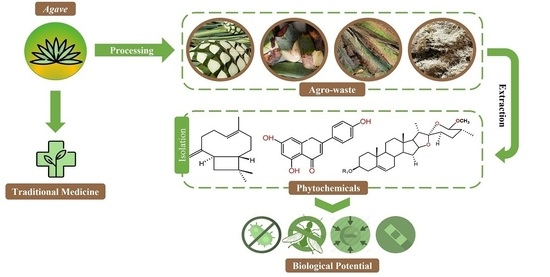
Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential
Abstract
:1. Introduction
Agave in Traditional Medicine
2. Extraction Methods Used to Recover Polyphenolic Compounds from Agave Agro-Waste
3. Bioactivity of Identified Phytochemicals from the Agave Genus
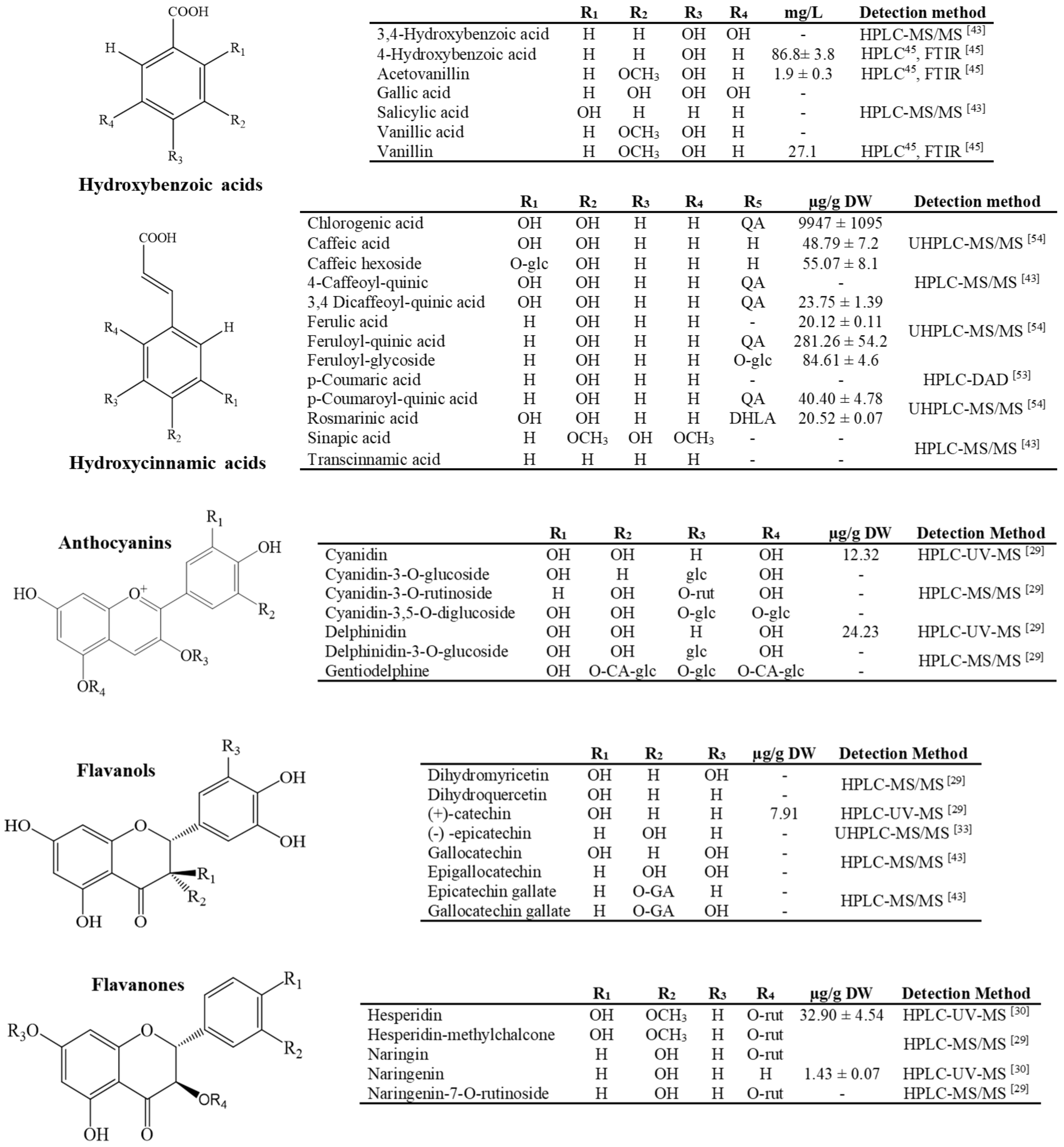
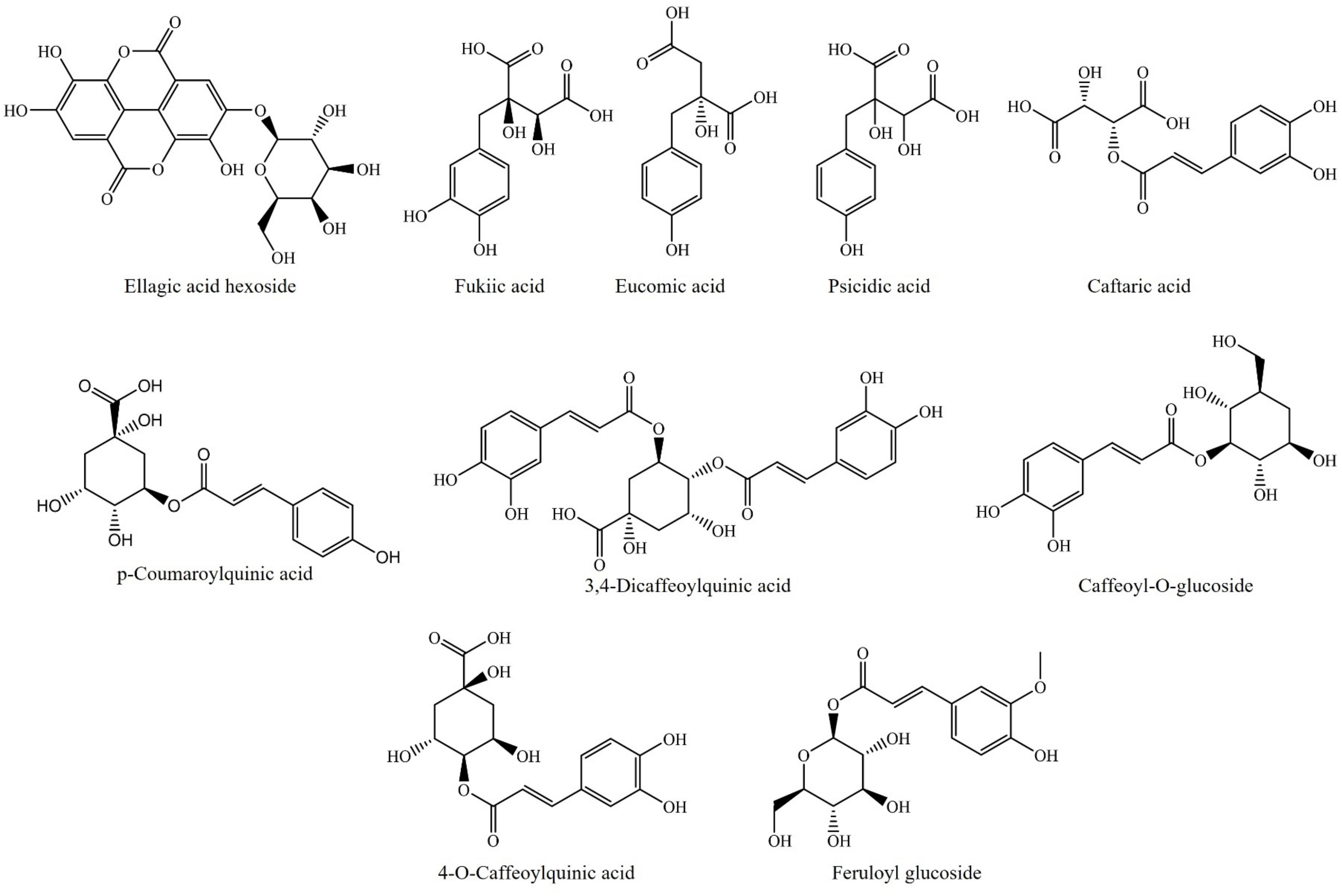
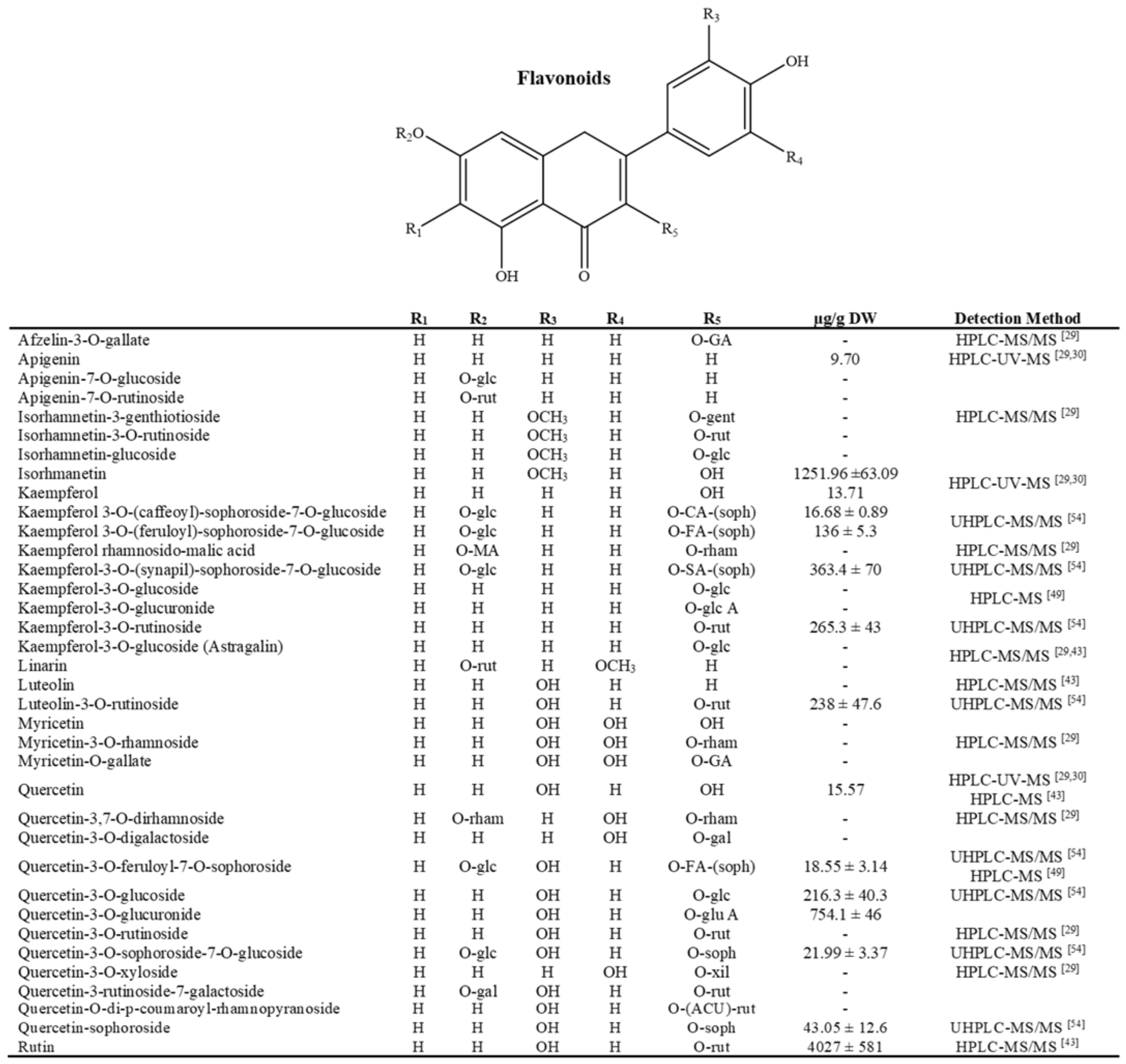
3.1. Polar Compounds: Phenols and Flavonoids
3.2. Non-Polar Compounds: Saponins
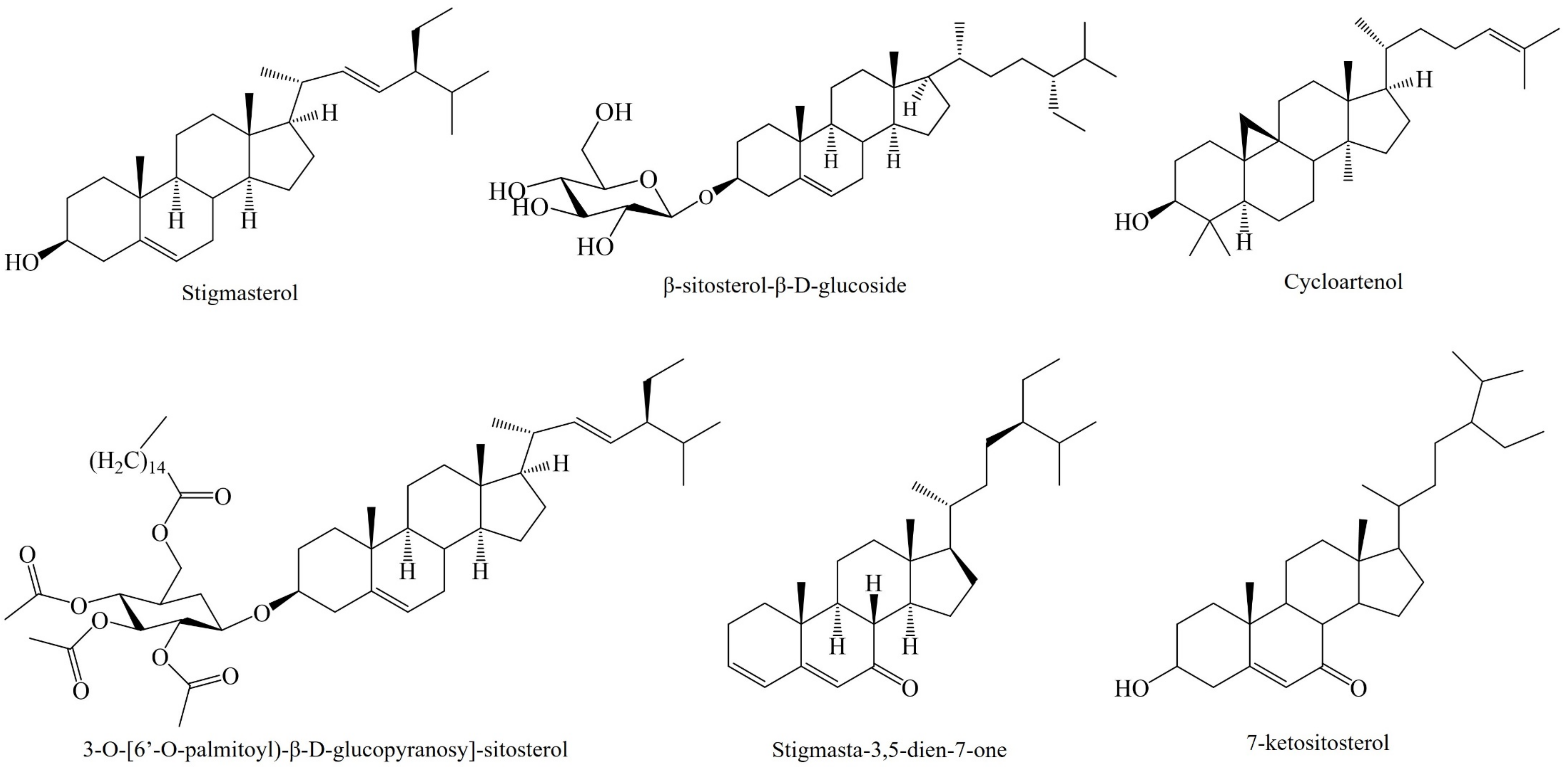
3.3. Phytosterols
4. The Role of Agave Extracts as Biopesticides
4.1. Insecticide Activity
4.2. Molluscicide Activity
4.3. Larvicide Activity
4.4. Nematicide Activity
4.5. Antifungal Activity
4.6. Antiparasitic Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Andrade, J.; De la Barrera, E.; Reyes, C.; Vargas-Soto, G.; Cervera, J. El Metabolismo Ácido de Las Crasuláceas: Diversidad, Fisiología Ambiental y Productividad. Boletín Soc. Botánica México 2007, 81, 37–50. [Google Scholar] [CrossRef] [Green Version]
- García Mendoza, A. Distribution of Agave (Agavaceae) in Mexico. Cact. Succul. J. 2002, 74, 177–187. [Google Scholar]
- García Mendoza, A.; Galván Villanueva, R. Riqueza de Las Familias Agavaceae y Nolinaceae En México. Bot. Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Gschaedler, A.C.; Gutiérrez Mora, A.; Contreras Ramos, S.M.; Davila Vazquez, G.; Gallardo Valdez, J. Panorama del Aprovechaminto de Los Agaves en Mexico; Gshchaedler Mathis, A.C., Villareal Hernández, S., Gutiérrez Mora, A., Ortiz Basurto, R.I., Moreno Terrazas Casildo, R., Lappe Oliveras, P.E., Larralde Corna, C.P., Contreras Ramos, S.M., Dávila Vázquez, G., Gallardo Váldez, J., Eds.; CONACYT, CIATEJ, AGARED: Guadalajara, Mexico, 2017; pp. 1–300. ISBN 9786079754853. [Google Scholar]
- Pérez-Zavala, M.D.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A Natural Renewable Resource with Multiple Applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef]
- Taylor, N.T.; Davis, K.M.; Abad, H.; McClung, M.R.; Moran, M.D. Ecosystem Services of the Big Bend Region of the Chihuahuan Desert. Ecosyst. Serv. 2017, 27, 48–57. [Google Scholar] [CrossRef]
- Castillo Quiroz, D.; Cano Pineda, A.; Berlanga Retes, C. Establecimiento y Aprovechamiento de Lechuguilla (Agave Lechuguilla Torr.); Comisión Nacional Forestal-Instituto Nacional de Investigaciones Forestales: Zapopan, Jalisco, México, 2012; pp. 1–33. [Google Scholar]
- Díaz-Jiménez, L.; Carlos-Hernandez, S.; Jasso de Rodríguez, D.; Rodríguez-García, R. Conceptualization of a Biorefinery for Guishe Revalorization. Ind. Crops Prod. 2019, 138, 111441. [Google Scholar] [CrossRef]
- Colunga-GarcíaMarín, P.; Torres-García, I.; Casas, A.; Figueredo-Urbina, C.J.; Rangel-Landa, S.; Delgado-Lemus, A.; Vargas, O.; Cabrera-Toledo, D.; Zizumbo-Villareal, D.; Aguirre-Dugua, X.; et al. Los Agaves y Las Prácticas Mesoamericanas de Aprovechamiento, Manejo y Domesticación. Domest. Cont. Am. 2017, 2, 273–309. [Google Scholar]
- Radding, C. The Children of Mayahuel: Agaves, Human Cultures, and Desert Landscapes in Northern Mexico. Environ. Hist. Durh. N. C. 2011, 17, 84–115. [Google Scholar] [CrossRef]
- Gentry, H.S. Agaves of Continental North America; University of Arizona Press: Arizona, North America, 1982; pp. 1–670. ISBN 9780816523955. [Google Scholar]
- Davidson, J.R.; De Montellano, B.R.O. The Antibacterial Properties of an Aztec Wound Remedy. J. Ethnopharmacol. 1983, 8, 149–161. [Google Scholar] [CrossRef]
- Irigoyen, F.; Paredes, A. Tarahumara Medicine: Ethnobotany and Healing among the Raramuri of Mexico; University of Oklahoma Press: Norman, OK, USA, 2015; pp. 1–416. ISBN 978-0-8061-4828-1. [Google Scholar]
