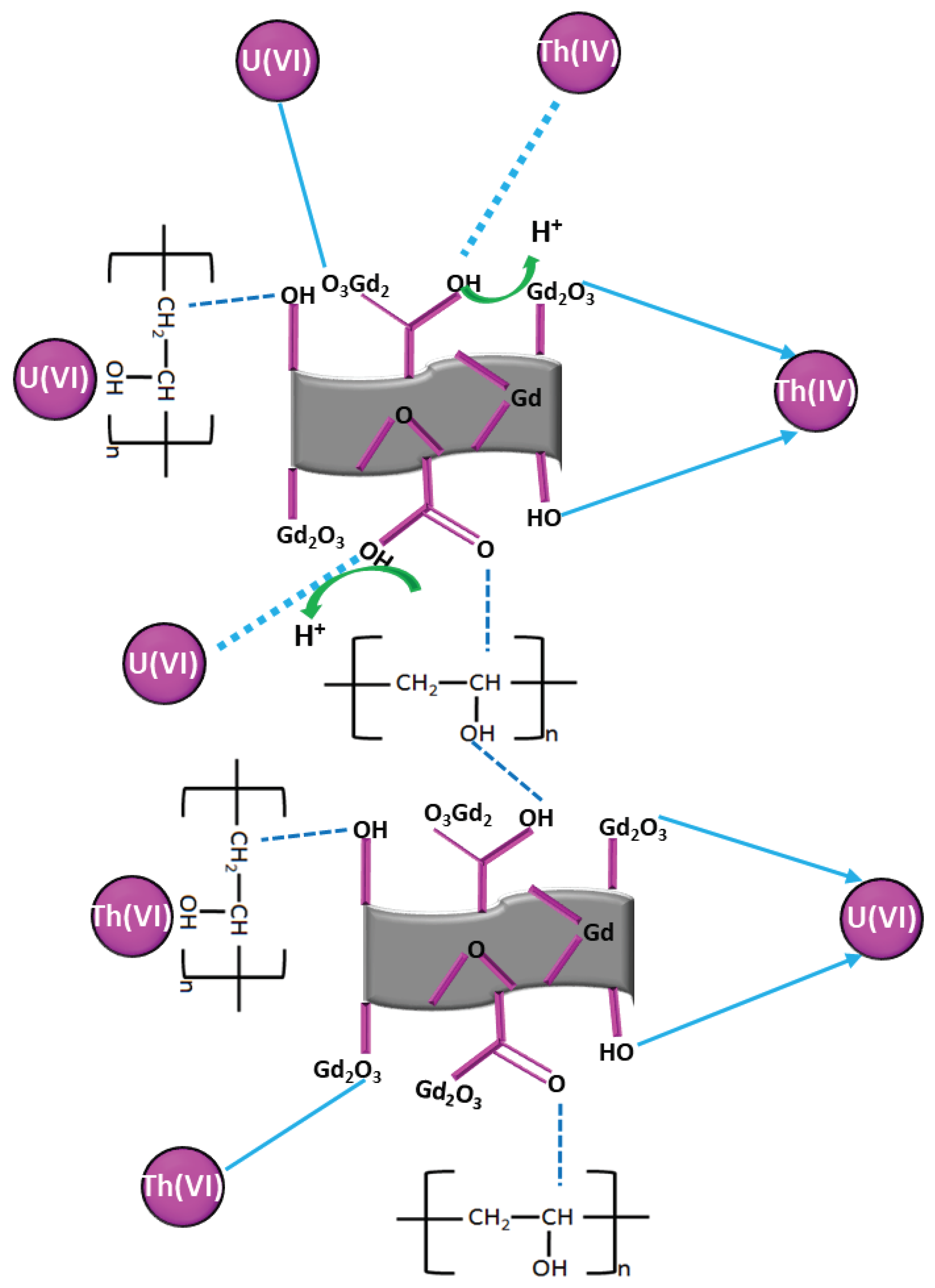
3.6. Adsorption Mechanism
3.7. Studying Environmental Relevance by Treating Metal Spiked Real Surface Water
4. Conclusions
- (1)
- Th(IV) and U(VI) absorption was comparably high in PGO–Gd, although Th(IV) adsorption was somewhat greater than U(VI) (VI).
