Direct Photoexcitation of Benzothiazolines: Acyl Radical Generation and Application to Access Heterocycles
Abstract
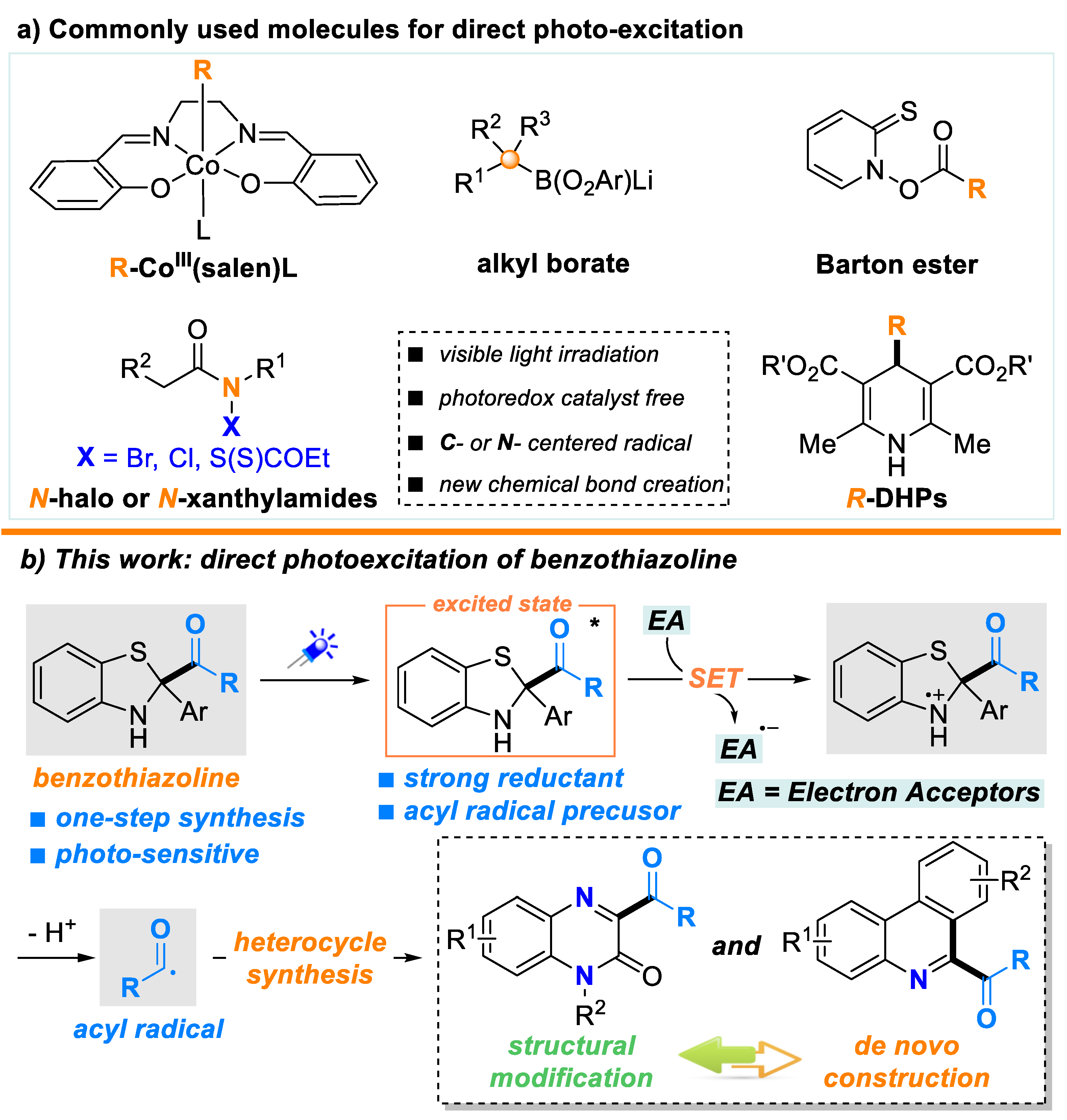
:1. Introduction
2. Results and Discussion
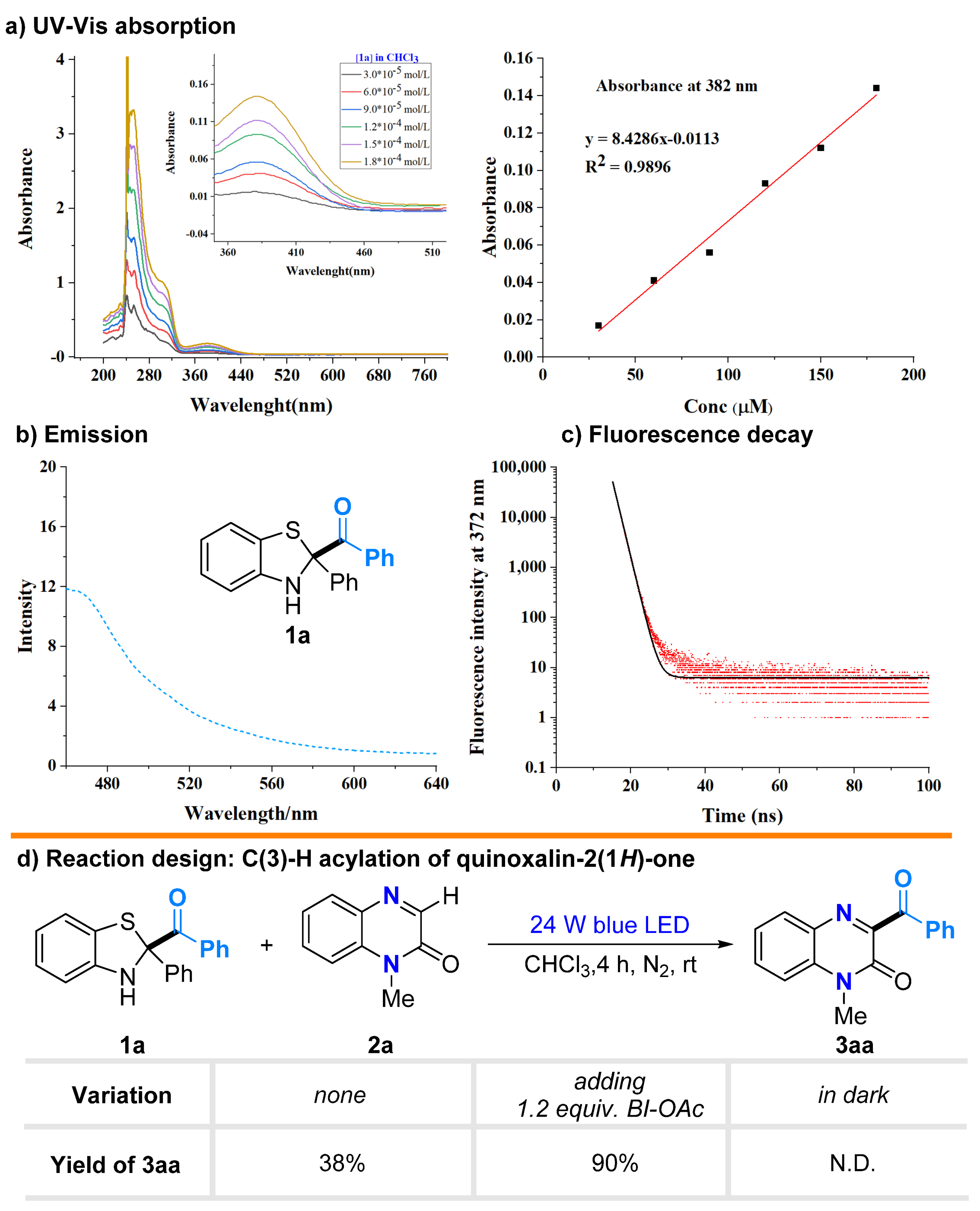
2.1. Photophysical Property of Benzothiazolines
2.2. Substrate Scopes for the Acylation of Quinoxaline-2(1H)-ones
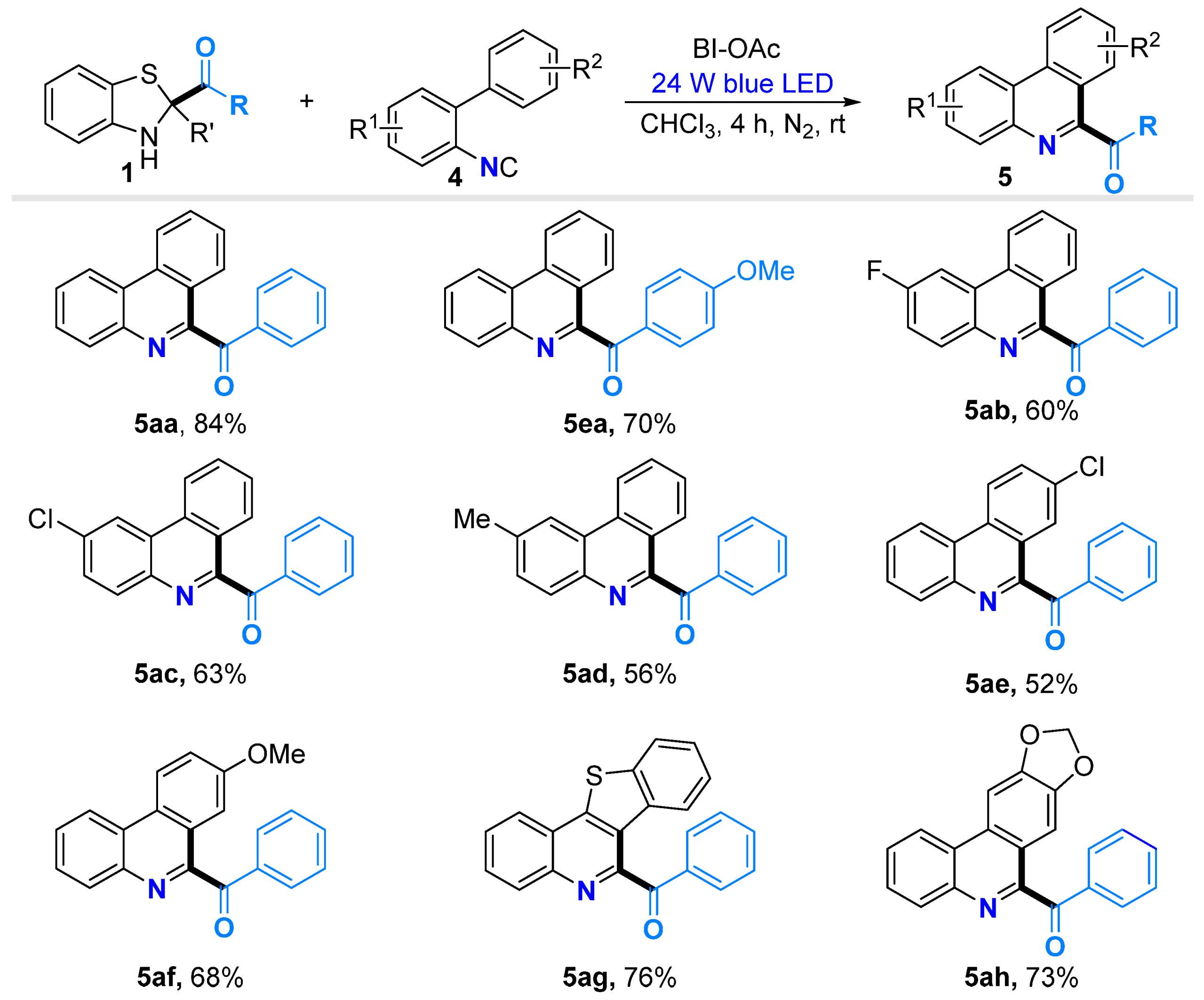
2.3. Substrate Scopes for the Synthesis of Phenanthridines
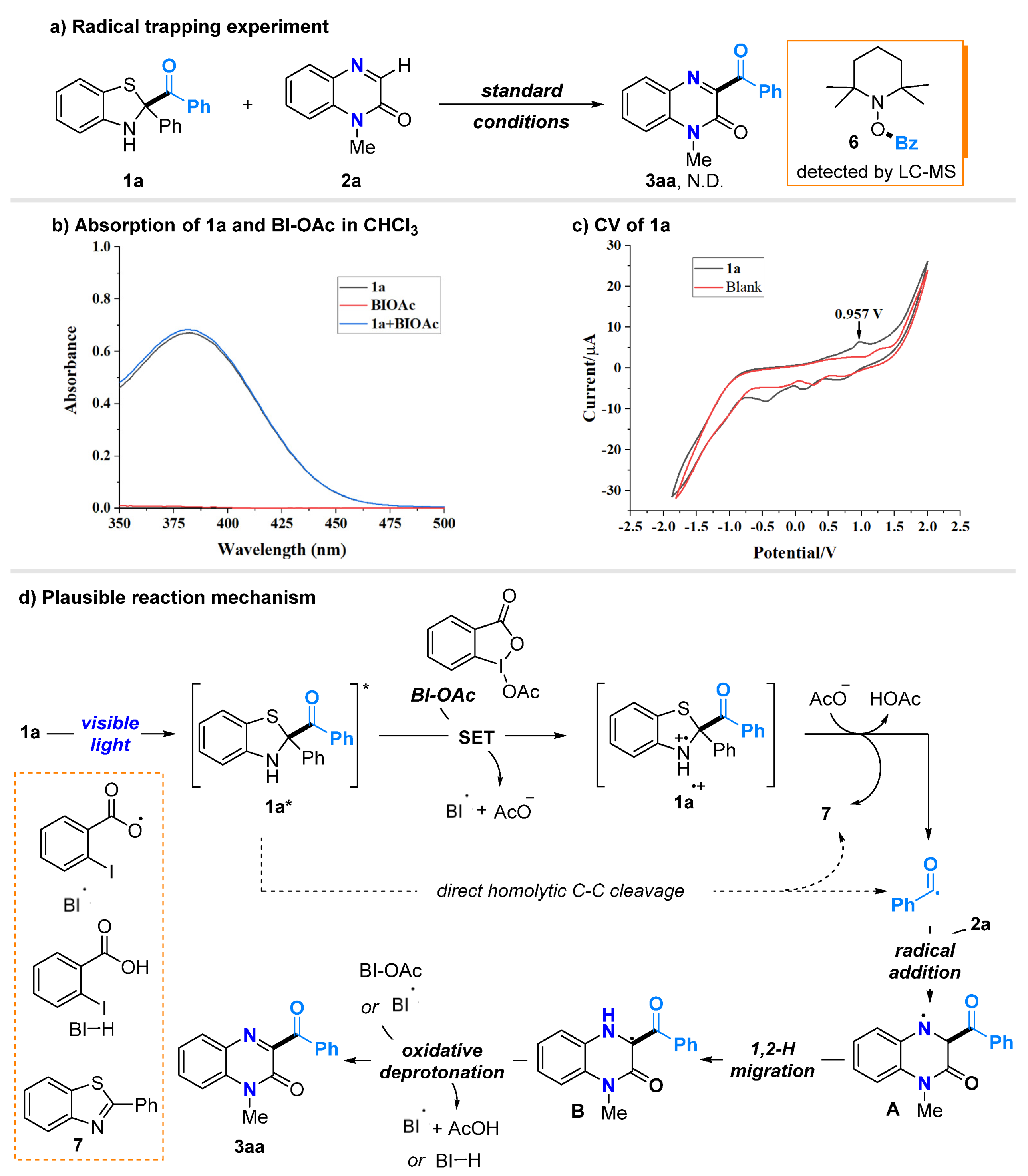
2.4. Study on the Mechanism
3. Materials and Methods
3.1. Generating Information
3.2. Experimental Procedures
3.2.1. Synthesis of Benzothiazolines
3.2.2. Synthesis of Quinoxalin-2(1H)-ones
3.2.3. Synthesis of 3-Acyl Quinoxaline-2(1H)-ones
3.2.4. Synthesis of Phenanthridines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Xiao, W.-J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Hopkinson, M.N.; Sahoo, B.; Li, J.-L.; Glorius, F. Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Chem. Eur. J. 2014, 20, 3874–3886. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.-G.; Xuan, J.; Xiao, W.-J. Visible Light-Mediated C-P Bond Formation Reactions. Sci. Bull. 2019, 64, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Visible Light-Driven Organic Photochemical Synthesis in China. Sci. China Chem. 2019, 62, 24–57. [Google Scholar] [CrossRef]
- Xuan, J.; He, X.-K.; Xiao, W.-J. Visible Light-Promoted Ring-Opening Functionalization of Three-Membered Carbo- and Heterocycles. Chem. Soc. Rev. 2020, 49, 2546–2556. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. [Google Scholar] [CrossRef]
