Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges
Abstract
:1. Introduction
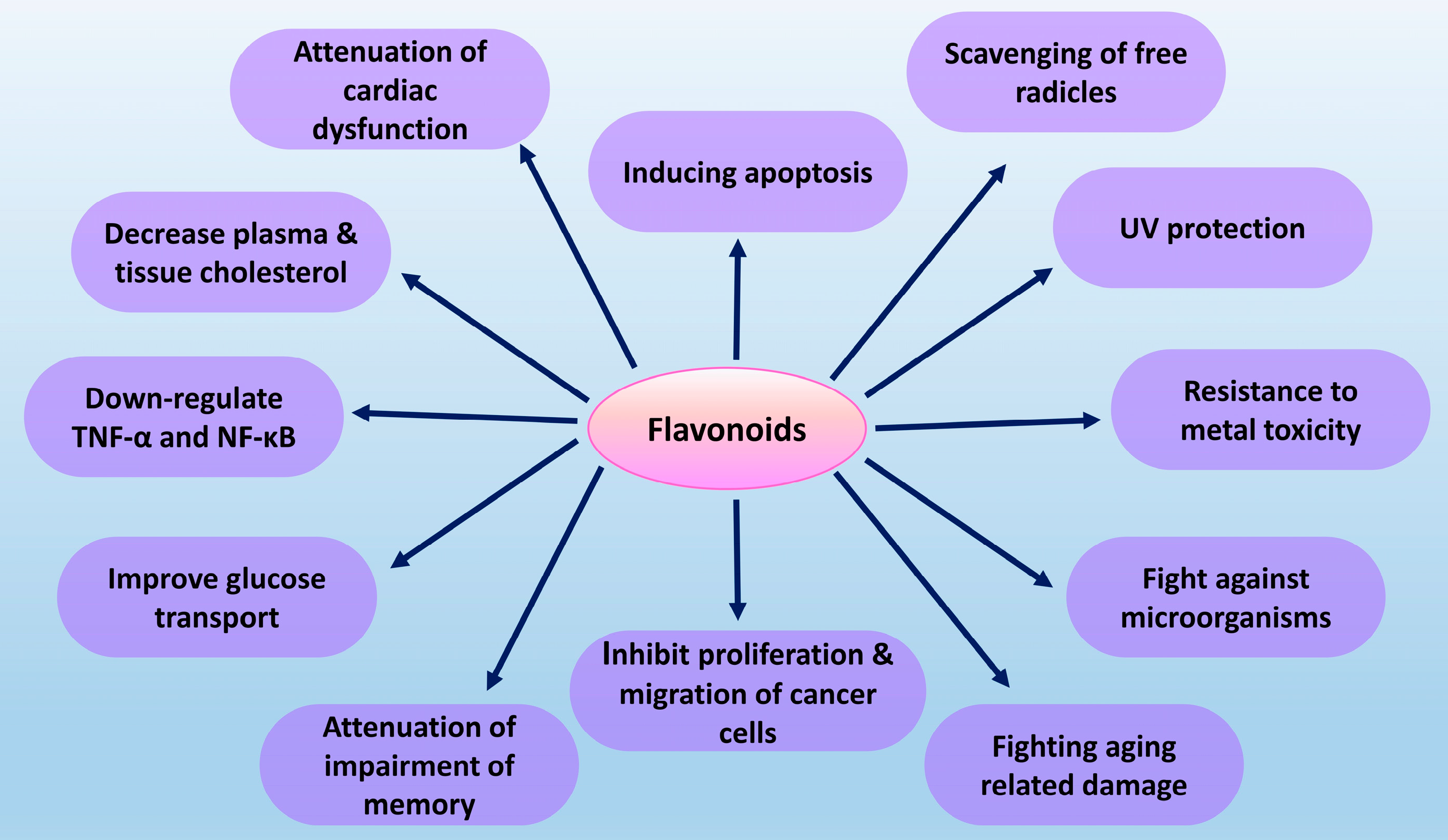
2. Flavonoids
3. Algal Flavonoids
3.1. Flavonol
3.1.1. Rutin
3.1.2. Quercetin
3.1.3. Kaempferol
3.1.4. Morin
3.2. Flavanol
3.2.1. Catechin
3.2.2. Epicatechin
3.2.3. Epigallocatechin-Gallate (EGCG)
3.3. Flavone
3.3.1. Apigenin
3.3.2. Dimethoxyflavon
3.4. Isoflavone
Genistein
3.5. Flavanone
Hesperidin
4. Prospects and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Kwok, H.F. Development of Marine-Derived Compounds for Cancer Therapy. Mar. Drugs 2021, 19, 342. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; Baart, G.J.E. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Bisol, Â.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phyther. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K. Induced Accumulation of Polyphenolics and Flavonoids in Cyanobacteria under Salt Stress Protects Organisms through Enhanced Antioxidant Activity. Am. J. Plant Sci. 2014, 5, 726–735. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Verma, S.; Meena, K.K.; Yandigeri, M. Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 2017, 7, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babić, O.; Kovač, D.; Rašeta, M.; Šibul, F.; Svirčev, Z.; Simeunović, J. Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J. Appl. Phycol. 2016, 28, 2333–2342. [Google Scholar] [CrossRef]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Ali, M.S. Spectral characterization of bioactive compounds from microalgae: N. Oculata and C. Vulgaris. Biocatal. Agric. Biotechnol. 2019, 19, 101094. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects in vitro toward hepatotoxicity model. Afr. J. Pharm. Pharmacol. 2009, 3, 133–139. [Google Scholar]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Klllç, C.S.; Sytar, O.; et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Rico, M.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ. Sci. Pollut. Res. 2015, 22, 14820–14828. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giu, D.; Oulad, Y.; Majdoub, E.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibitoye, O.B.; Uwazie, J.N.; Ajiboye, T.O. Bioactivity-guided isolation of kaempferol as the antidiabetic principle from Cucumis sativus L. fruits. J. Food Biochem. 2018, 42, e12479. [Google Scholar] [CrossRef]
- Prasad, R.; Prasad, S.B. Antitumor Activity of Rutin-Cisplatin in Combination and Its Protective Effect Against Hematotoxicity. Res. J. Life. Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 42–56. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Hsieh, Y.P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Rajput, S.A.; Wang, X.; Yan, H. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Trabelsi, L.; Mnari, A.; Abdel-Daim, M.M.; Abid-Essafi, S.; Aleya, L. Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement. Altern. Med. 2016, 16, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Yadavalli, R.; Peasari, J.R.; Mamindla, P.; Praveenkumar; Mounika, S.; Ganugapati, J. Phytochemical screening and in silico studies of flavonoids from Chlorella pyrenoidosa. Inform. Med. Unlocked 2018, 10, 89–99. [Google Scholar] [CrossRef]
- Blagojević, D.; Babić, O.; Rašeta, M.; Šibul, F.; Janjušević, L.; Simeunović, J. Antioxidant activity and phenolic profile in filamentous cyanobacteria: The impact of nitrogen. J. Appl. Phycol. 2018, 30, 2337–2346. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Tanwar, J.; Hameed, S.; Fatima, Z. Antimicrobial potential of epigallocatechin-3-gallate (EGCG): A green tea polyphenol. J. Biochem. Pharmacol. Res. 2014, 2, 167–174. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-rad, M.; Kr, D.; Sharifi-rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Yenjai, C.; Wanich, S.; Pitchuanchom, S.; Sripanidkulchai, B. Structural modification of 5,7-dimethoxyflavone from Kaempferia parviflora and biological activities. Arch. Pharm. Res. 2009, 32, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Stanisic, D.; Costa, A.F.; Favaro, W.J.; Tasic, L.; Seabra, A.B.; Duran, N. Anticancer Activities of Hesperidin and Hesperetin In vivo and their Potentiality against Bladder Cancer. J. Nanomed. Nanotechnol. 2018, 9, 1000515. [Google Scholar] [CrossRef]
- El-hadad, S.S.; Tikhomirova, N.A.; El-aziz, M.A. Biological activities of dihydroquercetin and its effect on the oxidative stability of butter oil. J. Food Process. Preserv 2020, 44, e14519. [Google Scholar] [CrossRef]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-baky, H.H.A.; El-Baz, F.K.; El-baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae. Afr. J. Biotechnol. 2009, 8, 7059–7067. [Google Scholar]
- Yadavalli, R.; Ratnapuram, H.; Motamarry, S.; Reddy, C.N.; Ashokkumar, V.; Kuppam, C. Simultaneous production of flavonoids and lipids from Chlorella vulgaris and Chlorella pyrenoidosa. Biomass Convers. Biorefin. 2020, 1–9. [Google Scholar] [CrossRef]
- Yoshie, Y.; Wang, W.; Petillo, D.; Suzuki, T. Distribution of catechins in Japanese seaweeds. Fish. Sci. 2000, 66, 998–1000. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phyther. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Oliveira, S.; Souza, G.A.; Eckert, C.R.; Silva, T.A.; Edmar Silva Sobra, E.S.; Fávero, O.P.; Ferreira, M.J.P.; Romoff, P.; Baader, W. Evaluation of Antiradical Assays Used in Determining The Antioxidant Capacity of Pure Compounds And Plant Extracts. Quim. Nov. 2014, 37, 497–503. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; Bulotta, S.; Allegri, L.; Pecce, V.; Abballe, L.; Damante, G.; Russo, D. Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocrine 2020, 67, 496–498. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 Cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. JBUON 2019, 24, 975–981. [Google Scholar]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Nie, Z.Y.; Yang, L.; Liu, X.J.; Yang, Z.; Yang, G.S.; Zhou, J.; Qin, Y.; Yu, J.; Jiang, L.L.; Wen, J.K.; et al. Morin inhibits proliferation and induces apoptosis by modulating the MIR-188-5p/PTEN/Akt regulatory pathway in CML cells. Mol. Cancer Ther. 2019, 18, 2296–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharjan, S.; Kwon, Y.S.; Lee, M.G.; Lee, K.S.; Nam, K.S. Cell cycle arrest-mediated cell death by morin in MDA-MB-231 triple-negative breast cancer cells. Pharmacol. Rep. 2021, 1–13. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.Z. Anticancer effects of catechin flavonoid in human glioma cells are mediated via autophagy induction, cell cycle arrest, inhibition of cell migration and invasion and targeting MAPK/ ERK signalling pathway. J. BU ON 2020, 25, 1084–1090. [Google Scholar]
- di Leo, N.; Battaglini, M.; Berger, L.; Giannaccini, M.; Dente, L.; Hampel, S.; Vittorio, O.; Cirillo, G.; Raffa, V. A catechin nanoformulation inhibits WM266 melanoma cell proliferation, migration and associated neo-angiogenesis. Eur. J. Pharm. Biopharm. 2017, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis induced by (−)-epicatechin in human breast cancer cells is mediated by reactive oxygen species. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef] [Green Version]
- Shariati, S.; Kalantar, H.; Pashmforosh, M.; Mansourif, E.; Khodayar, M.J. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed. Pharmacother. 2019, 114, 108776. [Google Scholar] [CrossRef]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Pinheiro, M.; Granja, A.; Farabegoli, F.; Reis, S.; Attar, R.; Sabitaliyevich, U.Y.; Xu, B.; Ahmad, A. EGCG Mediated Targeting of Deregulated Signaling Pathways and Non-Coding RNAs in Different Cancers: Focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL Mediated Signaling Pathways. Cancers 2020, 12, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shendge, A.K.; Chaudhuri, D.; Basu, T.; Mandal, N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Transl. Oncol. 2021, 23, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BU ON 2019, 24, 488–493. [Google Scholar]