- (2)
- The effect of varied pH values on metal intake revealed that increasing the pH enhanced metal ion absorption by PGO–Gd, reaching a maximum at pH 4.0. This finding also suggests that the surface charge and metal ion species have a significant impact on the adsorbent’s sorption capacity.
- (3)
- The qmax of PGO–Gd for U(VI) and Th(IV) was comparable and higher than those of the other absorbents.
- (4)
- The adsorption process is endothermic and thermodynamically favorable.
- (5)
- The PSO kinetic model and the Langmuir isotherm accurately explain the sequestration of U(VI) and Th(IV) by PGO–Gd, suggesting that the rate-limiting monolayer sorption process happened on the PGO–Gd homogeneous surface.
- (6)
- The characterization and adsorption studies concluded that the ions were adsorbed predominantly by surface complexation along with electrostatic interactions through adsorbent surface functionality.
- (7)
- The adsorbent can be reused up to four times without losing its original efficacy or stability. Hence, the use of PGO–Gd to remove radioactive waste from surface water is strongly recommended in this study.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkaya, R. Uranium and thorium adsorption from aqueous solution using a novel polyhydroxyethylmethacrylate-pumice composite. J. Environ. Radioact. 2013, 120, 58–63. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Zhao, W.; Xu, J. Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J. Mol. Liq. 2013, 188, 178–185. [Google Scholar] [CrossRef]
- Misaelides, P.; Godelitsas, A.; Filippidis, A.; Charistos, D.; Anousis, I. Thorium and uranium uptake by natural zeolitic materials. Sci. Total Environ. 1995, 173, 237–246. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J. Hazard. Mat. 2017, 326, 145–156. [Google Scholar] [CrossRef]
- Alam, A.; Moussa, M. Preparation of graphene/poly(vinyl alcohol) composite hydrogel films with enhanced electrical and mechanical properties. Polym. Compos. 2020, 41, 809–816. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Studies on removal of Pb(II) and Cr(III) using graphene oxide based inverse spinel nickel ferrite nano-composite as sorbent. Hydrometallurgy 2016, 165, 64–72. [Google Scholar] [CrossRef]
- Rouholah, Z.D.; Somayeh, M.F.; Ahmad, B.; Azadeh, T. Highly efficient simultaneous ultrasonic-assisted adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) ions from aqueous solutions by graphene oxide modified with 2,2′-dipyridylamine: Central composite design optimization. Ultrason. Sonochem. 2016, 32, 265–276. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Chang, Y.Y.; Koduru, J.R.; Yang, J.K. Porous graphene oxide based inverse spinel nickel ferrite nanocomposites for the enhanced adsorption removal of arsenic. RSC Adv. 2016, 6, 73776–73789. [Google Scholar] [CrossRef]
- Balasubramani, K.; Sivarajasekar, N.; Naushad, M. Effective adsorption of antidiabetic pharmaceutical (metformin) from aqueous medium using graphene oxide nanoparticles: Equilibrium and statistical modelling. J. Mol. Liq. 2020, 301, 112426. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide-based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Tran, T.Q.; Baskar, C.; Thomas, S.W.; Ramakrishna, S. Nanocomposites for electronic applications that can be embedded for textiles and wearables. Sci. China Tech. Sci. 2019, 62, 895–902. [Google Scholar] [CrossRef]