- Witzel, S.; Hashmi, A.S.K.; Xie, J. Light in Gold Catalysis. Chem. Rev. 2021, 121, 8868–8925. [Google Scholar] [CrossRef] [PubMed]
- Foster, R. Electron Donor-Acceptor Complexes. J. Phys. Chem. 1980, 84, 2135–2141. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Kochi, J.K. Fresh Look at Electron-Transfer Mechanisms via the Donor/Acceptor Bindings in the Critical Encounter Complex. Acc. Chem. Res. 2008, 41, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, Q.-Q.; Tan, F.; Lu, L.-Q.; Xiao, W.-J. Visible-Light-Driven Organic Photochemical Reactions in the Absence of External Photocatalysts. Synthesis 2019, 51, 3021–3054. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor—Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Ohmiya, H. Direct Excitation Strategy for Radical Generation in Organic Synthesis. Chem. Soc. Rev. 2021, 50, 6320–6332. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Windgassen, R.J. Alkylcobaloximes and Their Relation to Alkylcobalamins. J. Am. Chem. Soc. 1966, 88, 3738–3743. [Google Scholar] [CrossRef]
- Branchaud, B.P.; Meier, M.S.; Choi, Y. Alkyl-Alkenyl Cross Coupling via Alkyl Cobaloxime Radical Chemistry. An Alkyl Equivalent to the Heck Reaction Compatible with Common Organic Functional Groups. Tetrahedron Lett. 1988, 29, 167–170. [Google Scholar] [CrossRef]
- Kozlowski, P.M.; Kamachi, T.; Toraya, T.; Yoshizawa, K. Does Cob(II)alamin Act as a Conductor in Coenzyme B12 Dependent Mutases? Angew. Chem. Int. Ed. 2007, 46, 980–983. [Google Scholar] [CrossRef]
