Active Flavonoids from Colubrina greggii var. greggii S. Watson against Clinical Isolates of Candida spp.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extracts and Bioassay-Guided Fractionation against Candida spp.
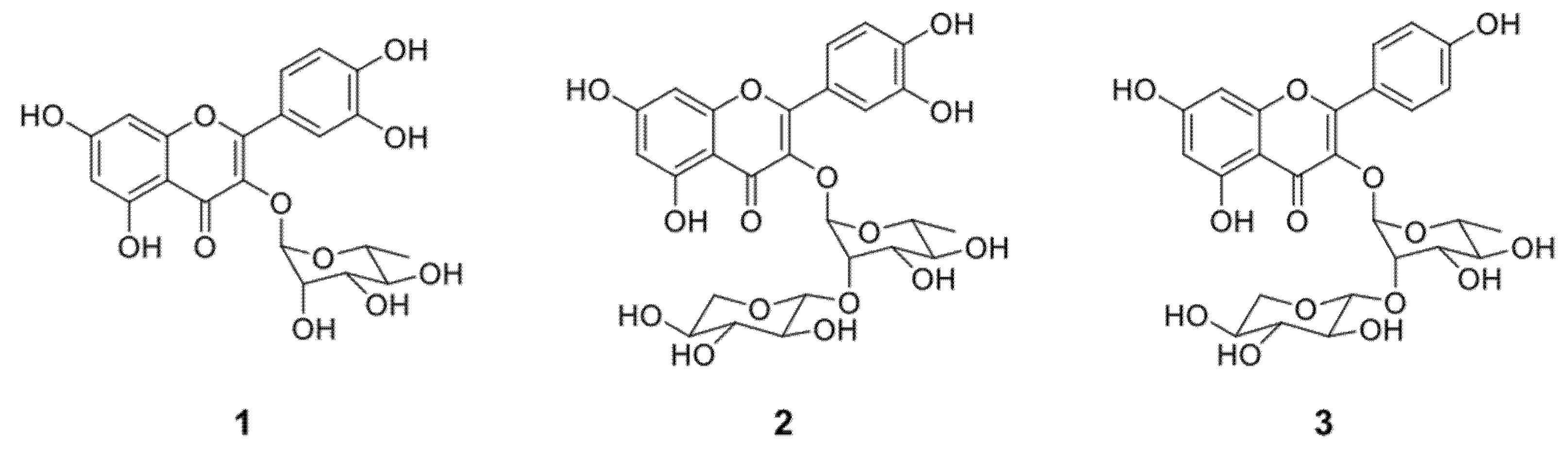
2.2. Structural Identification of the Compounds and Their Antifungal Activity
2.3. Cytotoxicity Aassay
3. Materials and Methods
3.1. General Procedures
3.2. Collection of the Plant Material
3.3. Preparation of Plant Extracts and Bioassay-Guided Fractionation
3.4. Candida spp. Isolates and Antifungal Activity Evaluation
3.5. Cytotoxicity Assay
3.5.1. Brine Shrimp Lethality Bioassay
3.5.2. In Vitro Cytotoxic Assay on Vero Cell Line
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Neumann, D.M.; Cammarata, A.; Backes, G.; Palmer, G.E.; Jursic, B.S. Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Tanida, T.; Okamoto, T.; Ueta, E.; Yamamoto, T.; Osaki, T. Antimicrobial peptides enhance the candidacidal activity of antifungal drugs by promoting the efflux of ATP from Candida cells. J. Antimicrob. Chemother. 2005, 57, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Nawange, S.R.; Warthe, N. In-vitro antifungal susceptibility reveals occurrence of azole and Allylamine resistance among clinical isolates of Candida albicans and Candida non albicans from Central India. Int. J. Pharm. Sci. Res. 2014, 5, 5267–5275. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, F.; Sabra, A.; El-Kirat-Chatel, S.; Pujol, S.; Fitton-Ouhabi, V.; Brèthes, D.; Dementhon, K.; Accoceberry, I.; Noël, T. Deletion of the Uracil Permease Gene Confers Cross-Resistance to 5-Fluorouracil and Azoles in Candida lusitaniae and Highlights Antagonistic Interaction between Fluorinated Nucleotides and Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 4476–4485. [Google Scholar] [CrossRef] [Green Version]
- Fera, M.T.; La Camera, E.; De Sarro, A. New triazoles and echinocandins: Mode of action, in vitroactivity and mechanisms of resistance. Expert Rev. Anti-Infect. Ther. 2009, 7, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Peri, A.M.; Mazzali, C.; Grande, R.; Cazzani, C.; Ricaboni, D.; Castelli, A.; Raimondi, F.; Magni, C.; Galli, M.; et al. Candidaemia Observed at a University Hospital in Milan (Northern Italy) and Review of Published Studies from 2010 to 2014. Mycopathologia 2014, 178, 227–241. [Google Scholar] [CrossRef] [PubMed]
- González, G.M.; Elizondo, M.; Ayala, J. Trends in Species Distribution and Susceptibility of Bloodstream Isolates of Candida Collected in Monterrey, Mexico, to Seven Antifungal Agents: Results of a 3-Year (2004 to 2007) Surveillance Study. J. Clin. Microbiol. 2008, 46, 2902–2905. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I. Activity of phenolic compounds from plant origin against Candida species. Ind. Crop. Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef] [Green Version]