- Alinia-Ahandani, E.; Alizadeh, Z.; Sheydaei, M. Some Pointed Medicinal Plants to Treat the Tick-Borne Disease. Open Access J. Bio. Sci. Res. 2020, 1. [Google Scholar] [CrossRef]
- Torre, L.D.L.; Cummins, I.; Logan-Hines, E. Agave Americana and Furcraea Andina: Key Species to Andean Cultures in Ecuador. Bot. Sci. 2018, 96, 246–266. [Google Scholar] [CrossRef] [Green Version]
- Chaachouay, N.; Benkhnigue, O.; Zidane, L. Ethnobotanical Study Aimed at Investigating the Use of Medicinal Plants to Treat Nervous System Diseases in the Rif of Morocco. J. Chiropr. Med. 2020, 19, 70–81. [Google Scholar] [CrossRef]
- García Mendoza, A.; Colunga-Garcia Marin, P.; Bye, R.J. Los Usos Del Agave Angustifolia Haw., Ancestro Silvestre Del Henequén En Su Área de Distribución Geográfica. Boletín Soc. Botánica México 1993, 62, 109–128. [Google Scholar] [CrossRef]
- Delgado-Lemus, A.; Casas, A.; Téllez, O. Distribution, Abundance and Traditional Management of Agave Potatorumin the Tehuacán Valley, Mexico: Bases for Sustainable Use of Non-Timber Forest Products. J. Ethnobiol. Ethnomed. 2014, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, S.; Venkatachalam, K.; Muruganandam, S. Ethnomedicinal Plants Used by the Malayali Tribals in Jawadhu Hills of Thiruvannamalai District, Tamil Nadu, India. J. Nat. Prod. Plant Resour. 2020, 4, 55–60. [Google Scholar]
- Rahmatullah, M.; Pk, S.R.; Al-Imran, M.; Jahan, R. The Khasia Tribe of Sylhet District, Bangladesh, and Their Fast-Disappearing Knowledge of Medicinal Plants. J. Altern. Complement. Med. 2013, 19, 599–606. [Google Scholar] [CrossRef]
- Phanuel, A.S. Plant Species in the Folk Medicine of Kit Mikayi Region, Western Kenya. Ethnobot. Leafl. 2010, 2010, 13. [Google Scholar]
- Debnath, M.; Pandey, M.; Sharma, R.; Thankur, G.; Lal, P. Biotechnological Intervention of Agave Sisalana: A Unique Fibe Yielding Plant with Medicinal Property. J. Med. Plant Res. 2010, 4, 177–187. [Google Scholar]
- Gidey, M.; Beyene, T.; Signorini, M.; Bruschi, P.; Yirga, G. Traditional Medicinal Plants Used by Kunama Ethnic Group in Northern Ethiopia. J. Med. Plants Res. 2015, 9, 494–509. [Google Scholar] [CrossRef] [Green Version]
- Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Uribe-Soto, J.N.; González-Valdez, L.S. The Phenols of the Genus Agave (Agavaceae). J. Biomater. Nanobiotechnol. 2013, 4, 33624. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.C.; Gulliya, B. Chemistry and Pharmacology of Flavonoids- A Review. Indian J. Pharm. Educ. Res. 2019, 53, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The Style and Substance of Plant Flavonoid Decoration; towards Defining Both Structure and Function. Phytochemistry 2020, 174. [Google Scholar] [CrossRef] [PubMed]
- Barriada-Bernal, L.G.; Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Gallardo-Velázquez, T.; Ávila-Reyes, J.A.; Torres-Morán, M.I.; González-Elizondo, M.D.S.; Herrera-Arrieta, Y. Flavonoid Composition and Antioxidant Capacity of the Edible Flowers of Agave Durangensis (Agavaceae). CYTA-J. Food 2014, 12, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Almaraz-Abarca, N.; González-Elizondo, M.D.S.; Campos, M.D.G.; Ávila-Sevilla, Z.E.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A. Variabilidad de Los Perfiles Fenólicos Foliares Del Complejo Agave Victoriae-Reginae (Agavaceae). Bot. Sci. 2013, 91, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Morreeuw, Z.P.; Escobedo-Fregoso, C.; Ríos-González, L.J.; Castillo-Quiroz, D.; Reyes, A.G. Transcriptome-Based Metabolic Profiling of Flavonoids in Agave Lechuguilla Waste Biomass. Plant Sci. 2021, 305, 110748. [Google Scholar] [CrossRef] [PubMed]