- Demarteau, J.; Debuigne, A.; Detrembleur, C. Organocobalt Complexes as Sources of Carbon-Centered Radicals for Organic and Polymer Chemistries. Chem. Rev. 2019, 119, 6906–6955. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Dowlatshahi, H.A.; Motherwell, W.B.; Villemin, D. A New Radical Decarboxylation Reaction for the Conversion of Carboxylic Acids into Hydrocarbons. J. Chem. Soc. Chem. Commun. 1980, 732–733. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Crich, D.; Motherwell, W.B. New and Improved Methods for the Radical Decarboxylation of Acids. J. Chem. Soc. Chem. Commun. 1983, 939–941. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Lacher, B.; Zard, S.Z. The Invention of Radical Reactions: Part XVI. Radical Decarboxylative Bromination and Iodination of Aromatic Acids. Tetrahedron 1987, 43, 4321–4328. [Google Scholar] [CrossRef]
- Schweitzer-Chaput, B.; Horwitz, M.A.; de Pedro Beato, E.; Melchiorre, P. Photochemical Generation of Radicals from Alkyl Electrophiles Using a Nucleophilic Organic Catalyst. Nat. Chem. 2019, 11, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Spinnato, D.; Schweitzer-Chaput, B.; Goti, G.; Ošeka, M.; Melchiorre, P. A Photochemical Organocatalytic Strategy for the α-Alkylation of Ketones by using Radicals. Angew. Chem. Int. Ed. 2020, 59, 9485–9490. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.Y.; Schuster, G.B. Photoalkylation of Dicyanoarenes with Alkyltriphenylborate Salts. J. Am. Chem. Soc. 1985, 107, 6710–6711. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gottschalk, P.; Davis, P.D.; Schuster, G.B. Electron-Transfer Reactions in Cyanine Borate Ion Pairs: Photopolymerization Initiators Sensitive to Visible Light. J. Am. Chem. Soc. 1988, 110, 2326–2328. [Google Scholar] [CrossRef]
