Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties
Abstract
:1. Introduction
2. Results and Discussion
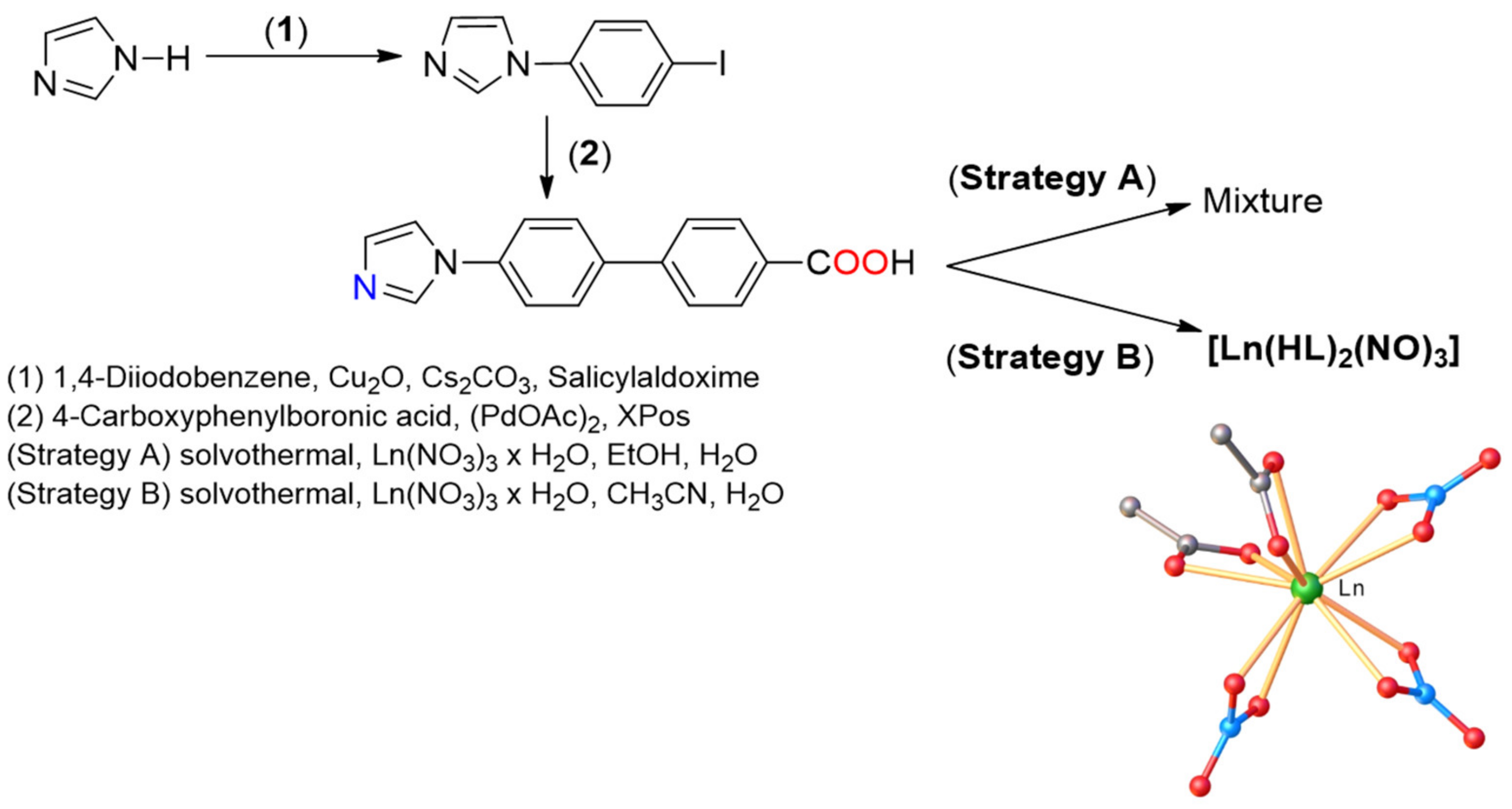
2.1. Synthesis and Characterization of [Ln(HL)2(NO3)3] Complexes
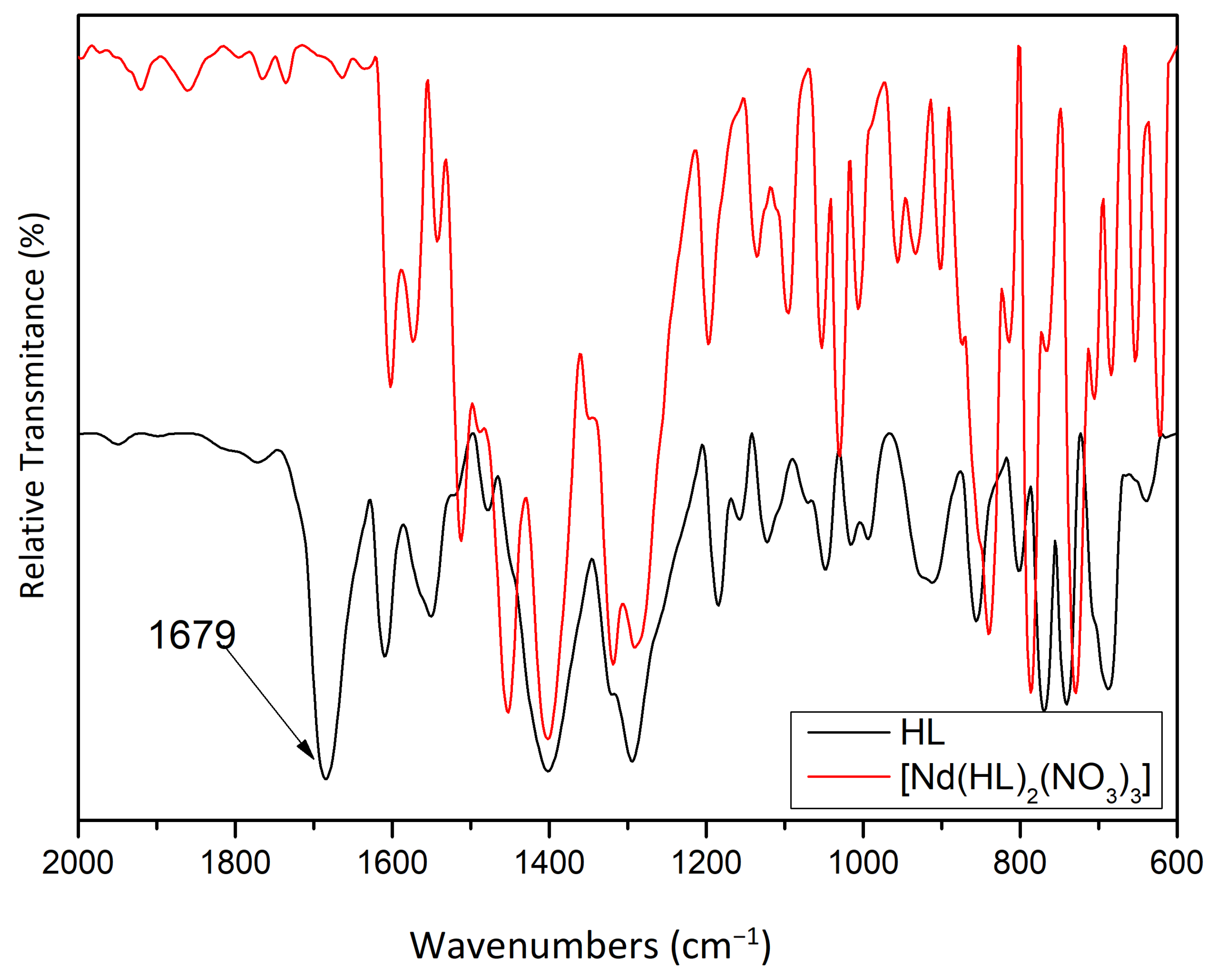
2.2. Spectroscopic Details
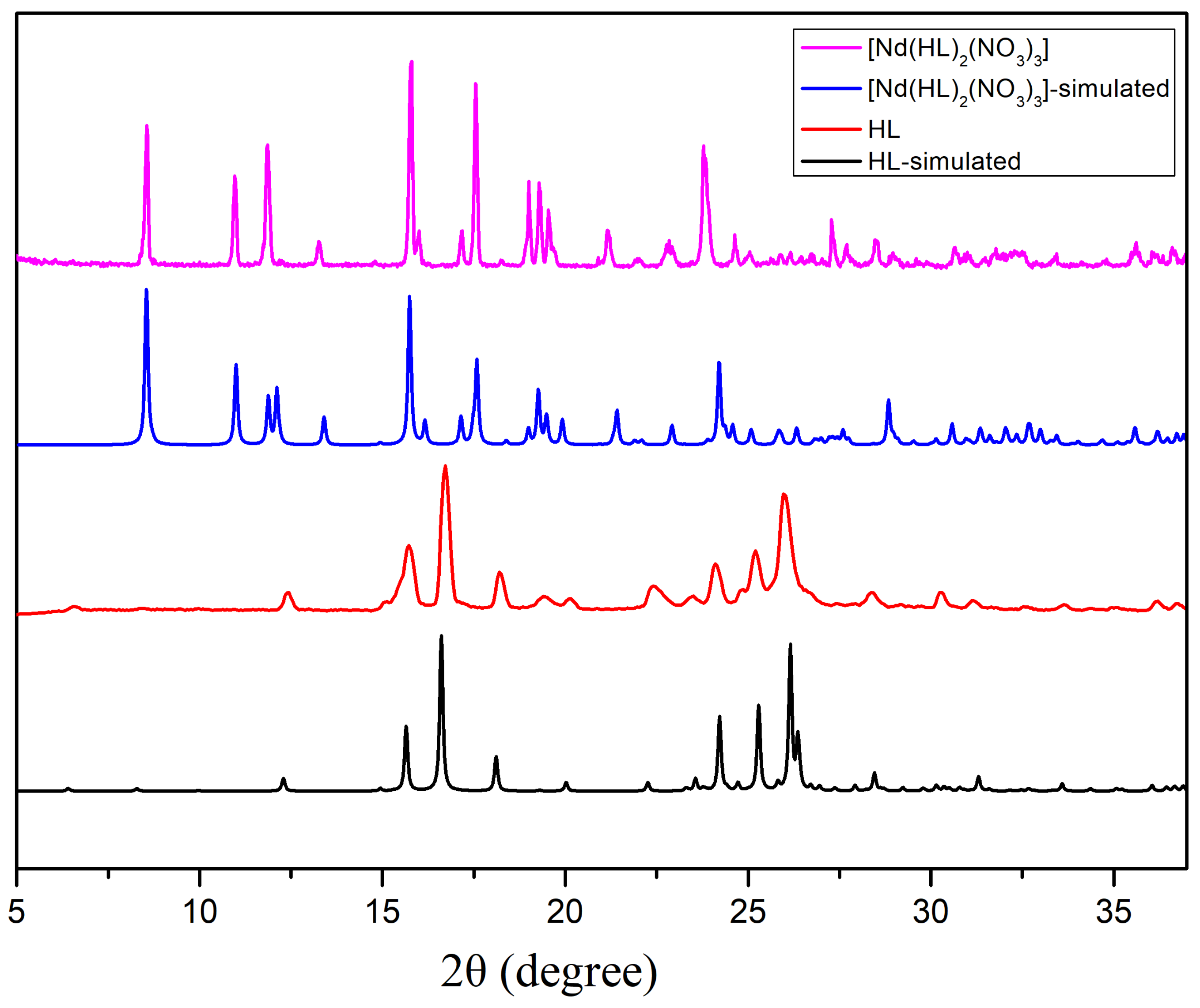
2.3. Powder X-ray Diffraction Studies (PXRD)
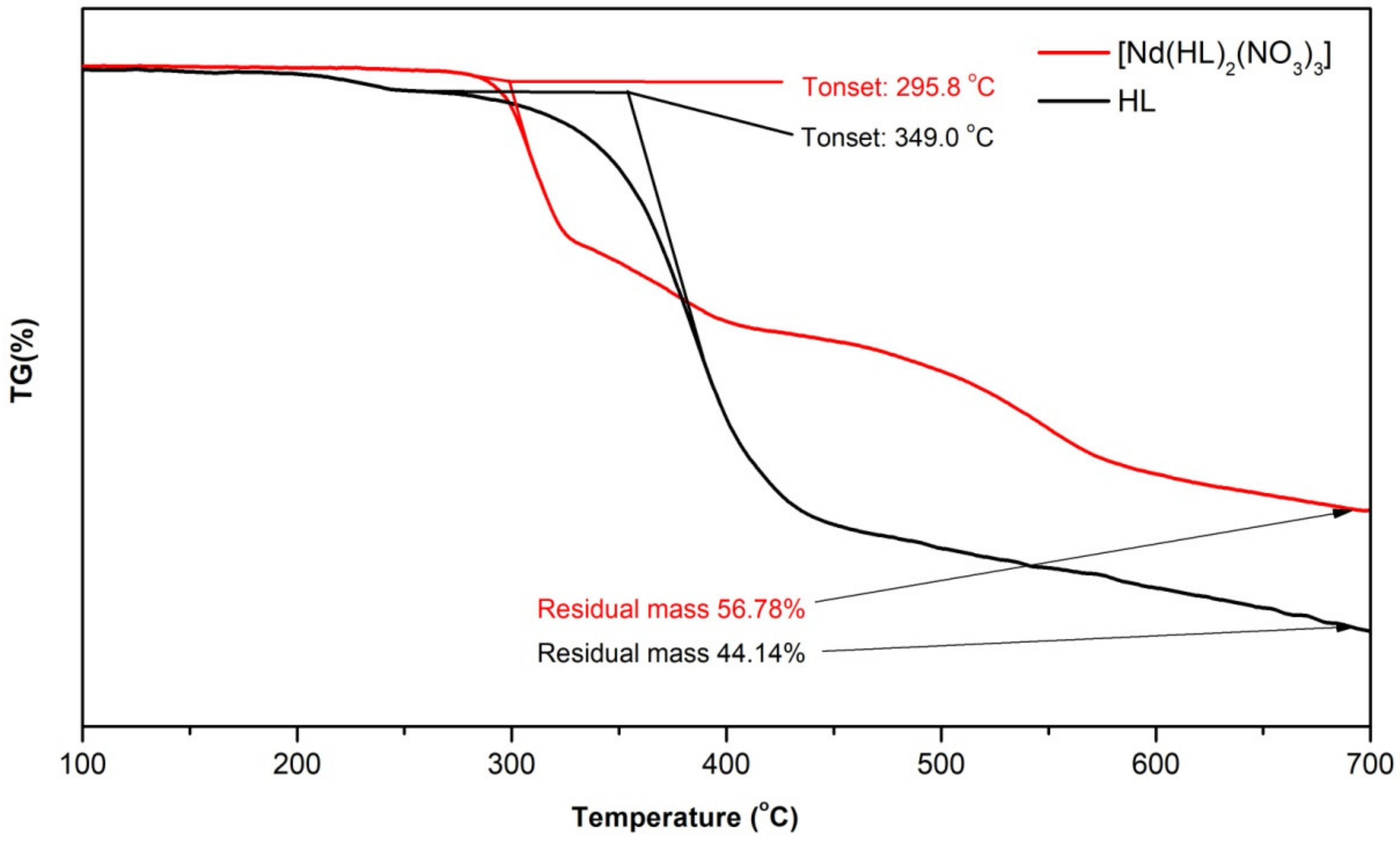
2.4. Thermogravimetric Analysis (TGA)
2.5. Luminiscence
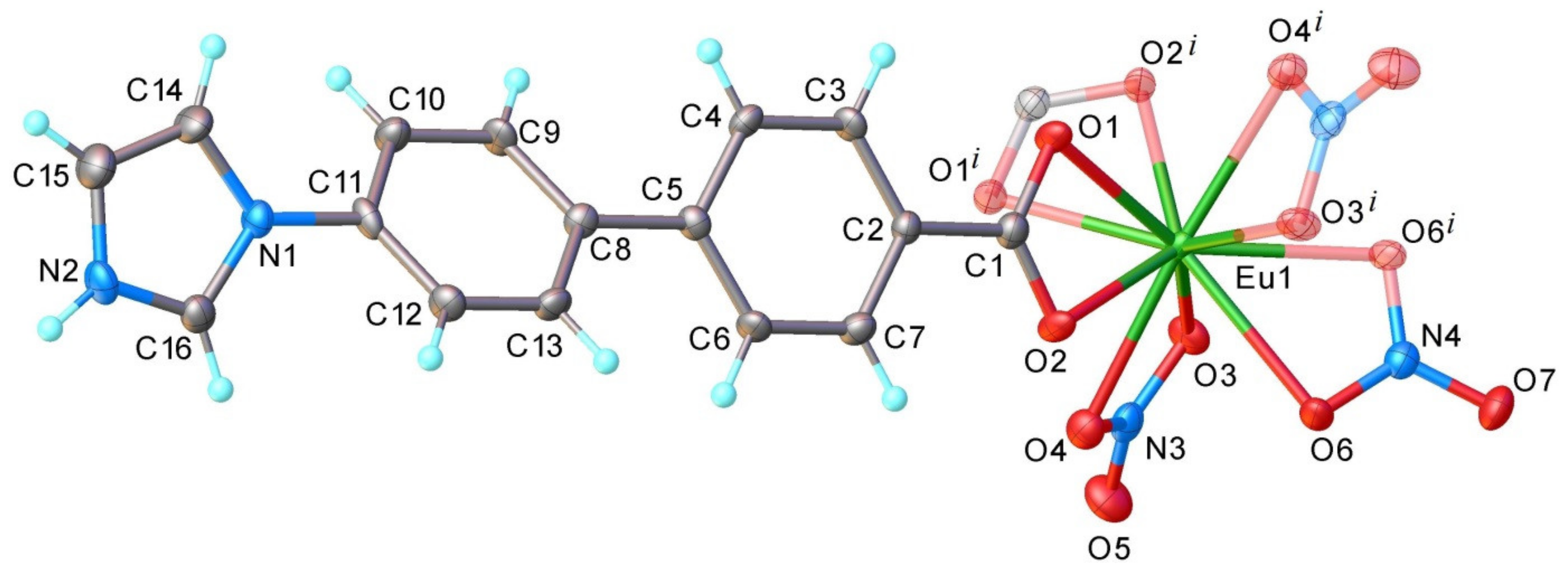
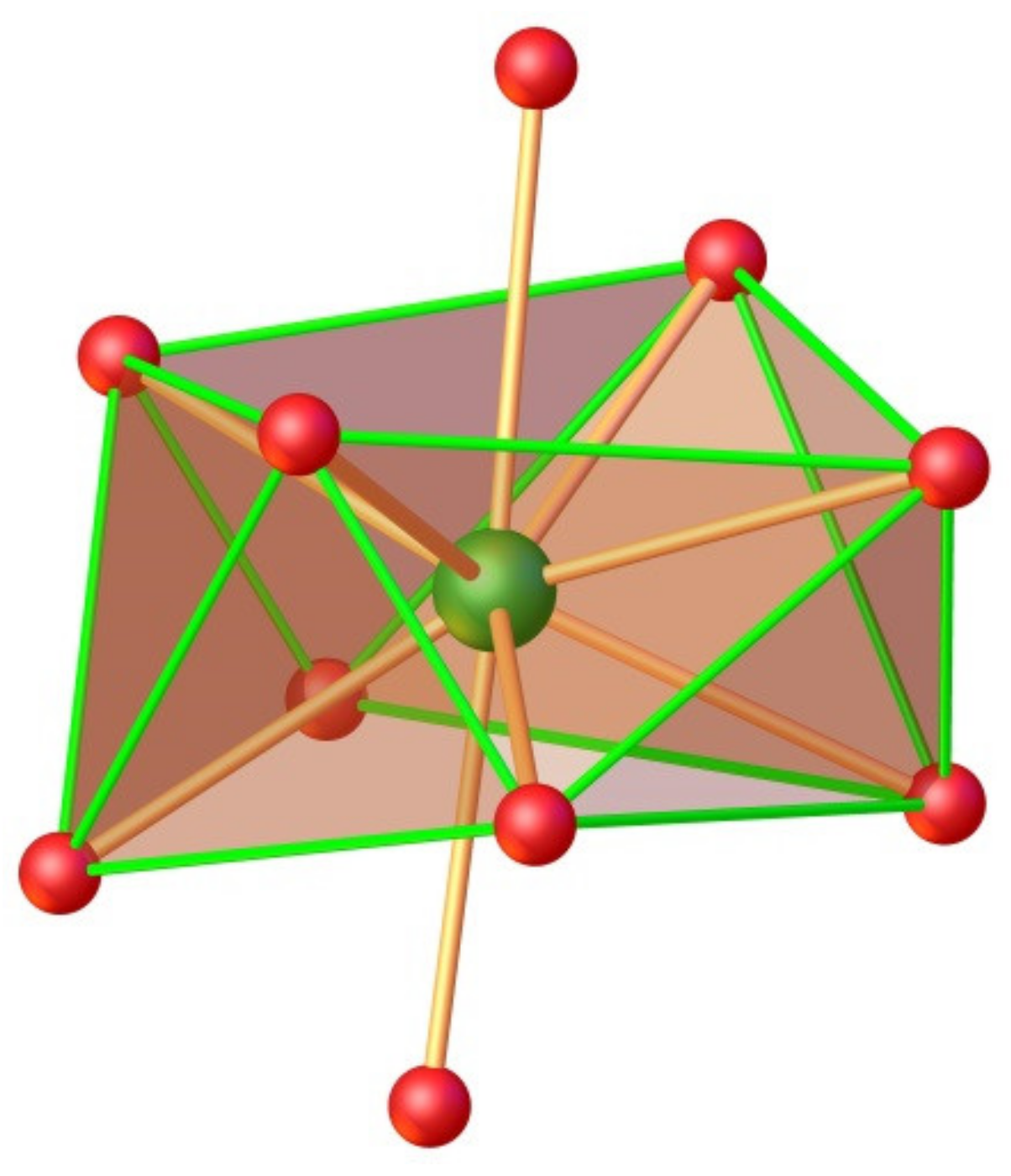
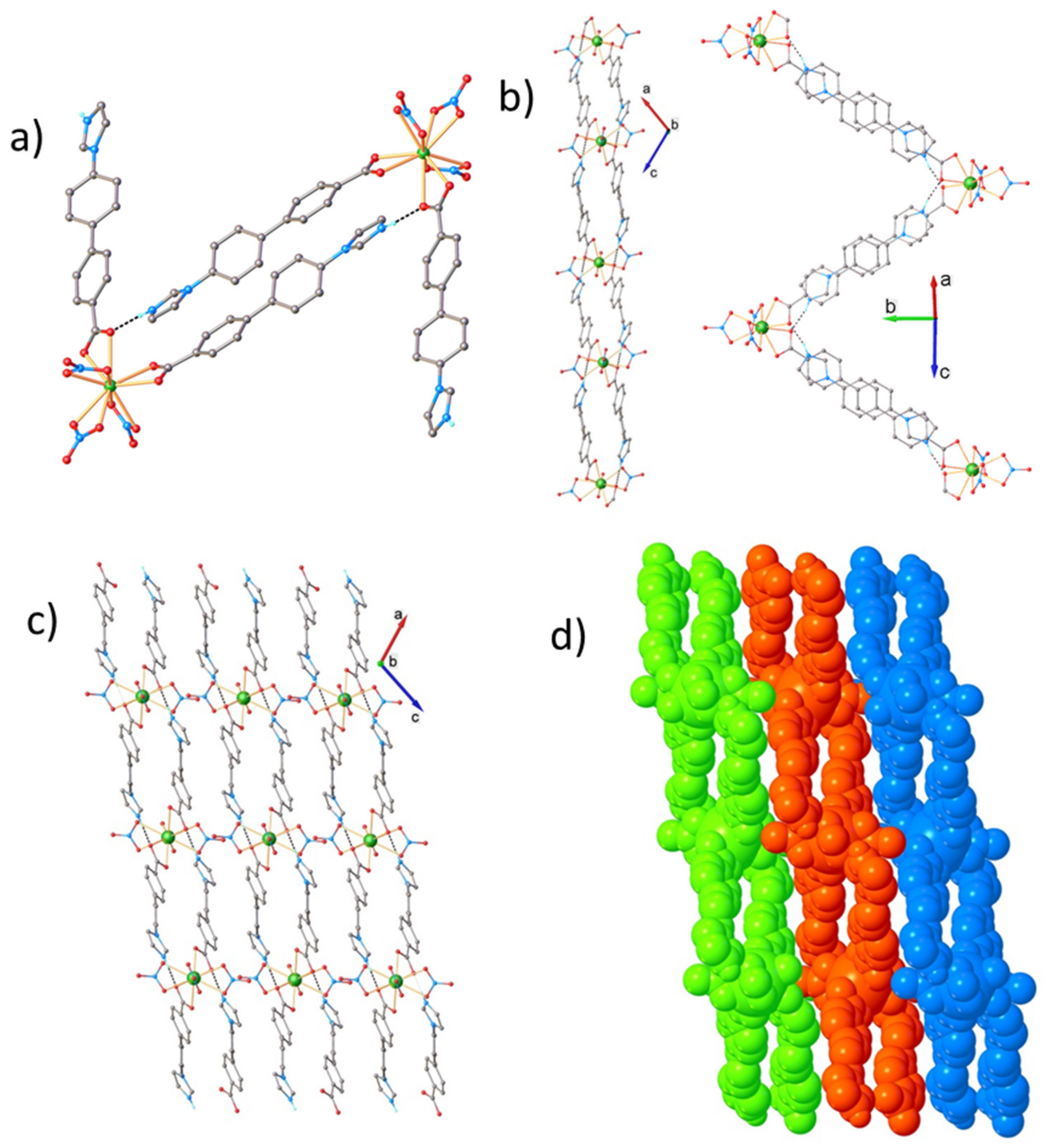
2.6. Crystallographic Studies
3. Experimental Section
3.1. Material and Methods
3.2. General Procedure for [Ln(HL)2(NO3)3]
Synthesis of the Lanthanide (III) Complexes
3.3. Single Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bao, G.; Wen, S.; Lin, G.; Yuan, J.; Lin, J.; Wong, K.-L.; Bünzli, J.-C.G.; Jin, D. Learning from lanthanide complexes: The development of dye-lanthanide nanoparticles and their biomedical applications. Coordin. Chem. Rev. 2021, 429, 213642. [Google Scholar] [CrossRef]
- Zhao, Q.-Q.; Ren, N.; Zhang, J.-J.; Geng, L.-N.; Wang, S.-P.; Shi, S.-K. Three novel Ho(III) complexes with different auxiliary ligands: Synthesis, crystal structures and thermal properties. Polyhedron 2017, 132, 78–89. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, C.; Yang, X.; Shen, B.; Sun, Y.; Chen, Y.; Xu, X.; Sun, H.; Yu, K.; Yang, B.; et al. pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.; Riccò, R.; Liang, K.; Bradshaw, D.; Falcaro, P. Metal-Organic Frameworks at the biointerface: Synthetic strategies and aplications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.; Chu, J.; Qi, Y.; Tang, M.; Hou, J.; Yang, G. Half-sandwich chiral rare-earth metal complexes with linked tridentate amido-indenyl ligand: Synthesis, characterization, and catalytic properties for intramolecular hydroamination. RSC Adv. 2017, 7, 1759–1765. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-H.; Silva, A.R.; Shi, F.-N. Two dimensional porous 3d–4f heterometallic coordination polymers constructed by pyridine2,3-dicarboxylic acid. CrystEngComm 2015, 17, 3852–3858. [Google Scholar] [CrossRef]