- Morreeuw, Z.P.; Castillo-Quiroz, D.; Ríos-González, L.J.; Martínez-Rincón, R.; Estrada, N.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R.; Reyes, A.G. High Throughput Profiling of Flavonoid Abundance in Agave Lechuguilla Residue-Valorizing under Explored Mexican Plant. Plants 2021, 10, 695. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Gutiérrez-Mora, A.; García-Lara, S. Micropropagation of Agave Salmiana: Means to Production of Antioxidant and Bioactive Principles. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in Vitro Drought Stress on Phenolic Acids, Flavonols, Saponins, and Antioxidant Activity in Agave Salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Morán-Velázquez, D.C.; Monribot-Villanueva, J.L.; Bourdon, M.; Tang, J.Z.; López-Rosas, I.; Maceda-López, L.F.; Villalpando-Aguilar, J.L.; Rodríguez-López, L.; Gauthier, A.; Trejo, L.; et al. Unravelling Chemical Composition of Agave Spines: News from Agave Fourcroydes Lem. Plants 2020, 9, 1642. [Google Scholar] [CrossRef]
- Iser, M.; Valdivie, M.; Figueredo, L.; Nuñez, E.; Más Toro, D.; Martínez, Y. Secondary Metabolites, Quality Indicators and Organoleptic Characteristics of Stems Meal from Agave Fourcroydes (Henequen). Cuba. J. Agric. Sci. 2020, 54, 1–10. [Google Scholar]
- Rahmani, H.; Benali, F.; Koudach, F.; Dif, M.M.; Mekhfi, N.; Nouredine, N.; Moumen, F.; Rahman, M. First Determination of Phenolic Compound Concentration and Antioxydant Activity of Agave Americana Leaves Extracts from Different Regions of Algeria (NW). J. Med. Plant Res. 2015, 3, 1–6. [Google Scholar]
- Rahmani, H.; Toumi Benali, F. Phenolic Quantification and Antioxidant Activity of Agave Americana Leaves Depending on Solvent and Geoclimatic Area. Adv. Environ. Biol. 2016, 9, 194–200. [Google Scholar]
- Ahumada-Santos, Y.P.; Montes-Avila, J.; de Jesús Uribe-Beltrán, M.; Díaz-Camacho, S.P.; López-Angulo, G.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Heredia, J.B.; Delgado-Vargas, F. Chemical Characterization, Antioxidant and Antibacterial Activities of Six Agave Species from Sinaloa, Mexico. Ind. Crops Prod. 2013, 49, 143–149. [Google Scholar] [CrossRef]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and Biological Studies of Agave Attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef]
- Delia, S.; Pérez-Herrera, A.; García-Sánchez, E.; Santiago Garcia, P. Identification and Quantification of Bioactive Compounds in Agave Potatorum Zucc. Leaves at Different Stages of Development and a Preliminary Biological Assay. Waste Biomass Valorization 2021, 12. [Google Scholar] [CrossRef]
- Medina-Galván, M.I.; Bernardino-Nicanor, A.; Castro-Rosas, J.; De La Luz Xochilt Negrete-Rodríguez, M.; Conde-Barajas, E.; González-Cruz, L. Antimicrobial and Antioxidant Activity of Flower Scape Extracts of Agave Salmiana: Effect of the Extraction Solvent and Development Stage. Res. J. Biotechnol. 2018, 13, 1–9. [Google Scholar]
- Ben Hamissa, A.M.; Seffen, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Converti, A. Phenolics Extraction from Agave Americana (L.) Leaves Using High-Temperature, High-Pressure Reactor. Food Bioprod. Process. 2012, 90, 17–21. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; Peña-Ramos, E.A.; Hernández, J.; González-Ríos, H. Antioxidant and Antimicrobial Activity of Agave Angustifolia Extract on Overall Quality and Shelf Life of Pork Patties Stored under Refrigeration. J. Food Sci. Technol. 2018, 55, 4413–4423. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Hernández, M.G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, J.G.; Rocha-Guzmán, N.E.; Lara-Ceniceros, T.E.; Contreras-Esquivel, J.C.; Prado Barragán, L.A.; Rutiaga-Quiñones, O.M. Effect of Ultrasound Pre-Treatment on the Physicochemical Composition of Agave Durangensis Leaves and Potential Enzyme Production. Bioresour. Technol. 2018, 249, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Avila-Gaxiola, E.; Avila-Gaxiola, J.; Velarde-Escobar, O.; Ramos-Brito, F.; Atondo-Rubio, G.; Yee-Rendon, C. Effect of Drying Temperature on Agave Tequilana Leaves: A Pretreatment for Releasing Reducing Sugars for Biofuel Production. J. Food Process Eng. 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Santana-Jiménez, A.Z.; Quintero-Ramos, A.; Sánchez-Madrigal, M.Á.; Meléndez-Pizarro, C.O.; Valdez-Cárdenas, M.D.; Orizaga-Heredia, M.D.; Méndez-Zamora, G.; Talamás-Abbud, R. Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave Tequilana Weber Var. Azul Extracts at Various PH Values. Processes 2020, 8, 841. [Google Scholar] [CrossRef]
- Anguiano-Sevilla, L.A.; Lugo-Cervantes, E.; Ordaz-Pichardo, C.; Rosas-Trigueros, J.L.; Jaramillo-Flores, M.E. Apoptosis Induction of Agave Lechuguilla Torrey Extract on Human Lung Adenocarcinoma Cells (SK-LU-1). Int. J. Mol. Sci. 2018, 19, 3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, V.D.; Taylor, C.M.; Bauer, S. Comprehensive Analysis of Monomeric Phenolics in Dilute Acid Plant Hydrolysates. Bioenergy Res. 2014, 7, 654–669. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Hamdi, S.H.; Belhadj, F.; Jemâa, J.M.; Messaoud, C.; Marzouki, M.N. Phytochemical Profile and Insecticidal Activity of Agave Americana Leaf Extract towards Sitophilus Oryzae (L.) (Coleoptera: Curculionidae). Environ. Sci. Pollut. Res. 2019, 26, 19468–19480. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros Guerra, J.G.; Chávez Cerna, E.; Ochoa Fuentes, Y.M.; Landeros Flores, J.; Aguirre Uribe, L.A.; Hernández Juárez, A. Insecticidal Activity of Plant Extracts against Whitefly Nymphs Bemisia Tabaci ( Hemiptera: Aleyrodidae ) in Laboratory. J. Entomol. Zool. Stud. 2020, 8, 595–599. [Google Scholar]
- El-Hawary, S.S.; El-Kammar, H.A.; Farag, M.A.; Saleh, D.O.; El Dine, R.S. Metabolomic Profiling of Five Agave Leaf Taxa via UHPLC/PDA/ESI-MS Inrelation to Their Anti-Inflammatory, Immunomodulatory and Ulceroprotective Activities. Steroids 2020, 160, 108648. [Google Scholar] [CrossRef]
- Sahnoun, M.; Saibi, W.; Brini, F.; Bejar, S. Apigenin Isolated from A. Americana Encodes Human and Aspergillus Oryzae S2 α-Amylase Inhibitions: Credible Approach for Antifungal and Antidiabetic Therapies. J. Food Sci. Technol. 2018, 55, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, M.; Bejar, S.; Daoud, L.; Ayadi, L.; Brini, F.; Saibi, W. Effect of Agave Americana L. on the Human, and Aspergillus Oryzae S2 α-Amylase Inhibitions. Nat. Prod. Res. 2019, 33, 755–758. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Santos-Zea, L.; Fajardo-Ramírez, O.R.; Romo-López, I.; Gutiérrez-Uribe, J.A. Fast Centrifugal Partition Chromatography Fractionation of Concentrated Agave (Agave Salmiana) Sap to Obtain Saponins with Apoptotic Effect on Colon Cancer Cells. Plant Foods Hum. Nutr. 2016, 71, 57–63. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; Tortoriello, J.; Zamilpa, A.; Alegría-Herrera, E.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Martínez-Duncker, I.; Monterrosas-Brisson, N. Anti-Neuroinflammatory Effect of Agaves and Cantalasaponin-1 in a Model of LPS-Induced Damage. Nat. Prod. Res. 2021, 35, 884–887. [Google Scholar] [CrossRef]