- Primer, D.N.; Molander, G.A. Enabling the Cross-Coupling of Tertiary Organoboron Nucleophiles through Radical-Mediated Alkyl Transfer. J. Am. Chem. Soc. 2017, 139, 9847–9850. [Google Scholar] [CrossRef]
- Sato, Y.; Nakamura, K.; Sumida, Y.; Hashizume, D.; Hosoya, T.; Ohmiya, H. Generation of Alkyl Radical through Direct Excitation of Boracene-Based Alkylborate. J. Am. Chem. Soc. 2020, 142, 9938–9943. [Google Scholar] [CrossRef]
- Schmidt, V.A.; Quinn, R.K.; Brusoe, A.T.; Alexanian, E.J. Site-Selective Aliphatic C–H Bromination Using N-Bromoamides and Visible Light. J. Am. Chem. Soc. 2014, 136, 14389–14392. [Google Scholar] [CrossRef]
- Czaplyski, W.L.; Na, C.G.; Alexanian, E.J. C–H Xanthylation: A Synthetic Platform for Alkane Functionalization. J. Am. Chem. Soc. 2016, 138, 13854–13857. [Google Scholar] [CrossRef]
- Na, C.G.; Alexanian, E.J. A General Approach to Site-Specific, Intramolecular C−H Functionalization Using Dithiocarbamates. Angew. Chem. Int. Ed. 2018, 57, 13106–13109. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, L.; Prieto, A.; Roy, S.R.; Melchiorre, P. Radical-Based C−C Bond-Forming Processes Enabled by the Photoexcitation of 4-Alkyl-1,4-dihydropyridines. Angew. Chem. Int. Ed. 2017, 56, 15039–15043. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Buzzetti, L.; Perego, L.A.; Melchiorre, P. A Redox-Active Nickel Complex that Acts as an Electron Mediator in Photochemical Giese Reactions. Angew. Chem. Int. Ed. 2019, 58, 4953–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfo, E.; Tang, X.; Raha Roy, S.; Melchiorre, P. Photo-chemical Asymmetric Nickel-Catalyzed Acyl Cross-Coupling. Angew. Chem. Int. Ed. 2019, 58, 16854–16858. [Google Scholar] [CrossRef]