- Reznichenko, A.L.; Hultzsch, K.C. Catalytic σ-bond metathesis. In Molecular Catalysis of Rare-Earth Elements, 1st ed.; Roesky, P.W., Ed.; Springer: Berlin, Germany, 2010; pp. 1–48. [Google Scholar]
- Ma, A.; Ke, F.; Jiang, J.; Yuan, Q.; Luo, Z.; Liu, J.; Kumar, A. Two lanthanide-based metal-organic frameworks for highly efficient adsorption and removal of fluoride ion from water. CrystEngComm 2017, 19, 2172–2177. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent extraction of rare earth elements with ionic liquids: Towards a selective and sustainable extraction of these valuable elements. Curr. Opin. Green Sustain. 2020, 27, 100428. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Yakushev, I.A.; Lyssenko, K.A.; Maximova, O.V.; Mironov, V.S.; Manakin, Y.V.; Kornev, A.B.; Vasiliev, A.N.; Yagubskii, E.B. Ten-coordinate lanthanide [Ln(HL)(L)] complexes (Ln = Dy, Ho, Er, Tb) with pentadentate N3O2-type Schiff-base ligands: Synthesis, structure and magnetism. Magnetochemistry 2020, 6, 60. [Google Scholar] [CrossRef]
- Guan, X.-F.; Zhao, H.-J.; Hao, Y.-J.; Guo, X.-R.; Yang, Z.-P.; Zhang, F.-Y.; Wang, W.-M. Structures, luminescence properties and single-molecule magnet behavior of four dinuclear lanthanide compounds. J. Mol. Struct. 2021, 1245, 131010. [Google Scholar] [CrossRef]
- Bar, A.K.; Kalita, P.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal-bipyramid Ln(III) complexes exhibiting single-ion-magnet behavior: A rational synthetic approach for a rigid equatorial plan. Inorg. Chem. 2018, 57, 2398–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Ren, Y.-N.; Li, J.-X.; Liu, B.-Q.; Dong, Y.-P. Syntheses, structures, and magnetic properties of two series of 3d-4f heterometallic coordination polymers derived from pyrazine-2,3-dicarboxylic acid. Eur. J. Inorg. Chem. 2018, 2018, 1099–1106. [Google Scholar] [CrossRef]