- Park, W.; Park, M.Y.; Song, G.; Lim, W. 5,7-Dimethoxyflavone induces apoptotic cell death in human endometriosis cell lines by activating the endoplasmic reticulum stress pathway. Phyther. Res. 2020, 34, 2275–2286. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibitory Effect of Hesperidin on the Expression of Programmed Death Ligand (PD-L1) in Breast Cancer. Molecules 2020, 25, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sisi, A.E.; Sokkar, S.S.; Ibrahim, H.A.; Hamed, M.F.; Abu-Risha, S.E. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3, and Ki-67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam. Clin. Pharmacol. 2020, 34, 458–475. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development—advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Zhao, Z.; Mertens, M.; Li, Y.; Muylaert, K.; Vankelecom, I.F.J. A highly efficient and energy-saving magnetically induced membrane vibration system for harvesting microalgae. Bioresour. Technol. 2020, 300, 122688. [Google Scholar] [CrossRef] [PubMed]

| Subclass | Flavonoids | Algae Source | Amount | Possible Bioactivity | Reference |
|---|---|---|---|---|---|
| Flavonol | Quercetin | Nostoc ellipsosporum | 23.8 ± 1.03 µg/g fresh wt | Anticancer, Antioxidant, Antimicrobial, Antidiabetic, anti-inflammatory, Neuroprotective, Hepatoprotective | [13,14,15,16,17,18] |
| Microcheate tenera | 18.4 ± 0.85 µg/g fresh wt | ||||
| Limnothrix obliqueacuminata | 12.4 ± 0.43 µg/g fresh wt | ||||
| Hapalosiphon fontinalis | 11.7 ± 0.66 µg/g fresh wt | ||||
| Myricetin | Dunaliella tertiolecta | 6.5 ± 0.6 attomol/cell | Anticancer, antidiabetic, anti-infectious, antioxidant, anti-inflammatory, anti-obesity, neuroprotective | [19,20] | |
| Tubinaria ornata | 346 ± 3.4 µg/g | ||||
| Kaempferol | Nannochloris sp. | 12.10 ± 1.32 µg/g | Anticancer, Antioxidant, Anti-inflammatory, Antidiabetic, Neuroprotective | [14,15,21,22,23] | |
| Microcheate tenera | 7.8 ± 0.7 µg/g fresh wt | ||||
| Nostoc ellipsosporum | 4.3 ± 0.6 µg/g fresh wt | ||||
| Hapalosiphon fontinalis. | 4.8 ± 0.6 µg/g fresh wt | ||||
| Westiellopsis prolific | 7.8 ± 0.46 µg/g fresh wt | ||||
| Rutin | Microcheate tenera | 29.4 ± 0.72 µg/g | Anticancer, antioxidant, antimicrobial, antidiabetic, anti Inflammatory, neuroprotective, cardioprotective, hepatoprotective, nephroprotective | [13,14,15,19,24] | |
| Dunaliella tertiolecta | 2.8 ± 0.3 attomol/cell | ||||
| Hapalosiphon intricatus | 9.61 µg/g fresh wt | ||||
| Calothrix geitonos | 12.0 ± 0.4 µg/g fresh wt | ||||
| Mastigocladus laminosus | 13.4 ± 0.46 µg/g fresh wt | ||||
| Lyngbya sp. | 8.8 ± 0.6 µg/g fresh wt | ||||
| Phormidium | 8.8 ± 0.6 µg/g | ||||
| Nostoc sp. | 4.52 mg/g | ||||
| Morin | Caulerpa serrulata | 3730 ± 23 µg/g | Anticancer, antioxidant, anti-microbial, antidiabetic, neuroprotective, anti-arthritis, anti-inflammatory, nephroprotective, cardio protective, hepatoprotective | [25,26] | |
| Flavanol | Catechin | Porphyra tenera | 128.8 ± 2.9 µg/g | Anticancer, Antioxidant, Antimicrobial, Anti-allergenic, anti-inflammatory, UV protection activity | [19,27,28,29,30,31] |