- Bieszczad, B.; Perego, L.A.; Melchiorre, P. Photochemical C−H Hydroxyalkylation of Quinolines and Isoquinolines. Angew. Chem. Int. Ed. 2019, 58, 16878–16883. [Google Scholar] [CrossRef] [Green Version]
- Henseler, A.; Kato, M.; Mori, K.; Akiyama, T. Chiral Phosphoric Acid Catalyzed Transfer Hydrogenation: Facile Synthetic Access to Highly Optically Active Trifluoromethylated Amines. Angew. Chem. Int. Ed. 2011, 50, 8180–8183. [Google Scholar] [CrossRef]
- Zhu, C.; Saito, K.; Yamanaka, M.; Akiyama, T. Benzothiazoline: Versatile Hydrogen Donor for Organocata-lytic Transfer Hydrogenation. Acc. Chem. Res. 2015, 48, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, W.; Li, Y.; Cheng, X. Visible-Light-Induced Difluoro-propargylation Reaction with Benzothiazoline as a Reductant. Adv. Synth. Catal. 2018, 360, 1466–1472. [Google Scholar] [CrossRef]
- Li, G.; Chen, R.; Wu, L.; Fu, Q.; Zhang, X.; Tang, Z. Alkyl Transfer from C-C Cleavage. Angew. Chem. Int. Ed. 2013, 52, 8432–8436. [Google Scholar] [CrossRef]
- Li, L.; Guo, S.; Wang, Q.; Zhu, J. Acyl Radicals from Benzothiazolines: Synthons for Alkylation, Alkenylation, and Alkynylation Reactions. Org. Lett. 2019, 21, 5462–5466. [Google Scholar] [CrossRef] [PubMed]
- Uchikura, T.; Moriyama, K.; Toda, M.; Mouri, T.; Ibáñez, I.; Akiyama, T. Benzothiazolines as Radical Transfer Reagents: Hydroalkylation and Hydroacylation of Alkenes by Radical Generation under Photoirradiation Conditions. Chem. Commun. 2019, 55, 11171–11174. [Google Scholar] [CrossRef] [PubMed]