- Lisowski, J.; Sessler, J.L.; Lynch, V.; Mody, T.D. lH NMR spectroscopic study of paramagnetic lanthanide(III) texaphyrins. Effect of axial ligation. J. Am. Chem. Soc. 1995, 117, 2273–2285. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Song, X.-Z.; Zhu, M.; Meng, X.; Wu, L.-L.; Feng, J.; Song, S.-Y.; Zhang, H.-J. Encapsulation of LnIII ions/dyes within a microporous anionic MOF by post-synthetic ionic exchange serving as a LnIII ion probe and two-color luminescent sensors chemistry. Chem. Eur. J. 2015, 21, 9748–9752. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, G.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Song, S.; Zhang, H. A Europium(III) based metal-organic framework: Bifunctional properties related to sensing and electronic conductivity. J. Mater. Chem. A 2014, 2, 237–244. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Li, J.-X.; Jiang, Y.; Liu, B.-Q.; Dong, Y.-P. Syntheses, structures, photoluminescence, and magnetism of a series of lanthanide 1,3-adamantanedicarboxylate coordination polymers. Polyhedron 2017, 13, 868–873. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wu, L.-L.; Feng, J.; Song, S.-Y.; Zhang, H.-J. An ideal detector composed of a 3D Gd-based coordination polymer for DNA and Hg2+ ion. Inorg. Chem. Front. 2016, 3, 376–380. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; He, L.; Liu, Y.; Liu, Y.; Chen, C.; Tang, Z. Core–shell upconversion nanoparticle@metal-organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv. Mater. 2015, 27, 4075–4080. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.-F.; Chen, Z.-Q.; Bian, Z.-Q.; Huang, C.-H. Sensitized luminescence from lanthanides in d–f bimetallic complexes. Coord. Chem. Rev. 2010, 254, 991–1010. [Google Scholar] [CrossRef]
- Guo, R.; Gao, L.; Zhou, W.; Zhang, Y.; Hu, T. A stable Eu (III) coordination polymer for sensitive and selective sensing of Fe3+/Cr2O72− ions in aqueous medium. Polyhedron 2021, 193, 114846. [Google Scholar] [CrossRef]
- Ran, J.; Zhao, X.; Hu, X.; Chen, Y.; Tian, Z. 3D Tb(III) and Eu(III) coordination polymers with mixed dicarboxylate ligands: Synthesis, structure and luminescence properties. Polyhedron 2021, 194, 114910. [Google Scholar] [CrossRef]