- Tran, T.Q.; Lee, J.K.Y.; Chinnappan, A.; Loc, N.H.; Tran, L.T.; Ji, D.; Jayathilaka, W.A.D.M.; Kumar, V.V.; Ramakrishna, S. High-performance carbon fiber/gold/copper composite wires for lightweight electrical cables. J. Mater. Sci. Technol. 2020, 42, 46–53. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; Fernández, M.J.; Fernández, M.D. Graphene based poly(vinyl alcohol) nanocomposites prepared by in situ green reduction of graphene oxide by ascorbic acid: Influence of graphene content and glycerol plasticizer on properties. Nanomaterials 2018, 8, 1013. [Google Scholar] [CrossRef] [Green Version]
- Finch, C.A. (Ed.) Poly(Vinyl Alcohol): Properties and Applications; John Wiley & Sons: London, UK, 1973; ISBN 047125892X 9780471258926. [Google Scholar]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly(vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Modarress, H. Viscometric study of aqueous poly(vinyl alcohol) (PVA) solutions as a binder in adhesive formulations. J. Adhes. Sci. Technol. 2006, 20, 1273–1280. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Musetti, A.; Paderni, K.; Fabbri, P.; Pulvirenti, A.; Al-Moghazy, M.; Fava, P. Poly(vinyl alcohol)-based film potentially suitable for antimicrobial packaging applications. J. Food Sci. 2014, 79, E577–E582. [Google Scholar] [CrossRef]
- Hyon, S.H.; Cha, W.I.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.D.; Ren, P.G.; Chen, J.; Zhang, W.Q.; Ji, X.; Li, Z.M. High barrier graphene oxide nanosheet/poly(vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409, 156–163. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Sadasivuni, K.K.; Ponnamma, D.L.; Chidambaram, K. Synergistic effect of vanadium pentoxide and graphene oxide in polyvinyl alcohol for energy storage application. Eur. Polym. J. 2016, 76, 14–27. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, J.; Lv, W.; Song, Y.; Zheng, Q. Multifunctional graphene/poly(vinyl alcohol) aerogels: In situ hydrothermal preparation and applications in broad-spectrum adsorption for dyes and oils. Carbon 2017, 123, 354–363. [Google Scholar] [CrossRef]
- Dai, J.; Huang, T.; Tian, S.Q.; Xiao, Y.J.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhou, Z. High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for wastewater treatment. Mater. Des. 2016, 107, 187–197. [Google Scholar] [CrossRef]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadeler, F.J. Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Cat. Today 2019, 335, 437–451. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.Y.; Kang, S.; Yang, J.K. Facile synthesis of flowered mesoporous graphene oxide–lanthanum fluoride nanocomposite for adsorptive removal of arsenic. J. Mol. Liq. 2019, 279, 32–42. [Google Scholar] [CrossRef]
- Wu, J.; Fujii, K.; Yashima, M.; Staykov, A.; Akbay, T.; Ishihara, T.; Kilner, J.A. A systematic evaluation of the role of lanthanide elements in functional complex oxides; implications for energy conversion devices. J. Mater. Chem. A 2018, 6, 11819–11829. [Google Scholar] [CrossRef] [Green Version]
- Boopathi, G.; Karthikeyan, G.G.; Jaimohan, S.M.; Pandurangan, A.; de Barros, A.L.F.; de Barros, A.L. Dopant effects of Gd3+ on the electrochemical pseudocapacitive characteristics of electroactive mesoporous NiO electrodes for supercapacitors. J. Phy. Chem. C 2018, 122, 9257–9274. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.M.; Natarajan, K.; Karuppiah, C.; Yang, C.C.; Muthuraj, V. 3D flower-like gadolinium molybdate catalyst for efficient detection and degradation of organophosphate pesticide (fenitrothion). ACS Appl. Mater. Interfaces 2018, 10, 15652–15664. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mbianda, X.Y.; Mishra, A.K.; Mishra, A. Gadolinium nanoparticle-decorated multiwalled carbon nanotube/titania nanocomposites for degradation of methylene blue in water under simulated Solar Light. Environ. Sci. Pollu. Res. 2014, 21, 5597–5609. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.S.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K.; Karri, R.R.; Koduru, J.R. Process modeling and optimization of an iron oxide immobilized graphene oxide gadolinium nanocomposite for arsenic adsorption. J. Mol. Liq. 2020, 299, 112261. [Google Scholar] [CrossRef]
- Lee, S.H.; Lingamdinne, L.P.; Yang, J.K.; Chang, Y.Y.; Koduru, J.R. Potential electromagnetic column treatment of heavy metal contaminated water using porous Gd2O3-doped graphene oxide nanocomposite: Characterization and surface interaction mechanisms. J. Water Process Eng. 2021, 41, 102083. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Roh, H.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Adsorption removal of Co(II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar] [CrossRef]
- Prasad, C.V.; Reddy, M.S.P.; Rajagopal Reddy, V.R.; Park, C. Effect of annealing on chemical, structural and electrical properties of Au/Gd2O3/n-GaN heterostructure with a high-k rare-earth oxide interlayer. Appl. Surf. Sci. 2018, 427, 670–677. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Lee, S.; Choi, J.S.; Lebaka, V.R.; Durbaka, V.R.P.; Koduru, J.R. Potential of the magnetic hollow sphere nanocomposite (graphene oxide-gadolinium oxide) for arsenic removal from real field water and antimicrobial applications. J. Hazard. Mater. 2021, 402, 123882. [Google Scholar] [CrossRef]
- Qian, B.; Zou, H.; Meng, D.; Zhou, X.; Song, Y.; Zheng, K.; Miao, C.; Sheng, Y. Columnar Gd2O3: Eu3+/Tb3+ phosphors: Preparation, luminescence properties and growth mechanism. CrystEngComm 2018, 20, 7322–7328. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Zhang, D.; Yang, S. Structural, luminescence and magnetic properties of Yb3+-Er3+ codoped Gd2O3 hierarchical architectures. CrystEngComm 2015, 17, 1106–1114. [Google Scholar] [CrossRef]
- Veerapandian, M.; Neethirajan, S. Graphene oxide chemically decorated with Ag–Ru/chitosan nanoparticles: Fabrication, electrode processing and immunosensing properties. RSC Adv. 2015, 5, 75015–75024. [Google Scholar] [CrossRef]
- Ruyter-Hooley, M.; Larsson, A.C.; Johnson, B.B.; Antzutkin, O.N.; Angove, M.J. Surface complexation modeling of inositol hexaphosphate sorption onto gibbsite. J. Colloid Interface Sci. 2015, 440, 282–291. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.S.; Angaru, G.K.R.; Karri, R.R.; Yang, J.K.; Chang, Y.Y.; Koduru, J.R. Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 2021, 286, 131776. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Choi, J.S.; Lingamdinne, L.P.; Chang, Y.Y.; Koduru, J.R.; Ha, J.H.; Yang, J.K. Removal of U (VI) by sugar-based magnetic pseudo–graphene oxide and its application to authentic groundwater using electromagnetic system. Environ. Sci. Pollut. Res. 2019, 26, 22323–22337. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Shelar-Lohar, G.; Joshi, S. Comparative study of uranium and thorium metal ion adsorption by gum ghatti grafted poly (acrylamide) copolymer composites. RSC Adv. 2019, 9, 41326–41335. [Google Scholar] [CrossRef] [Green Version]