- Alanís-Garza, B.; González-González, G.; Salazar-Aranda, R.; de Torres, N.W.; Rivas-Galindo, V. Screening of antifungal activity of plants from the northeast of Mexico. J. Ethnopharmacol. 2007, 114, 468–471. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Pérez-López, L.A.; López-Arroyo, J.; Alanís-Garza, B.A.; De Torres, N.W. Antimicrobial and Antioxidant Activities of Plants from Northeast of Mexico. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, R.F. Flora del Bajio y de Regiones Adyacentes; Instituto de Ecología: Mexico City, Mexico, 1996; pp. 17–19. [Google Scholar]
- Schmourlo, G.; Mendonça-Filho, R.R.; Alviano, C.S.; Costa, S.S. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J. Ethnopharmacol. 2005, 96, 563–568. [Google Scholar] [CrossRef]
- Mendieta, R.M. Plantas Medicinales del Estado de Yucatán. Boletín de la Sociedad Botánica de México 1982, 43, 94–95. [Google Scholar]
- García-Sosa, K.; Villarreal-Alvarez, N.; Lübben, P.; Peña-Rodríguez, L.M. Chrysophanol, an antimicrobial anthraquinone from the root extract of Colubrina greggii. J. Mex. Chem. Soc. 2006, 50, 76–78. [Google Scholar]
- Clinical and Laboratory Standards Institute M27-A3. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; pp. 5–10. [Google Scholar]
- Kaan, Ö.; Koç, A.N.; Atalay, M.A.; Mutlu, S.F. Molecular epidemiology, antifungal susceptibility and virulence factors of Candida glabrata complex strains in Kayseri/Turkey. Microb. Pathog. 2021, 154, 104870. [Google Scholar] [CrossRef]
- Butassi, E.; Svetaz, L.A.; Ivancovich, J.J.; Feresin, G.E.; Tapia, A.; Zacchino, S.A. Synergistic mutual potentiation of antifungal activity of Zuccagnia punctata Cav. and Larrea nitida Cav. extracts in clinical isolates of Candida albicans and Candida glabrata. Phytomedicine 2015, 22, 666–678. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Singh, S.; Tewari, R.; Bhatt, V.; Sharma, J.; Maurya, I. Phytochemical analysis and mode of action against Candida glabrata of Paeonia emodi extracts. Journal de Mycologie Médicale 2018, 28, 443–451. [Google Scholar] [CrossRef]
- Yunus, S.N.M.; Zolkeflee, N.K.Z.; Jaafar, A.H.; Abas, F. Metabolite identification in different fractions of Ficus auriculata Loureiro fruit using the 1H-NMR metabolomics approach and UHPLC-MS/MS. S. Afr. J. Bot. 2021, 138, 348–363. [Google Scholar] [CrossRef]