- Kateshali, A.F.; Dogaheh, S.G.; Soleimannejad, J.; Blake, A.J. Structural diversity and applications of Ce(III)-based coordination polymers. Coord. Chem. Rev. 2020, 419, 213392. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Zhang, L.; Yu, B.; Wang, Y.; Zhang, W. Multiporous terbium phosphonate coordination polymer microspheres as fluorescent probes for trace anthrax biomarker detection. ACS Appl. Mater. Interfaces 2019, 11, 15998–16005. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Song, Y.; Li, Y.-X.; Zuo, J.-L.; Gao, S.; You, X.-Z. Three-dimensional lanthanoid-containing coordination frameworks: Structure, magnetic and fluorescent properties. Eur. J. Inorg. Chem. 2005, 2005, 766–772. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Zhang, J.; Chen, Y.-M.; Yang, G.-Y. Porous lanthanide–organic open frameworks with helical tubes constructed from interweaving triple-helical and double-helical chains. Angew. Chem. Int. Ed. 2005, 44, 5814–5817. [Google Scholar] [CrossRef]
- Haldar, R.; Bhattacharyya, S.; Maji, T.K. Luminescent metal-organic frameworks and their potential applications. J. Chem. Sci. 2020, 132, 99. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Growing a nucleotide/lanthanide coordination polymer shell on liposomes. Langmuir 2019, 35, 11217–11224. [Google Scholar] [CrossRef]
- Kobayashi, A.; Suzuki, Y.; Ohba, T.; Noro, S.-I.; Chang, H.-C.; Kato, M. Ln-Co-based rock-salt-type porous coordination polymers: Vapor response controlled by changing the lanthanide ion. Inorg. Chem. 2011, 50, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, X.; Cen, P.; Jin, X.; Yang, J.; Zhang, Y.-Q.; Ferrando-Soria, J.; Pardo, E.; Liu, X. A series of lanthanide(III) metal-organic frameworks derived from pyridyl-dicarboxylate ligand: Single-molecule magnet behaviour and luminescent properties. Dalton Trans. 2020, 49, 14123–14132. [Google Scholar] [CrossRef]
- Dascalu, I.A.; Mikhalyova, E.A.; Shova, S.; Bratanovici, B.I.; Ardeleanu, R.; Marangoci, N.; Lozan, V.; Roman, G. Synthesis, crystal structure and luminescent properties of isoreticular lanthanide–organic frameworks based on a tetramethyl-substituted terphenyldicarboxylic acid. Polyhedron 2021, 194, 114929. [Google Scholar] [CrossRef]
