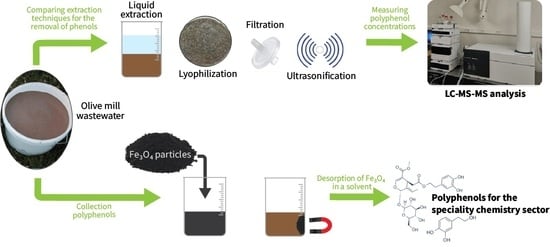
The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector
Abstract
:1. Introduction
2. Results
2.1. Identification of the Polyphenol Content in OMWW from Slovenian Istria
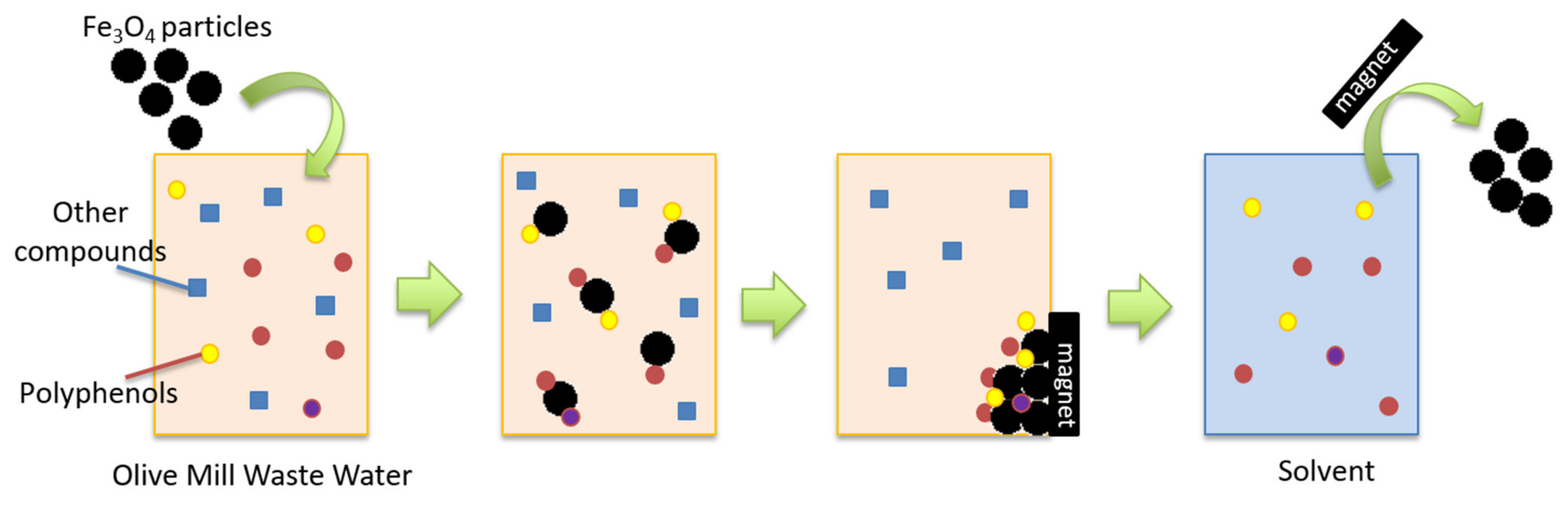
2.2. Removal of Polyphenolic Compounds from OMWW by Fe3O4 Particles
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Sample Collection
4.3. Extraction Methods to Determine the Polyphenol Content in OMWW
- 20 mL of OMWW was freeze-dried. The residue was shaken (20 min, 200 rpm) using 20 mL MeOH. To remove particles, the MeOH extract was filtered through a 0.2 pore size filter before measurement.
- 20 mL of OMWW was freeze-dried. The residue was shaken (20 min, 200 rpm) using 20 mL of water:MeOH (1:1) mixture. To remove particles, the MeOH extract was filtered through a 0.2 pore size filter before measurement.