- Freitas, L.B.; Boaventura, M.A.D.; Santos, W.L.; Stehmann, J.; Júnior, D.D.; Lopes, M.T.; Magalhães, T.F.; da Silva, D.L.; de Resende, M.A. Allelopathic, cytotoxic and antifungic activities of new dihydrophenanthrenes and other constituents of leaves and roots extracts of Banisteriopsis anisandra (Malpighiaceae). Phytochem. Lett. 2015, 12, 9–16. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.M.; De Torres, N.W. Activity of Polyphenolic Compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar] [CrossRef] [PubMed]
- Soicke, H.; Görler, K.; Waring, H. Flavonol Glykoside aus Moghania faginea. Planta Medica 1990, 56, 410–412. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Olsen, C.E.; Møller, B.L. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Bilia, A.R.; Morelli, I. Phytochemical investigations of Licania genus. Flavonoids and triterpenoids from Licania pittieri. Pharm. Acta Helvetiae 1995, 70, 223–226. [Google Scholar] [CrossRef]
- Rodrigues, J.; Rinaldo, D.; Santos, L.; Vilegas, W. An unusual C6“–”C6′′ linked flavonoid from Miconia cabucu (Melastomataceae). Phytochemistry 2007, 68, 1781–1784. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Markham, K.R.; Ternai, B. 13C NMR of flavonoids II. Flavonoids others than flavones and flavonol aglycones. Tetrahedron 1976, 32, 2607–2611. [Google Scholar] [CrossRef]
- Bloor, S.J. Blue flower colour derived from flavonol—anthocyanin co-pigmentation in Ceanothus papillosus. Phytochemistry 1997, 45, 1399–1405. [Google Scholar] [CrossRef]
- Bisignano, G.; Sanogo, R.; Marino, A.; Aquino, R.P.; ’Angelo, V.D.; Germanò, M.P.; De Pasquale, R.; Pizza, C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett. Appl. Microbiol. 2000, 30, 105–108. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Braguini, W.L.; Alves, B.B.; Pires, N.V. Toxicity assessment of Lavandula officinalis extracts in Brine Shrimp (Artemia salina). Toxicol. Mech. Methods 2019, 29, 411–420. [Google Scholar] [CrossRef]
- de Souza Corrêa, J.G.; Bianchin, M.; Lopes, A.P.; Silva, E.; Ames, F.Q.; Pomini, A.M.; Carpes, S.T.; Rinaldi, J.D.C.; Melo, R.C.; Kioshima, E.S.; et al. Chemical profile, antioxidant and anti-inflammatory properties of Miconia albicans (Sw.) Triana (Melastomataceae) fruits extract. J. Ethnopharmacol. 2021, 273, 113979. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E.; Goetz, C.M.; McLaughlin, J.L.; Suffness, M. A blind comparison of simple bench-top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991, 2, 107–111. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
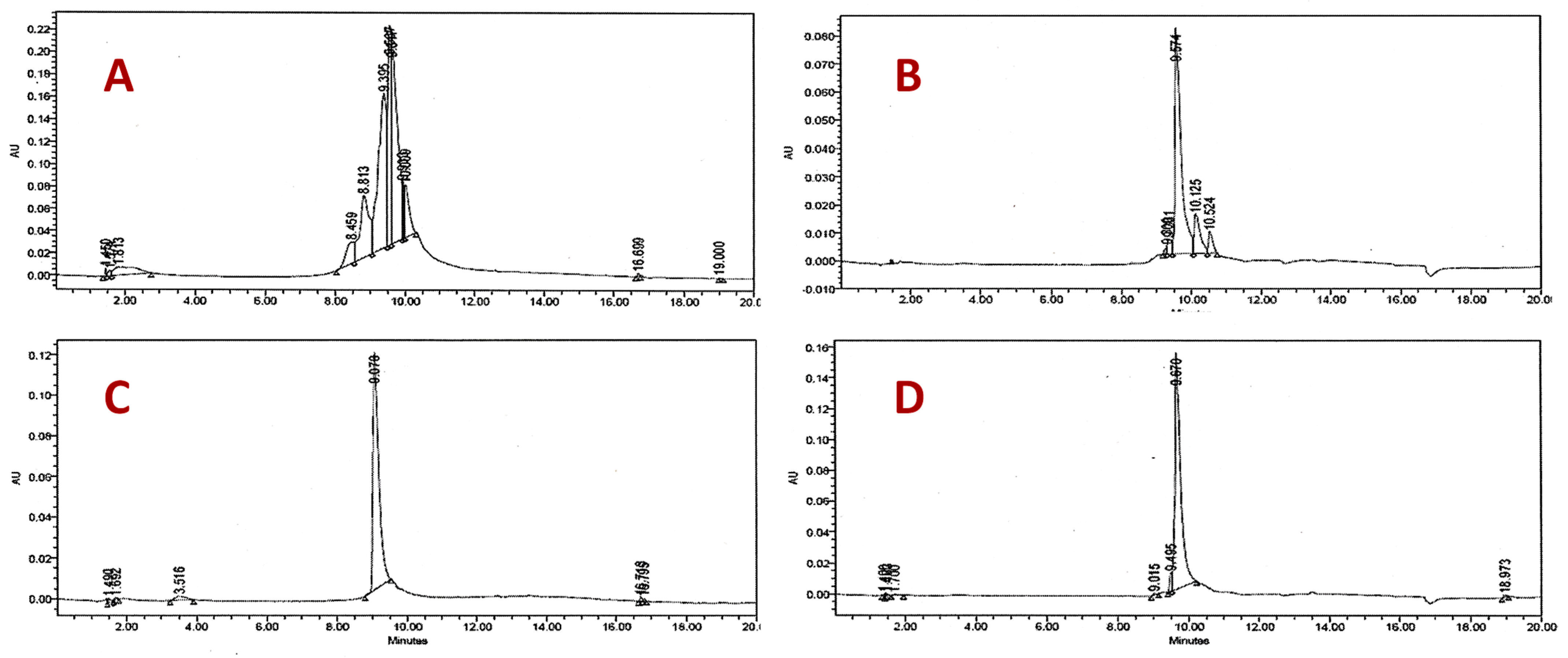
| Extracts, Fractions, and Compounds | MIC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cg | Cg | Cp | Cp | Ca | Ca | Ct | Ct | Ck | Ck | |
| 83 | 84 | 95 | 96 | 97 | 98 | 105 | 166 | 137 | 168 | |
| Ethyl Acetate extract | 16 | 16 | 32 | 32 | 32 | 125 | 63 | 63 | 16 | 16 |
| Butanol extract | 2 | 2 | 63 | 63 | 63 | 63 | 250 | 125 | 16 | 16 |