- Bejan, D.; Bahrin, L.G.; Shova, S.; Marangoci, N.L.; Kökҫam-Demir, Ü.; Lozan, V.; Janiak, C. New microporous lanthanide organic frameworks. Synthesis, structure, luminescence, sorption, and catalytic acylation of 2-naphthol. Molecules 2020, 25, 3055. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P.V.D. Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Hao, Z.-M.; Song, X.-Z.; Meng, X.; Zhao, S.-N.; Song, S.-Y.; Zhang, H.-J. A new type of double-chain based 3D lanthanide(III) metal-organic framework demonstrating proton conduction and tunable emission. Chem. Commun. 2014, 50, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, A.; Mondal, S.S.; Wruck, A.; Kelling, A.; Schilde, U.; Brandt, P.; Janiak, C.; Schönfeld, S.; Weber, B.; Rybakowski, L.; et al. Hydrogen-bonded supramolecular metal-imidazolate frameworks: Gas sorption, magnetic and UV/Vis spectroscopic properties. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef] [Green Version]
- Bahrin, L.G.; Bejan, D.; Shova, S.; Gdaniec, M.; Fronc, M.; Lozan, V.; Janiak, C. Alkali- and alkaline-earth metal–organic networks based on a tetra(4-carboxyphenyl)bimesitylene-linker. Polyhedron 2018, 173, 114128. [Google Scholar] [CrossRef]
- Amani, V.; Rafizadeh, M. Two-dimensional lanthanide(III) cyclic coordination polymers complexes containing dimethyl phosphate ligand: Synthesis, spectroscopic characterization, thermal analysis, and crystal structures. J. Mol. Struct. 2021, 1229, 129834. [Google Scholar] [CrossRef]
- Xing, S.; Janiak, C. Design and properties of multiple-emitter luminescent metalorganic frameworks. Chem. Commun. 2020, 56, 12290–12306. [Google Scholar] [CrossRef]
- Ma, Z.L.; Shi, J.Y.; Wang, M.C.; Tian, L. Lanthanide-organic complex with uncoordinated lewis basic triazolyl sites as multi-responsive sensor for nitrobenzene, Cu2+ and MnO4−. Dye. Pigm. 2020, 108930. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Lin, W. Rational synthesis of noncentrosymmetric metal-organic frameworks for second-order nonlinear optics. Chem. Rev. 2012, 112, 1084–1104. [Google Scholar] [CrossRef]