- Figueroa, L.M.; Santos-Zea, L.; Escalante, A.; Gutiérrez-Uribe, J.A. Mass Spectrometry-Based Metabolomics of Agave Sap (Agave Salmiana) after Its Inoculation with Microorganisms Isolated from Agave Sap Concentrate Selected to Enhance Anticancer Activity. Sustainability 2017, 9, 2095. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.J.; Sánchez-Mendoza, E.; Zamilpa, A.; González-Cortazar, M.; Herrera-Ruiz, M.; Almanza-Pérez, J.C.; Terán-Cabanillas, E.; Condé, R.; Domínguez-Ramírez, L.; Arcos, E.M.; et al. Steroidal Saponin from Agave Marmorata Roezl Modulates Inflammatory Response by Inhibiting NF-ΚB and AP-1. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.V.A.; Botura, M.B.; dos Santos, J.D.G.; Argolo, D.S.; da Silva, V.D.A.; da Silva, G.D.; de Lima, H.G.; Braz Filho, R.; Vieira, I.J.C.; Branco, A.; et al. Saponin-Rich Fraction from Agave Sisalana: Effect against Malignant Astrocytic Cells and Its Chemical Characterisation by ESI-MS/MS. Nat. Prod. Res. 2018, 33, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; dos Santos, M.O.; Barbon, F.F.; Manjerona, B.A.; Meirelles, B.R.; de Oliva Neto, P.; da Silva, P.I.; dos Santos, L.; Camargo, I.C.C.; de Souza, E.B. Analysis of Antioxidant, Cytotoxic and Mutagenic Potential of Agave Sisalana Perrine Extracts Using Vero Cells, Human Lymphocytes and Mice Polychromatic Erythrocytes. Biomed. Pharmacother. 2018, 98, 873–885. [Google Scholar] [CrossRef]
- Pereira, G.M.; Ribeiro, M.G.; da Silva, B.P.; Parente, J.P. Structural Characterization of a New Steroidal Saponin from Agave angustifolia Var. Marginata and a Preliminary Investigation of Its in Vivo Antiulcerogenic Activity and in Vitro Membrane Permeability Property. Bioorg. Med. Chem. Lett. 2017, 27, 4345–4349. [Google Scholar] [CrossRef]
- Mina, S.; Melek, F.R.; Abdel-Khalik, S.M.; El-Shaarawy, F.S.; Eskander, J. Pharmacological Activities of Agave Seemanniana and Isolation of Three Steroidal Saponins. European J. Med. Plants 2014, 4, 271–283. [Google Scholar] [CrossRef]
- Gutiérrez Nava, Z.J.; Jiménez-Aparicio, A.R.; Herrera-Ruiz, M.L.; Jiménez-Ferrer, E. Immunomodulatory Effect of Agave Tequilana Evaluated on an Autoimmunity Like-SLE Model Induced in Balb/c Mice with Pristane. Molecules 2017, 22, 848. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Valle, E.; Herrera-Ruiz, M.; Salgado, G.R.; Zamilpa, A.; Ocampo, M.L.A.; Aparicio, A.J.; Tortoriello, J.; Jiménez-Ferrer, E. Anti-Inflammatory Effect of 3-O-[(6′-O-Palmitoyl)-β-d-Glucopyranosyl Sitosterol] from Agave Angustifolia on Ear Edema in Mice. Molecules 2014, 19, 15624–15637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and Bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Mannasaheb, B.; Kulkarni, P.; Sangreskopp, M.; Savant, C.; Mohan, A. Protective Effect of Agave Americana Linn. Leaf Extract in Acetic Acid-Induced Ulcerative Colitis in Rats. Int. Q. J. Res. Ayurveda 2015, 36, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannasaheb, B.; Kulkarni, V.; Shaikh, I.; Sangreskopp, M.; Savant, C. Gastro Protective Effect of Agave Americana Linn. Leaf Extract in Indomethacin-Induced Enterocolitis in Rats. Int. J. Green Pharm. 2015, 9, 229–235. [Google Scholar]