- 20 mL of OMWW was freeze-dried. The residue was sonicated for 15 min using 20 mL MeOH. To remove particles, the MeOH extract was filtered through a 0.2 pore size filter before measurement.
- 20 mL OMWW was defatted with hexane (1:1, v/v). The 2 layers were separated by centrifugation (4000 rpm, 15min) and the hexane layer was removed. Phenolic compounds in the OMWW were three times extracted using a liquid–liquid extraction method by adding ethyl acetate (1:1, v/v) to the OMWW. The mixture was shaken for 20 min at 200 rpm. Layers were separated by 10 min of centrifugation at 4000 rpm and the ethyl acetate extracts were collected. Ethyl acetate was removed by vacuum evaporation at 40 °C and the oily residue was dissolved in 10 mL of MeOH before measurement [29].
- The pH of 20 mL OMWW was adjusted to pH 2 using HCl (2 M). OMWW was defatted with hexane (1:1, v/v). The 2 layers were separated by centrifugation (4000 rpm, 15min) and the hexane layer was removed. Phenolic compounds in the OMWW were three times extracted using a liquid–liquid extraction method by adding ethyl acetate (1:1, v/v) to the OMWW. The mixture was shaken for 20 min at 200 rpm. Layers were separated by 10 min of centrifugation at 4000 rpm and the ethyl acetate extracts were collected. Ethyl acetate was removed by vacuum evaporation at 40 °C and the oily residue was dissolved in 10 mL of MeOH before measurement [12].
- Filtration through paper filters; dissolving the obtained residue in methanol and filtering it through 0.2 µm pore size filters. Sum the polyphenol concentration found in the filtrate and the MeOH fraction.
- Filtration through 0.2 µm pore size filters
- The pH of 20 mL of OMWW was adjusted to pH 2 using HCl (2 M), raised to pH 8 using NaOH (2 M) or remained at its original pH (pH 5).
- The pH of 20 mL of OMWW was adjusted to pH 2 using HCl (2 M), raised to pH 8 using NaOH (2 M) or remained at its original pH (pH 5). OMWW was sonicated for 5, 20 and 40 min.
4.4. LC-MS/MS Analysis
4.5. OMWW Treatment with Fe3O4 Particles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Burgos, E.; Pilar Gómez-Serranillos, M. Effect of phenolic compounds on human health. Nutrients 2021, 13, 3922. [Google Scholar] [CrossRef]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Politi, M.; Foteinis, S.; Chatzisymeon, E.; Mantzavinos, D. Recovery of antioxidants from olive mill waste waters: A viable solution that promotes their overall sustainable management. J. Environ. Manag. 2013, 128, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Azaizeh, H.; Halahlih, F.; Najami, N.; Brunner, D.; Faulstich, M.; Tafesh, A. Antioxidant activity of phenolic fractions in olive mill wastewater. Food Chem. 2012, 134, 2226–2234. [Google Scholar] [CrossRef]