- George, M.R.; Critchley, P.E.; Whitehead, G.F.S.; Bailey, A.J.; Cuda, F.; Murdin, B.N.; Grossel, M.C.; Curry, R.J. Modified pyridine-2,6-dicarboxylate acid ligands for sensitization of near-infrared luminescence from lanthanide ions (Ln3+ = Pr3+, Nd3+, Gd3+, Dy3+, Er3+). J. Lumin. 2021, 230, 117715. [Google Scholar] [CrossRef]
- Du, J.-Q.; Dong, J.-L.; Xie, F.; Yang, R.-X.; Lan, H.-M.; Wang, D.-Z. Lanthanide complexes supported via benzimidazole carboxylic acid ligand: Synthesis, luminescence and magnetic properties. J. Mol. Struct. 2020, 1202, 127345. [Google Scholar] [CrossRef]
- Hemmilä, I.; Laitala, V. Progress in lanthanides as luminescent probes. J. Fluoresc. 2005, 15, 529–542. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid detection of the biomarkers for carcinoid tumors by a water stable luminescent lanthanide metal–organic framework sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, A.R.; Yang, X.; Fu, M.; Wang, R.; Chi, L.; Zhai, G. Current advances in versatile metal-organic frameworks for cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102266. [Google Scholar] [CrossRef]
- Bejan, D.; Bahrin, L.G.; Shova, S.; Sardaru, M.; Clima, L.; Nicolescu, A.; Marangoci, N.; Lozan, V.; Janiak, C. Spontaneous resolution of non-centrosymmetric coordination polymers of zinc(II) with achiral imidazole–biphenyl–carboxylate ligands. Inorg. Chim. Acta 2018, 482, 275–283. [Google Scholar] [CrossRef]
- Gaye, M.; Tamboura, F.B.; Sall, A.S. Spectroscopic studies of some lanthanide(III) nitrate complexes synthesized from a new ligand 2,6-bis-(salicylaldehyde hydrazone)-4-chlorophenol. Bull. Chem. Soc. Ethiop. 2003, 17, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Bejan, D.; Bahrin, L.G.; Cojocaru, C.; Trandabat, A.F.; Marangoci, N.L.; Rotaru, A.; Shova, S. The use of C1 symmetry imidazole-carboxylate building block and auxiliary acetate co-ligand for assembly of a 2D wave-like zinc(II) coordination polymer: Experimental and theoretical study. J. Coord. Chem. 2020, 73, 2250–2264. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2015.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]

| Parameter | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Empirical Formula | C32H24LaN7O13 | C32H24CeN7O13 | C32H24N7NdO13 | C32H24EuN7O13 | C32H24GdN7O13 | C32H24DyN7O13 | C32H24HoN7O13 |