- Santos Cerqueira, G.; Dos Santos E Silva, G.; Rios Vasconcelos, E.; Fragoso De Freitas, A.P.; Arcanjo Moura, B.; Silveira MacEdo, D.; Lopes Souto, A.; Barbosa Filho, J.M.; De Almeida Leal, L.K.; De Castro Brito, G.A.; et al. Effects of Hecogenin and Its Possible Mechanism of Action on Experimental Models of Gastric Ulcer in Mice. Eur. J. Pharmacol. 2012, 683, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Santos-Zea, L.; Gutierrez-Uribe, J.A.; Benedito, J. Effect of Solvent Composition on Ultrasound-Generated Intensity and Its Influence on the Ultrasonically Assisted Extraction of Bioactives from Agave Bagasse (Agave Salmiana). Food Eng. Rev. 2020. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Benedito, J. Effect of Ultrasound Intensification on the Supercritical Fluid Extraction of Phytochemicals from Agave Salmiana Bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging Roles for Conjugated Sterols in Plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; González-Cortazar, M.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Identification and Quantification of β-Sitosterol β-d-Glucoside of an Ethanolic Extract Obtained by Microwave-Assisted Extraction from Agave Angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef] [Green Version]
- Olvera-García, V.; Martín del Campo, S.T.; Gutiérrez-Uribe, J.A.; Cardador-Martínez, A. GC-MS and HPLC-MS-TOF Characterization of Agave Atrovirens Extracts. A Preliminary Study. Ind. Crops Prod. 2015, 78, 39–47. [Google Scholar] [CrossRef]
- Martínez-Aguilar, J.F.; Peña-Álvarez, A. Characterization of Five Typical Agave Plants Used to Produce Mezcal through Their Simple Lipid Composition Analysis by Gas Chromatography. J. Agric. Food Chem. 2009, 57, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, M.M.; El-Sayed, M.M.; Abdel-Hameed, E.S. Molluscicidal Steroidal Saponins and Lipid Content of Agave Decipiens. Fitoterapia 1999, 70, 371–381. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rodríguez, I.M.; del Río, J.C. Chemical Composition of Lipophilic Extractives from Sisal (Agave sisalana) Fibers. Ind. Crops Prod. 2008, 28, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.S.; Oliveira, L.G.; Costa, A.F.; Correia, M.T.S.; Lima, E.A.L.-A.; Lima, V.L.M. Efficacy of Libidibia Ferrea Var. Ferrea and Agave Sisalana Extracts against Dactylopius Opuntiae (Hemiptera: Coccoidea). J. Agric. Sci. 2018, 10, 255. [Google Scholar] [CrossRef]
- Guimarães de Oliveira, L.H.; Alexandria Paiva Silva de Sousa, P.; Felipe Hilario, F.; Joventino Nascimento, G.; Saraiva Morais, J.P.; Paulo de Medeiros, E.; Francisco de Sousa, M.; da Cruz Nunes, F. Agave sisalana Extract Induces Cell Death in Aedes Aegypti Hemocytes Increasing Nitric Oxide Production. Asian Pac. J. Trop. Biomed. 2016, 6, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.F.; Tomás, J.; Osuna, A.; Brandão, H.N.; Haraguchi, M.; Alberto, C. Composição Química e Toxicidade Foliar de Extratos Do Resíduo Líquido de Sisal Chemical Composition and Foliar Toxicity of the Extracts from the Waste Liquid of Sisal Introdução Material e Métodos. MAGISTRA 2014, 26, 372–384. [Google Scholar]
- Chrinius, H.; Musa, M.; Olalekan, B.A.; Usman Jajere, M.; Erphaim Akuaden, A.; Suleiman, B. Efficacy of Agave Sisalana N-Hexane Extract in the Control of Callosobruchus maculatus (Fabricius) (Colloptera: Bruchidae) Pest. J. Appl. Biol. Biotechnol. 2015, 3, 001–003. [Google Scholar] [CrossRef]
- Pereira, A.J.; Cardoso, I.M.; Araújo, H.D.; Santana, F.C.; Carneiro, A.P.; Coelho, S.P.; Pereira, F.J. Control of Brevicoryne Brassicae (Hemiptera: Aphididade) with Extracts of Agave americana Var. Marginata Trel. in Brassica oleracea Crops. Ann. Appl. Biol. 2018, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Fuertes, C.M.; Jurado, B.; Gordillo, G.C.; Negrón, L.P.; Núñez, E.; Esteban, M.; Távara, A. Estudio Integral de Plantas Biocidas Del Algodonero. Cienc. Investig. 2010, 13, 34–41. [Google Scholar] [CrossRef]