- Guerra, R. Ecotoxicological and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere 2001, 44, 1737–1747. [Google Scholar] [CrossRef]
- EL Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, H.; Serbaji, M.M.; Karray, B.; Medhioub, K. Olive mill waste water management study by using principal component analysis. Int. J. Geosci. 2013, 4, 29413. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Diaz-Rubio, E.M.; Saura-Calixto, F. Non-extractable polyphenols in plant foods: Nature, isolation, and analysis. In Polyphenols in Plants. Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press-Elsevier: San Diego, CA, USA, 2014; pp. 203–218. [Google Scholar]
- Vavouraki, A. Removal of polyphenols from olive mill wastewater by FPX 66 resin: Part II. Adsorption kinetics and equilibrium studies. Int. J. Waste Resour. 2020, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Papaoikonomou, L.; Labanaris, K.; Kaderides, K.; Goula, A.M. Adsorption–desorption of phenolic compounds from olive mill wastewater using a novel low-cost biosorbent. Environ. Sci. Pollut. Res. 2021, 28, 24230–24244. [Google Scholar] [CrossRef]
- Annab, H.; Fiol, N.; Villaescusa, I.; Essamri, A. A proposal for the sustainable treatment and valorisation of olive mill wastes. J. Environ. Chem. Eng. 2019, 7, 102803. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martínez-Férez, A. About the recovery of the phenolic fraction from olive mill wastewater by micro and ultracentrifugation membranes. Chem. Eng. Trans. 2017, 60, 271–276. [Google Scholar]
- Garcia-Castello, E.M.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Jahangiri, M.; Rahimpour, A.; Nemati, S.; Alimohammady, M. Recovery of polyphenols from olive mill wastewater by nano-filtration. Cellul. Chem. Technol. 2016, 50, 961–966. [Google Scholar]
- Mudimu, O.A.; Peters, M.; Brauner, F.; Braun, G. Overview of membrane processes for the recovery of polyphenols from olive mill wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar]
- Boudissa, F.; Kadi, H. Transfer of phenolic compounds from olive mill wastewater to olive cake oil. J. Am. Oil Chem. Soc. 2013, 90, 717–723. [Google Scholar] [CrossRef]
- Dammak, I.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Recovery of polyphenols from olive mill wastewater using drowning-out crystallization based separation process. Innov. Food Sci. Emerg. Technol. 2016, 34, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Yahiaouia, N.; Kadia, H.; Moussaouia, R.; Sebaouia, O.; Fiallo, M. Treatment and valorization of olive mill wastewater by hydroxyapatite co-precipitation using experimental design. Desalin. Water Treat. 2020, 195, 232–239. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Allouche, N.; Fki, A.I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive mill waste extracts: Polyphenols content, antioxidant, and antimicrobial activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Al-Natsheh, M.S.; Yaghmoor, R.; Al-Rimawi, F. Enrichment of phenolic compounds from olive mill wastewater and in vitro evaluation of their antimicrobial activities. Evid. Based Complement. Altern. Med. 2017, 2017, 3706915. [Google Scholar] [CrossRef] [Green Version]