| Fw | 853.49 | 854.70 | 858.82 | 866.54 | 871.83 | 877.08 | 879.51 |
| Space Group | P2/n | P2/n | P2/n | P2/n | P2/n | P2/n | P2/n |
| a [Å] | 11.7434(7) | 11.6491(5) | 11.6264(8) | 11.6229(7) | 11.6154(7) | 11.5987(4) | 11.6138(3) |
| b [Å] | 10.0978(5) | 10.1346(4) | 10.1166(6) | 10.0972(5) | 10.0797(5) | 10.0803(3) | 10.0845(2) |
| c [Å] | 14.0667(9) | 14.0436(7) | 14.0056(8) | 14.0020(8) | 13.9905(7) | 13.9615(5) | 14.0087(4) |
| α [°] | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| β [°] | 108.771(7) | 109.849(5) | 109.845(7) | 109.798(7) | 109.817(7) | 109.871(4) | 109.726(3) |
| γ [°] | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| V [Å3] | 1579.36(17) | 1559.48(13) | 1549.51(17) | 1546.14(16) | 1541.00(16) | 1535.17(10) | 1544.41(7) |
| Z | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| rcalcd [g cm−3] | 1.795 | 1.820 | 1.841 | 1.861 | 1.879 | 1.897 | 1.891 |
| Crystal Size [mm] | 0.25 × 0.05 × 0.02 | 0.20 × 0.10 × 0.05 | 0.25 × 0.10 × 0.05 | 0.30 × 0.05 × 0.05 | 0.30 × 0.05 × 0.05 | 0.10 × 0.05 × 0.05 | 0.25 × 0.05 × 0.05 |
| T [K] | 293 | 180 | 180 | 180 | 180 | 180 | 180 |
| μ [mm−1] | 1.436 | 1.544 | 1.761 | 2.114 | 2.238 | 2.520 | 2.647 |
| 2Θ range [°] | 4.034 to 50.054 | 3.942 to 58.712 | 3.952 to 50.052 | 3.954 to 58.262 | 3.956 to 50.048 | 3.962 to 50.054 | 3.956 to 50.044 |
| Reflections Collected | 5552 | 7773 | 5738 | 7681 | 5840 | 11,123 | 22,162 |
| Independent Reflections | 2786[Rint = 0.0333] | 3657[Rint = 0.0356] | 2743[Rint = 0.0401] | 3599[Rint = 0.0387] | 2712[Rint = 0.0267] | 2713[Rint = 0.0431] | 2727[Rint = 0.0557] |
| Data/Restraints/Parameters | 2786/0/241 | 3657/0/241 | 2743/0/241 | 3599/0/241 | 2712/0/241 | 2713/0/241 | 2727/0/241 |
| R1 a | 0.0326 | 0.0341 | 0.0365 | 0.0419 | 0.0300 | 0.0301 | 0.0304 |
| wR2 b | 0.0483 | 0.0668 | 0.0609 | 0.0700 | 0.0467 | 0.0622 | 0.0764 |
| GOF c | 0.976 | 1.050 | 1.007 | 1.042 | 1.057 | 1.070 | 1.040 |
| Largest Diff. Peak/Hole [e Å−3] | 0.51/−0.37 | 0.63/−0.50 | 0.48/−0.76 | 0.82/−0.71 | 0.49/−0.42 | 0.55/−0.60 | 0.86/−0.66 |