| Hexane extract | 63 | 63 | 125 | 125 | 250 | >500 | >500 | >500 | 125 | 125 |
| Fraction AE-F1 | >500 | >500 | >500 | >500 | 250 | >500 | >500 | >500 | >500 | >500 |
| Fraction AE-F2 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction AE-F3 | 16 | 16 | 125 | 125 | 500 | 500 | >500 | >500 | >500 | >500 |
| Fraction AE-F4 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction AE-F5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction AE-F6 | 63 | 63 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 16 |
| Fraction But-F1 | 2 | >500 | >500 | 64 | >500 | >500 | >500 | 125 | >500 | 64 |
| Fraction But-F2 | 2 | >500 | >500 | 250 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction But-F3 | 4 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction But-F4 | 2 | 2 | 63 | 63 | 63 | 63 | 63 | 63 | 32 | 32 |
| Compound 1 | 16 | 16 | 125 | 125 | 500 | 500 | >500 | >500 | 500 | 500 |
| Compound 2 | 63 | 63 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Compound 3 | 16 | 16 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fluconazol | 32 | 63 | 0.5 | 1 | 4 | 4 | 2 | 2 | 32 | 4 |
| Compounds | Vero Cell Line CC50 (µg/mL) a | Artemia Salina LC50 (µg/mL) a |
|---|---|---|
| 1 | 1659.6 ± 81 | >500 |
| 2 | >2000 | 322.8 ± 0.009 |
| 3 | >2000 | 254.8 ± 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Martínez, E.M.; Tamez-Fernández, J.F.; González-González, G.M.; Silva-Mares, D.A.; Waksman-Minsky, N.; Pérez-López, L.A.; Rivas-Galindo, V.M. Active Flavonoids from Colubrina greggii var. greggii S. Watson against Clinical Isolates of Candida spp. Molecules 2021, 26, 5760. https://doi.org/10.3390/molecules26195760
Melchor-Martínez EM, Tamez-Fernández JF, González-González GM, Silva-Mares DA, Waksman-Minsky N, Pérez-López LA, Rivas-Galindo VM. Active Flavonoids from Colubrina greggii var. greggii S. Watson against Clinical Isolates of Candida spp. Molecules. 2021; 26(19):5760. https://doi.org/10.3390/molecules26195760
Chicago/Turabian StyleMelchor-Martínez, Elda M., Juan F. Tamez-Fernández, Gloria María González-González, David A. Silva-Mares, Noemí Waksman-Minsky, Luis Alejandro Pérez-López, and Verónica M. Rivas-Galindo. 2021. "Active Flavonoids from Colubrina greggii var. greggii S. Watson against Clinical Isolates of Candida spp." Molecules 26, no. 19: 5760. https://doi.org/10.3390/molecules26195760
APA StyleMelchor-Martínez, E. M., Tamez-Fernández, J. F., González-González, G. M., Silva-Mares, D. A., Waksman-Minsky, N., Pérez-López, L. A., & Rivas-Galindo, V. M. (2021). Active Flavonoids from Colubrina greggii var. greggii S. Watson against Clinical Isolates of Candida spp. Molecules, 26(19), 5760. https://doi.org/10.3390/molecules26195760