- De Marco, E.; Sa Varese, M.; Paduano, A.; Sacchi, R. Characterization and fractionnement of phenolic compounds extracted from olive mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Bedouhene, S.; Hurtado-Nedelec, M.; Sennani, N.; Marie, J.-C.; El-Benna, J.; Moulti-Mati, F. Polyphenols extracted from olive mill wastewater exert a strong antioxidant effect in human neutrophils. Int. J. Waste Resour. 2014, 4, 1000161. [Google Scholar] [CrossRef] [Green Version]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- El Hadrami, A.; Belaqziz, M.; El Hassni, M.; Hanifi, S.; Abbad, S.; Capasso, R.; El Hadrami, I. Physico-chemical characterization and effects of olive oil mill wastewaters fertirrigation on the growth of some mediterranean crops. J. Agron. 2004, 3, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Belaqziz, M.; Tan, S.P.; EL Abbassi, A.; Kiai, H.; Hafidi, A.; Donovan, O.O.; McLoughlin, P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE 2017, 12, e0182622. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’Antona, N. Poly-phenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antiox-idants 2019, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Frascari, D.; Bacca, A.E.M.; Zama, F.; Bertin, L.; Fava, F.; Pinelli, D. Olive mill wastewater valorisation through phenolic compounds adsorption in a continuous flow column. Chem. Eng. J. 2016, 283, 293–303. [Google Scholar] [CrossRef]
- Galanakis, C.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valori-zation of olive mill wastewater by membrane processes to recover natural antioxidant compounds for cosmeceutical and nutraceutical applications or functional foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Savarese, M.; De Marco, E.; Falco, S.; D’Antuoni, I.; Sacchi, R. Biophenol extracts from olive oil mill wastewaters by membrane separation and adsorption resin. Int. J. Food Sci. Technol. 2016, 51, 2386–2395. [Google Scholar] [CrossRef]
- Aytar, P.; Gedikli, S.; Çelikdemir, M.; Uzuner, S.; Farizoğlu, B.; Şam, M.; Çabuk, A.; Sağlam, N. Dephenolization of olive mill wastewater by pellets of some white rot fungi. J. Biol. Chem. 2011, 39, 379–390. [Google Scholar]
- Bazoti, F.N.; Gikas, E.; Skaltsounis, A.L.; Tsarbopoulos, A. Development of a liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI MS/MS) method for the quantification of bioactive substances present in olive oil mill wastewaters. Anal. Chim. Acta 2006, 573, 258–266. [Google Scholar] [CrossRef] [PubMed]