| Compound | Strategy | Metal Source | Ligand (mmol) | Salt (mmol) | EtOH (mL) | MeCN (mL) | H2O (mL) | Yield/ Color |
|---|---|---|---|---|---|---|---|---|
| [La(HL)2(NO3)3] (1) | A | La(NO3)3·6H2O | 0.06 | 0.17 | 3.80 | - | 1.0 | 47%/ Yellow (Mixture) |
| [La(HL)2(NO3)3] (1) | B | La(NO3)3·6H2O | 0.026 | 0.82 | - | 28.6 | 2.25 | 46%/ Dark Yellow |
| [Ce(HL)2(NO3)3] (2) | B | Ce(NO3)3·6H2O | 0.026 | 0.72 | - | 3.20 | 0.25 | 64%/ Yellowish |
| [Nd(HL)2(NO3)3] (3) | A | Nd(NO3)3·6H2O | 0.03 | 0.09 | 1.90 | - | - | 82% Yellowish (Mixture) |
| [Nd(HL)2(NO3)3] (3) | B | Nd(NO3)3·6H2O | 0.15 | 0.50 | - | 19.0 | 1.5 | 25%/ Yellow |
| [Eu(HL)2(NO3)3] (4) | B | Eu(NO3)3·5H2O | 0.18 | 0.53 | - | 28.6 | 2.25 | 49%/ Brown |
| [Gd(HL)2(NO3)3] (5) | A | Gd(NO3)3·6H2O | 0.08 | 0.25 | 1.30 | - | 1.0 | 3%/ Yellow (Mixture) |
| [Gd(HL)2(NO3)3] (5) | B | Gd(NO3)3·6H2O | 0.19 | 0.53 | - | 28.6 | 2.25 | 47%/ Brown |
| [Dy(HL)2(NO3)3] (6) | A | Dy(NO3)3·5H2O | 0.12 | 0.36 | 1.90 | - | 0.50 | 29%/ Yellow (Mixture) |
| [Dy(HL)2(NO3)3] (6) | B | Dy(NO3)3·5H2O | 0.12 | 0.37 | - | 9.50 | 0.75 | 43%/ Dark Yellow |
| [Ho(HL)2(NO3)3] (7) | A | Ho(NO3)3·5H2O | 0.02 | 0.06 | 1.90 | - | - | 75%/ Yellow (Mixture) |
| [Ho(HL)2(NO3)3] (7) | B | Ho(NO3)3·5H2O | 0.12 | 0.34 | - | 19.0 | 1.50 | 25%/ Dark Yellow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardaru, M.-C.; Marangoci, N.L.; Shova, S.; Bejan, D. Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties. Molecules 2021, 26, 6942. https://doi.org/10.3390/molecules26226942
Sardaru M-C, Marangoci NL, Shova S, Bejan D. Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties. Molecules. 2021; 26(22):6942. https://doi.org/10.3390/molecules26226942
Chicago/Turabian StyleSardaru, Monica-Cornelia, Narcisa Laura Marangoci, Sergiu Shova, and Dana Bejan. 2021. "Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties" Molecules 26, no. 22: 6942. https://doi.org/10.3390/molecules26226942
APA StyleSardaru, M.-C., Marangoci, N. L., Shova, S., & Bejan, D. (2021). Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties. Molecules, 26(22), 6942. https://doi.org/10.3390/molecules26226942