- Mirzabe, G.H.; Keshtkar, A.R. Application of response surface methodology for thorium adsorption on PVA/Fe3O4/SiO2/APTES nanohybrid adsorbent. J. Ind. Eng. Chem. 2015, 26, 277–285. [Google Scholar] [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium (VI) and thorium (IV) removal from aqueous solution. Chem. Eng. J. 2013, 220, 161–171. [Google Scholar] [CrossRef]


| PFO | PSO | qe,exp., mg g−1 | ||||
|---|---|---|---|---|---|---|
| qe,model, mg g−1 | K1 | R2 | qe,model, mg g−1 | K2 | R2 | |
| 38.56 | 0.063 | 0.937 | 44.46 | 0.0019 | 0.991 | 43.25 |
| Temperature, K | Metal Ion | Langmuir | Frendlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax,, mg g−1 | KL, L mg−1 | R2 | Kf, mg g−1(L mg−1)1/n | n | R2 | B, g L−1 | Kt, L mg−1 | R2 | ||
| 298 | U(VI) | 427.50 | 0.22 | 0.999 | 132.56 | 3.02 | 0.902 | 188.11 | 5.26 | 0.955 |
| Th(IV) | 455.00 | 0.24 | 0.998 | 154.25 | 3.25 | 0.896 | 219.04 | 1.77 | 0.971 | |
| 313 | U(VI) | 465.23 | 0.35 | 0.999 | 141.56 | 2.89 | 0.845 | 199.45 | 4.49 | 0.925 |
| Th(IV) | 469.67 | 0.38 | 0.997 | 163.25 | 3.12 | 0.912 | 259.58 | 2.18 | 0.976 | |
| 333 | U(VI) | 479.20 | 0.56 | 0.997 | 150.23 | 2.75 | 0.897 | 205.27 | 5.04 | 0.964 |
| Th(IV) | 487.56 | 0.49 | 0.995 | 174.36 | 2.95 | 0.876 | 287.80 | 3.76 | 0.965 | |
| Absorbent | Experimental Conditions | qmax mg g−1 | Ref. | |
|---|---|---|---|---|
| Initial Con mg L−1, Dosage mg L−1 and pH | U(VI) | Th(IV) | ||
| Reduced graphene oxide based inverse spinel nickel ferrite (rGONF) | 2–30, 0.3 and 3.5 | 200 | 126.58 | [4] |
| Magnetized watermelon rind biochar (MWBC) | 10–200, 0.2 and 4 | 233.56 | - | [42] |
| Sugar-based magnetic graphene oxide (SMGO) | 2–30, 1.0 and 4 | 28.2 | - | [43] |
| Three-dimensional layered double hydroxide/graphene hybrid material | 20–130, 0.01 and 4 | 277.8 | - | [44] |
| Gum-g-poly(AAm) composite | 25–1000, 0.05 and 6 | 367.65 | 125.95 | [45] |
| PVA/Fe3O4/SiO2/APTES nanohybrid | 30–500, 1.0 and 5 | - | 112.4 | [46] |
| PVA/TiO2/TMPTMS nanofiber | 30–500, 1.0 and 5 | 187.6 | 222.2 | [47] |
| PGO–Gd | 10–100, 0.1 and 4 | 427.50 | 455.0 | This work |
| PGO | 10–100, 0.1 and 4 | 105.65 | 125.00 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.-Y.; Naushad, M.; Yang, J.-K. Polyvinyl Alcohol Polymer Functionalized Graphene Oxide Decorated with Gadolinium Oxide for Sequestration of Radionuclides from Aqueous Medium: Characterization, Mechanism, and Environmental Feasibility Studies. Polymers 2021, 13, 3835. https://doi.org/10.3390/polym13213835
Lingamdinne LP, Koduru JR, Chang Y-Y, Naushad M, Yang J-K. Polyvinyl Alcohol Polymer Functionalized Graphene Oxide Decorated with Gadolinium Oxide for Sequestration of Radionuclides from Aqueous Medium: Characterization, Mechanism, and Environmental Feasibility Studies. Polymers. 2021; 13(21):3835. https://doi.org/10.3390/polym13213835
Chicago/Turabian StyleLingamdinne, Lakshmi Prasanna, Janardhan Reddy Koduru, Yoon-Young Chang, Mu. Naushad, and Jae-Kyu Yang. 2021. "Polyvinyl Alcohol Polymer Functionalized Graphene Oxide Decorated with Gadolinium Oxide for Sequestration of Radionuclides from Aqueous Medium: Characterization, Mechanism, and Environmental Feasibility Studies" Polymers 13, no. 21: 3835. https://doi.org/10.3390/polym13213835
APA StyleLingamdinne, L. P., Koduru, J. R., Chang, Y.-Y., Naushad, M., & Yang, J.-K. (2021). Polyvinyl Alcohol Polymer Functionalized Graphene Oxide Decorated with Gadolinium Oxide for Sequestration of Radionuclides from Aqueous Medium: Characterization, Mechanism, and Environmental Feasibility Studies. Polymers, 13(21), 3835. https://doi.org/10.3390/polym13213835