- DeLisi, R.; Saiano, F.; Pagliaro, M.; Ciriminna, R. Quick assessment of the economic value of olive mill waste water. Chem. Central J. 2016, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Jebabli, H.; Nsir, H.; Taamalli, A.; Abu-Reidah, I.; Álvarez-Martínez, F.J.; Losada-Echeberria, M.; Catalán, E.B.; Mhamdi, R. Industrial-scale study of the chemical composition of olive oil process-derived matrices. Processes 2020, 8, 701. [Google Scholar] [CrossRef]
- Sedej, I.; Milczarek, R.; Wang, S.C.; Sheng, R.; de Jesús Avena-Bustillos, R.; Dao, L.; Takeoka, G. Spray drying of a phenol-ic-rich membrane filtration fraction of olive mill wastewater: Optimisation and dried product quality. Int. J. Food Sci. Technol. 2016, 51, 1900–1909. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with con-ventional methods. J. Agric. Food Chem. 2011, 59, 12725–12731. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859−3872. [Google Scholar] [CrossRef]
- Hamza, M.; Khoufi, S.; Sayadi, S. Fungal enzymes as a powerful tool to release antioxidants from olive mill wastewater. Food Chem. 2012, 131, 1430–1436. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.; Cataldi, T.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Tesh, S.J.; Scott, T.B. Iron nanoparticles for water treatment: Is the future free or fixed? In Iron Oxides, from Nature to Applications; Faivre, D., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016; pp. 473–522. [Google Scholar]
- Catalano, S.; Lawrence, J.; Seadeek, C.; Palazzolo, J.; Wesorick, S.; Cotton, S.; TerBeek, E. Visual Encyclopedia of Chemical Engineering: Adsorbers (n.d.). Available online: http://encyclopedia.che.engin.umich.edu/Pages/SeparationsChemical/Adsorbers/Adsorbers.html (accessed on 12 October 2021).
- Gentile, L.; Uccella, N.A.; Sivakumar, G. Soft-MS and computational mapping of oleuropein. Int. J. Mol. Sci. 2017, 18, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, A.; Forouhar, A.; Ursu, A.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, characterization, and applications of pectins from plant by-products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Miklavčič Višnjevec, A.; Baker, P.; Charlton, A.; Preskett, D.; Peeters, K.; Tavzes, Č.; Kramberger, K.; Schwarzkopf, M. Developing an olive biorefinery in slovenia: Analysis of phenolic compounds found in olive mill pomace and wastewater. Molecules 2020, 26, 7. [Google Scholar] [CrossRef]
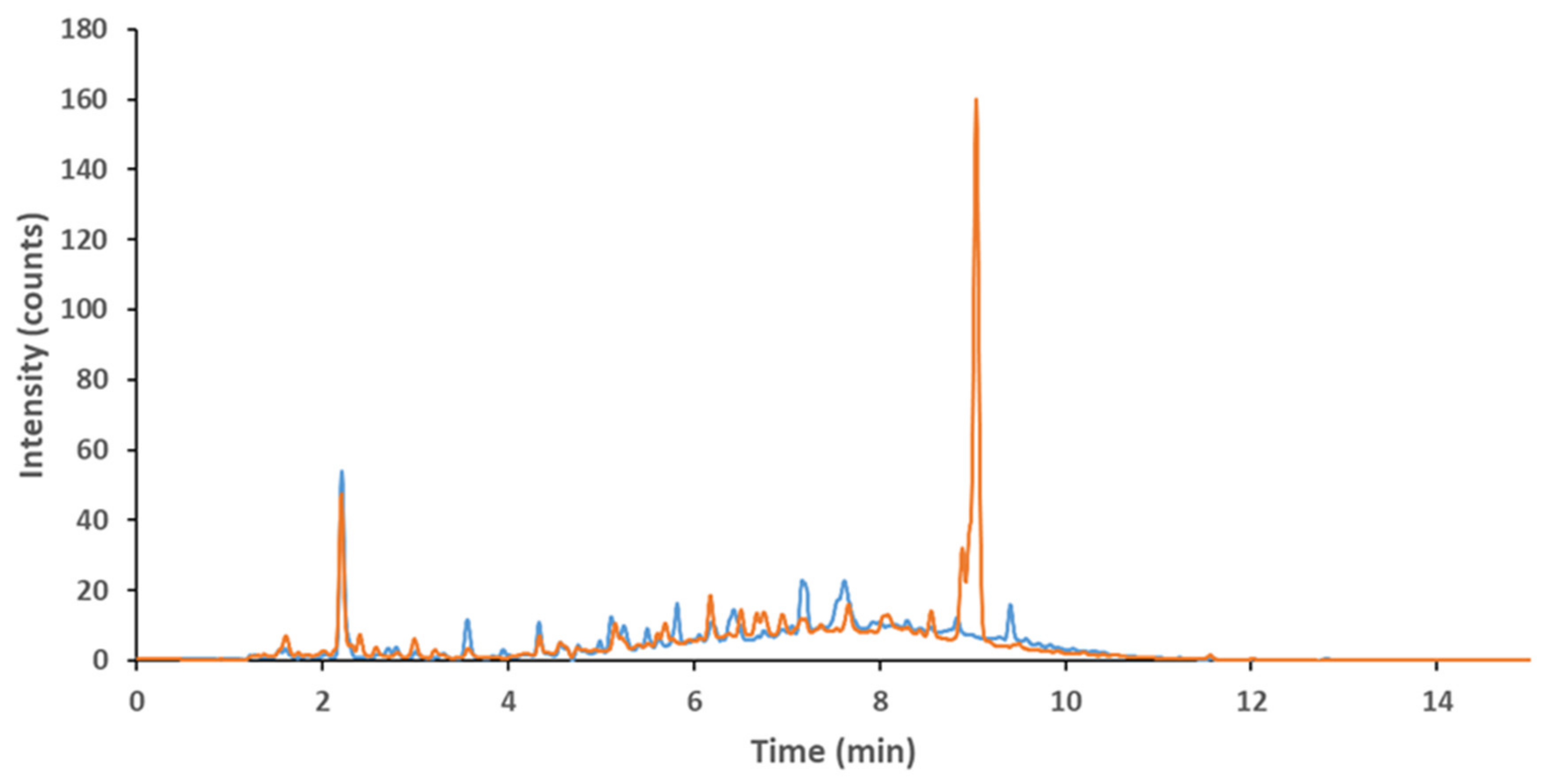
| Phenolic Compound | Freeze Dry MeOH, Shake | Freeze Dry MeOH:H2O | Freeze Dry, MeOH, US | Acidfied EtAc | EtAc | OMWW Filtered + Residue | OMWW Filtered |
|---|---|---|---|---|---|---|---|
| Oleoside isomers | 1,445,574 | 1,110,863 | 2,289,053 | <LOD | <LOD | 645,128 | 328,597 |
| Hydroxytyrosol glucoside | 5,66,618 | 304,694 | 1,960,374 | 132,473 | 209,382 | 242,807 | 73,459 |