- Cunha Pereira, R.; Faria Barbosa, W.; Pereira Lima, M.A.; Vieira, J.O.L.; Carvalho Guedes, R.N.; Rodrigues da Silva, B.K.; Dias Barbosa, G.M.; Lemes Fernandes, F. Toxicity of Botanical Extracts and Their Main Constituents on the Bees Partamona helleri and Apis mellifera. Ecotoxicology 2020, 29, 246–257. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, T.M.B.; Reis, P.R.; de Oliveira, D.F.; Carvalho, G.A.; de Carvalho, D.A. Evaluation of Aqueous Extract of Plants for the Control of the Mite Oligonychus ilicis (McGregor, 1917) (Acari: Tetranychidae) on coffee tree. Simpósio Pesqui. Cafés Bras. 2009, 3, 94–103. [Google Scholar]
- Veronez, B.; Sato, M.E.; Nicastro, R.L. Toxicidade de Compostos Sintéticos e Naturais Sobre Tetranychus Urticae e o Predador Phytoseiulus Macropilis. Pesqui. Agropecu. Bras. 2012, 47, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Herbert-Doctor, L.A.; Saavedra-Aguilar, M.; Villarreal, M.L.; Cardoso-Taketa, A.; Vite-Vallejo, O. Insecticidal and Nematicidal Effects of Agave tequilana Juice against Bemisia Tabaci and Panagrellus Redivivus. Southwest. Entomol. 2016, 41, 27–40. [Google Scholar] [CrossRef]
- Barrêto, A.; Araújo, E.; Bonifácio, B. Eficiência de Extratos de Agave sisalana (Perrine) Sobre o Ácaro Rajado Tetranychus Urticae (Koch) e Ocorrência de Fitotoxidez Em Plantas de Algodoeiro (Gossypium Hirsutum L. r Latifolium Hutch). Rev. Bras. Agroecol. 2010, 5, 207–215. [Google Scholar]
- Marieta, C.; Saba, L. The Impact of Agave attenuata Extracts: Biotic Resistance Esponses of Wheat and Their Ability to Act as Repellents/Insecticides against the Russian Wheat Aphid. J. Plant Physiol. Pathol. 2019, 7. [Google Scholar] [CrossRef]
- Rawi, S.M.; Al-Hazmi, M.; Al Nassr, F.S. Comparative Study of the Molluscicidal Activity of Some Plant Extracts on the Snail Vector of Schistosoma mansoni, Biomphalaria alexandrina. Int. J. Zool. Res. 2011, 7, 169–189. [Google Scholar] [CrossRef]
- Bakry, F.A.; Hamdi, S.A. The Molluscicidal Activity of Some Plant Extracts Against Biomphalaria alexandrina Snails. Egypt. J. Exp. Biol. 2006, 2, 99–106. [Google Scholar]
- El-Sayed, M.M.; Abdel-Hadi, M.; El-Nahas, H.A. Molluscicidal Activity and Clinico-Pathological Effect of Agave lophantha. Arch. Pharmal. Res. 1991, 14, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, T.D. Gross Histopathological Effects of an Extract of Agave Attenuata on the Epithelium of the Digestive Tract of Bulinus africanus. Ann. Trop. Med. Parasitol. 1999, 93, 519–526. [Google Scholar] [CrossRef]
- Nodarse, M.; Castellanos, L.; Herrera, N.; Morfa, M. Acción Molusquicida de Extractos Vegetales de Tres Especies de La Familia Agavaceae Contra Praticolella griseola (Pfeiffer). Rev. Protección Veg. 2017, 32. [Google Scholar]
- Innacone, J.; La Torre, M.I.; Alvariño, L.; Cepeda, C.; Ayala, H.; Argota, G. Toxicity of the Biopesticides Agave americana, Furcraea andina (Asparagacease) and Sapindus saponaria (Sapindaceae) on Invader Snail Melanoides tuberculata (Thiaridae). Neotrop. Helminthol. 2013, 7, 231–241. [Google Scholar]
- Sukumaran, D.; Parashar, B.D.; Rao, K.M. Molluscicidal Properties of Agave americana and Balanites aegyptica. Int. J. Pharmacol. 1994, 32, 232–238. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.-B.; Zhong, Q.-H.; Zhang, J.-E.; Luo, M.-Z.; Zhao, B.-L.; Qin, Z. Toxicological Effect of Agave sisalana Perrine Extract on Golden Apple Snail (Pomacea Canaliculata Lamarck). Chin. J. Eco-Agric. 2012, 20, 69–74. [Google Scholar] [CrossRef]
- Hamed, R.R.; Maharem, T.M.; Farid, N.M.; Ramadan, K.; Abdel Aziz, M.H. Effect of Agave attenuata Extracts on Detoxification Enzymes of Biomphlaria alexandrina. Environmentalist 2006, 26, 157–164. [Google Scholar] [CrossRef]