- Uchikura, T.; Toda, M.; Mouri, T.; Fujii, T.; Moriyama, K.; Ibáñez, I.; Akiyama, T. Radical Hydroalkylation and Hydroacylation of Alkenes by the Use of Benzothiazoline under Thermal Conditions. J. Org. Chem. 2020, 85, 12715–12723. [Google Scholar] [CrossRef]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent Advances in the Direct Functionalization of Quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar] [CrossRef]
- Rostoll-Berenguer, J.; Blay, G.; Pedro, J.R.; Vila, C. Recent Advances in Photocatalytic Functionalization of Quinoxalin-2-ones. Eur. J. Org. Chem. 2020, 2020, 6148–6172. [Google Scholar] [CrossRef]
- Kavarnos, G.J. Fundamentals of Photo-Induced Electron Transfer; VCH-Publishers: Weinheim, Germany, 1993. [Google Scholar]
- Balzani, V. (Ed.) Electron Transfer in Chemistry; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- He, X.-K.; Lu, J.; Zhang, A.-J.; Zhang, Q.-Q.; Xu, G.-Y.; Xuan, J. BI-OAc-Accelerated C3–H Alkylation of Quinoxalin-2(1H)-ones under Visible-Light Irradiation. Org. Lett. 2020, 22, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Feng, J.-G.; Wu, L.; Zhang, Y.-Q. Aliphatic Aldehydes: Novel Radical Alkylating Reagents. Adv. Synth. Catal. 2019, 361, 1700–1709. [Google Scholar] [CrossRef]
- Lu, H.; Yu, T.-Y.; Xu, P.-F.; Wei, H. Selective Decarbonylation via Transition-Metal-Catalyzed Carbon–Carbon Bond Cleavage. Chem. Rev. 2021, 121, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ruchelman, A.L.; Zhou, N.; Liu, A.A.; Liu, L.F.; LaVoie, E.J. Esters and Amides of 2,3-dimethoxy-8,9-methylenedioxy-benzo[i]phenanthridine-12-carboxylic acid: Potent Cytotoxic and Topoisomerase I-Targeting Agents. Bioorg. Med. Chem. 2005, 13, 6782–6794. [Google Scholar] [CrossRef]
- Bernardo, P.H.; Wan, K.-F.; Sivaraman, T.; Xu, J.; Moore, F.K.; Hung, A.W.; Mok, H.Y.K.; Yu, V.C.; Chai, C.L.L. Structure−Acivity Relationship Studies of Phenanthridine-Based Bcl-XL Inhibitors. J. Med. Chem. 2008, 51, 6699–6710. [Google Scholar] [CrossRef]
- Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Studer, A. 6-Trifluoromethyl-Phenanthridines through Radical Trifluoromethylation of Isonitriles. Angew. Chem. Int. Ed. 2013, 52, 10792–10795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent Advances in the Synthesis of Nitrogen Heterocycles via Radical Cascade Reactions using Isonitriles as Radical. Acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Stu-der, A. 6-Perfluoroalkylated Phenanthridines via Radical Perfluoroalkylation of Isonitriles. Org. Lett. 2014, 16, 3990–3993. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.-K.; Lu, J.; Ye, H.-B.; Li, L.; Xuan, J. Direct Photoexcitation of Benzothiazolines: Acyl Radical Generation and Application to Access Heterocycles. Molecules 2021, 26, 6843. https://doi.org/10.3390/molecules26226843
He X-K, Lu J, Ye H-B, Li L, Xuan J. Direct Photoexcitation of Benzothiazolines: Acyl Radical Generation and Application to Access Heterocycles. Molecules. 2021; 26(22):6843. https://doi.org/10.3390/molecules26226843
Chicago/Turabian StyleHe, Xiang-Kui, Juan Lu, Hai-Bing Ye, Lei Li, and Jun Xuan. 2021. "Direct Photoexcitation of Benzothiazolines: Acyl Radical Generation and Application to Access Heterocycles" Molecules 26, no. 22: 6843. https://doi.org/10.3390/molecules26226843
APA StyleHe, X.-K., Lu, J., Ye, H.-B., Li, L., & Xuan, J. (2021). Direct Photoexcitation of Benzothiazolines: Acyl Radical Generation and Application to Access Heterocycles. Molecules, 26(22), 6843. https://doi.org/10.3390/molecules26226843