| Hydroxytyrosol | 575,656 | 604,716 | 51,584 | <LOD | 86,154 | 304,692 | 247,739 |
| Elenolic acid glucoside isomers | 1,029,428 | 321,183 | 1,180,572 | 75,025 | 65,159 | 698,553 | 584,125 |
| Sacolagonoside | 3,869,429 | 132,231 | 3,324,623 | 138,370 | 130,652 | 445,311 | 290,300 |
| Trans p-coumaric acid 4-glucoside | 34,388 | <LOD | <LOD | <LOD | <LOD | 70,226 | 35,977 |
| β-OH-verbascoside isomers | 945,209 | 535,374 | 998,241 | 329,091 | 145,549 | 528,836 | 341,598 |
| Vanilin | 463,172 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Verbascoside isomers | 674,206 | 474,729 | 633,051 | 128,349 | 86,880 | 295,769 | 201,862 |
| Demethyloleuropein | 344,815 | 323,718 | 336,136 | 22,328 | 62,148 | 129,393 | 94,889 |
| Rutin | 161,112 | <LOD | 93,293 | 38,197 | 61,715 | <LOD | <LOD |
| Luteolin-O-glucoside isomers | <LOD | 382,343 | <LOD | 69,310 | <LOD | 79,061 | <LOD |
| Luteolin rutinoside | <LOD | <LOD | <LOD | 58,880 | <LOD | 114,975 | <LOD |
| Nuzhenide Isomers | 538,060 | 580,076 | 882,461 | 78,569 | <LOD | 359,262 | 298,155 |
| Caffeoyl-6-secologanoside | 610,945 | 646,526 | 579,465 | 61,018 | <LOD | 343,308 | 222,196 |
| Oleuropein isomers | 453,752 | 845,967 | 483,535 | 143,477 | <LOD | 745,436 | 707,012 |
| Hydroxytyrosol acetate | <LOD | <LOD | <LOD | 29,961 | <LOD | <LOD | <LOD |
| 3,4-DHPEA-EDA | 1,751,318 | 634,046 | 1,520,368 | 115,301 | 125,972 | 510,237 | 363,454 |
| Oleuropein aglycone Isomers | 1,475,716 | 749,689 | 1,440,826 | 625,458 | 395,710 | 1,164,560 | 1,008,817 |
| Oleuropein/Oleuroside | 1,241,321 | 411,952 | 385,577 | 328,181 | 112,339 | 508,792 | 441,105 |
| p-HPEA-EDA | <LOD | <LOD | 158,551 | <LOD | <LOD | 69,982 | <LOD |
| Ligstroside | 1,041,186 | 532,238 | 1,007,165 | 130,851 | 112,607 | 692,672 | 663,710 |
| Apigenin | 362,484 | <LOD | <LOD | 37,981 | 31,983 | 85,286 | <LOD |
| Total (mg/mL) | 10.2 ± 0.7 | 4.99 ± 0.35 | 10.1 ± 0.7 | 1.48 ± 0.10 | 0.95 ± 0.07 | 4.67 ± 0.33 | 3.43 ± 0.24 |
| Phenolic Compound | OMWW (pH 2) | OMWW (pH 5) | OMWW (pH 8) | OMWW (pH2 + US) | OMWW (pH5 + US) | OMWW (pH8 + US) |
|---|---|---|---|---|---|---|
| Oleoside isomers | 309,970 | 370399 | 427,345 | 1,697,180 | 289,116 | 466,507 |
| Hydroxytyrosol glucoside | 471,096 | 284219 | 21,786 | 5,227,070 | 388,312 | 21,006 |
| Hydroxytyrosol | 293,461 | 260768 | 68,9939 | 3,141,870 | 314,009 | 341,010 |
| Elenolic acid glucoside isomers | 568,689 | 715803 | 506,267 | 2,481,760 | 717,451 | 535,038 |
| Trans p-coumaric acid 4-glucoside | 99,950 | 169716 | 74,408 | 728,910 | 114,826 | 62,571 |
| β-OH-verbascoside isomers | 399,555 | 351,584 | 223,935 | 4,395,790 | 351,633 | 172,049 |
| Verbascoside isomers | 270,546 | 227,444 | 186,890 | 2,518,070 | 272,648 | 183,198 |
| Demethyloleuropein | 69,378 | 39,526 | 37,856 | <LOD | 63,493 | 45,920 |