| Spirulina platensis | 22.7 ± 2.3 µg/g | ||||
| Nannochloris sp. | 33.47 ± 3.14 µg/g | ||||
| Dunaliella tertiolecta | 36.1 ± 0.8 attomol/cell | ||||
| Euglena cantabrica | 71.4 µg/g | ||||
| Leptolyngbya sp. | 2.6 ± 0.2 mg/g | ||||
| Anabaena sp. | 35.19 µg/g DW | ||||
| Epicatechin | Dunaliella tertiolecta | 24.4 ± 0.1 attomol/cell | Anticancer, Antioxidant, Antidiabetic, anti-inflammatory, Cardio-protective, Neuroprotective | [19,29,30] | |
| Spirulina platensis | 27.5 ± 1.3 µg/g | ||||
| Porphyra tenera | 16.4 ± 0.7 µg/g | ||||
| Hizikia fusiformis | 8.2 ± 0.1 µg/g | ||||
| Epigallocatechin-gallate | Undaria pinnatifida | 7.5 ± 0.1 µg/g | Anticancer, Antioxidant, Antimicrobial, Anti-allergic, Antidiabetic, anti-inflammatory, Cardio-protective, Neuroprotective | [32,33] | |
| Porphyra tenera | 4.0 ± 0.1 µg/g | ||||
| Flavone | Apigenin | Leptolyngbya sp. | 0.4 ± 0.02 mg/g | Anticancer, antidiabetic, neuroprotective, anti-arthritis, anti-depressant, anti-inflammatory | [27,34] |
| Luteolin-7-glucoside | Diacronema lutheri | 0.8 ± 0.06 ng/g | Anticancer, anti-inflammation, anti-allergy and antioxidant | [9,27,35] | |
| Leptolyngbya sp. | 0.4 ± 0.01 mg/g | ||||
| Dimethoxyflavon | Phaeodactylum tricornutum | 28.38 ± 2.90 µg/g | Anticancer, antifungal | [22,36] | |
| Tetraselmis suecica | 19.01 ± 1.58 µg/g | ||||
| Isoflavone | Genistein | Phaeodactylum tricornutum | 1.42 ± 0.14 ng/g | Anticancer, Antioxidant, Antimicrobial, Antidiabetic, Cardioprotective | [9,37] |
| Daidzein | Phaeodactylum tricornutum | 5.9 ± 0.6 ng/g | Anticancer, antidiabetic, Anti-Osteoporosis, anti-aging, antioxidant, anti-microbial, anti-inflammatory | [9,38] | |
| Flavanone | Naringenin | Leptolyngbya sp. | 4.1 ± 0.01 mg/g | Anticancer, antioxidant, antimicrobial, anti-inflammatory, antiadipogenic, anti-diabetic, cardioprotective, eye-protective | [9,12,27] |
| Diacronema lutheri | 0.60 ± 0.06 ng/g | ||||
| Hesperidin | Gracilaria texorii | 119000 ± 1800 µg/g | Anticancer, anti-allergic, anti-oxidant and anti-inflammatory | [25,39] | |
| Flavanonol | Dihydroquercetin | Haematococcus pluvialis | 1.6 ± 0.16 ng/g | Anticancer, antioxidant, anti-bacterial | [9,30,40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. https://doi.org/10.3390/molecules26226844
Ferdous UT, Balia Yusof ZN. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules. 2021; 26(22):6844. https://doi.org/10.3390/molecules26226844
Chicago/Turabian StyleFerdous, Umme Tamanna, and Zetty Norhana Balia Yusof. 2021. "Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges" Molecules 26, no. 22: 6844. https://doi.org/10.3390/molecules26226844
APA StyleFerdous, U. T., & Balia Yusof, Z. N. (2021). Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules, 26(22), 6844. https://doi.org/10.3390/molecules26226844