| Nuzhenide Isomers | 167,438 | 138,946 | <LOD | 379,690 | 220,018 | <LOD |
| Caffeoyl-6-secologanoside | 266,821 | 268,048 | 222,335 | 2,786,090 | 213,464 | 214,685 |
| Oleuropein | 365,061 | 355,250 | 129,169 | 1,500,900 | 361,880 | 162,743 |
| Hydroxytyrosol acetate | <LOD | <LOD | <LOD | 177,910 | 31,639 | <LOD |
| 3,4-DHPEA-EDA | 780,943 | 336,025 | <LOD | 5,456,340 | 375,536 | <LOD |
| Oleuropein aglycone Isomers | 819,442 | 607,781 | <LOD | 4,189,430 | 674,198 | <LOD |
| p-HPEA-EDA | 17,413 | 68,905 | <LOD | 578,420 | 70,888 | <LOD |
| Total (mg/mL) | 3.83 ± 0.27 | 3.29 ± 0.23 | 1.98 ± 0.14 | 27.6 ± 1.9 | 3.48 ± 0.24 | 1.96 ± 0.14 |
| Phenolic Compounds | Polyphenol Content in 1st MeOH Fraction | Polyphenol Content in 15th MeOH Fraction | Soluble Polyphenol Content in OMWW -Before Treatment | Soluble Polyphenol Concentration in OMWW—After Treatment |
|---|---|---|---|---|
| Oleoside isomers | 17,173 | 18,190 | 322,365 | 322,726 |
| Hydroxytyrosol glucoside | 3995 | 6876 | 71,549 | 72,656 |
| Hydroxytyrosol | 6955 | 1527 | 177,540 | 45,708 |
| Caffeic acid | 6246 | 7554 | 151,334 | 93,235 |
| Elenolic acid glucoside isomers | 7967 | 1889 | 51,519 | 48,253 |
| β-OH-verbascoside isomers | 8275 | 7939 | 129,286 | 179,532 |
| Demethyloleuropein | 562 | <LOD | 24,056 | <LOD |
| Rutin | 607 | 657 | 10,704 | 5970 |
| Verbascoside isomers | 6474 | <LOD | 148,867 | <LOD |
| Luteolin rutinoside | 1060 | 1347 | 17,369 | 11,588 |
| Caffeoyl-6-secologanoside | 7943 | 7883 | 128,738 | 104,644 |
| Luteolin-O-glucoside isomers | 4042 | 3529 | 23,156 | 15,171 |
| Oleuropein isomers | <LOD | 426 | <LOD | <LOD |
| 3,4-DHPEA-EDA | <LOD | 423 | <LOD | <LOD |
| Oleuropein/Oleuroside | 951 | < LOD | 49,132 | < LOD |
| p-HPEA-EDA | 261 | <LOD | 7963 | 3539 |
| Apigenin | 1990 | 1325 | <LOD | <LOD |
| Oleuropein aglycone isomers | 369 | <LOD | 6813 | <LOD |
| Total (mg/mL) | 0.23 ± 0.02 | 0.19 ± 0.02 | 3.86 ± 0.12 | 2.68 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, K.; Miklavčič Višnjevec, A.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules 2021, 26, 6946. https://doi.org/10.3390/molecules26226946
Peeters K, Miklavčič Višnjevec A, Esakkimuthu ES, Schwarzkopf M, Tavzes Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules. 2021; 26(22):6946. https://doi.org/10.3390/molecules26226946
Chicago/Turabian StylePeeters, Kelly, Ana Miklavčič Višnjevec, Esakkiammal Sudha Esakkimuthu, Matthew Schwarzkopf, and Črtomir Tavzes. 2021. "The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector" Molecules 26, no. 22: 6946. https://doi.org/10.3390/molecules26226946
APA StylePeeters, K., Miklavčič Višnjevec, A., Esakkimuthu, E. S., Schwarzkopf, M., & Tavzes, Č. (2021). The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules, 26(22), 6946. https://doi.org/10.3390/molecules26226946