- Nunes, F.C.; Leite, J.A.; Oliveira, L.H.G.; Sousa, P.A.P.S.; Menezes, M.C.; Moraes, J.P.S.; Mascarenhas, S.R.; Braga, V.A. The Larvicidal Activity of Agave sisalana against L4 Larvae of Aedes Aegypti Is Mediated by Internal Necrosis and Inhibition of Nitric Oxide Production. Parasitol. Res. 2015, 114, 543–549. [Google Scholar] [CrossRef]
- Singh, R.K.; Mittal, P.K.; Kumar, G.; Dhiman, R.C. Evaluation of Mosquito larvicidal Efficacy of Leaf Extract of a Cactus Plant, Agave sisalana. J. Entomol. Zool. Stud. 2014, 2, 83–86. [Google Scholar]
- Pizarro, A.P.B.; Oliveira Filho, A.M.; Parente, J.P.; Melo, M.T.V.; Dos Santos, C.E.; Lima, P.R. O Aproveitamento Do Residuo Da Industria Do Sisal No Controle de Larvas de Mosquitos. Rev. Soc. Bras. Med. Trop. 1999, 32, 23–29. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Urs, K.C.D. Growth Regulator Activity of the Xerophytic Perennial Plant, Agave cantala Roxb. on Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Int. J. Trop. Insect Sci. 1991, 12, 439–442. [Google Scholar] [CrossRef]
- Fábio, J.N.; Damasceno, J.C.A.; Barbosa, D.H.S.; Malheiro, R.; Pereira, J.A.; Soares, A.C.F. Control of the Banana Burrowing Nematode Using Sisal Extract. Agron. Sustain. Dev. 2015, 35, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Damasceno, J.C.; Soares, A.C.; Jesus, F.N.; Sant’Ana, R.S. Sisal Leaf Decortication Liquid Residue for Controlling Meloidogyne javanica in Tomato Plants. Hortic. Bras. 2015, 33, 155–162. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, J.; Silva Sousa, C.D.; Fermino Soares, A.C.; Sousa Lima, F.D.; Gomes Barbosa Silveira, D.H. Actinobacteria and Organic Fertilizer for Managment of the Nematode Scutellomena Bradys in Yam Plants. Rev. Caatinga 2016, 29, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Potenza, M.R.; Junior, J.J.; Alves, J.N. Evaluation of Contact Activities of Plant Extracts against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). In Proceedings of the 9th International Working Conference on Stored-Product Protection, Campinas, São Paulo, Brazil, 15–18 October 2006; pp. 811–815. [Google Scholar]
- Kajla, M.; Bhattacharya, K.; Gupta, K.; Banerjee, U.; Kakani, P.; Gupta, L.; Kumar, S. Identification of the Temperature Induced Larvicidal Efficacy of Agave angustifolia against Aedes, Culex, and Anopheles Larvae. Front. Public Health 2016, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, M.; Rodriguez, M. Evaluation of the Molluscicidal action of Agave Legrelliana on Fossaria cubensis (Molllusca: Lymnaeidae) Main Vecgtor of Fascioliasis in Cuba. Parasitol. Día 1994, 18, 46–49. [Google Scholar]
- Abdel-Gawad, M.; El-Nahas, H.A.; Osman, N.S. Molluscicidal Activity of Steroidal Saponins Isolated from Agave angustifolia. Glob. J. Pharmacol. 2015, 9, 138–143. [Google Scholar] [CrossRef]
- Mina, S.A.; Mina, S.A.; Melek, F.R.; Abdel-khalik, S.M.; Gabr, N.M. Two Steroidal Saponins from Agave franzosinii and Agave angustifolia Leaves and Biological Activities of Agave franzosinii. J. Nat. Prod. 2013, 6, 188–197. [Google Scholar]
- Lubian, C.; Martinha, D.D.; Portz, R.; Gonçalves, M.P.; Holz, S.; Marcelino, W.L.; Nogueira, A.C.C.; Thomé, R.M.; Missio, V.C.; Cordeiro, J.; et al. Anthelmintic Activity of Plant Aqueous Extracts against Panagrellus redivivus in Vitro. Arq. Inst. Biol. 2019, 86, 1–11. [Google Scholar] [CrossRef]
- Nandi, B. Evaluation of Nematicidal Properties and Inhibition of Egg Hatching Activity of Some Medicinal Plant Extracts Against Meloidogyne Incognita. Res. Artic. NBU J. Anim. Sc 2016, 10, 89–94. [Google Scholar]
- Silveira, R.X.; Ana Carolina, S.; Mariana, B.; María José, M.; Luciana Morita, K.; Claudia María, L.; Alexsandro, M.; Elene de Alencar, A.; Simone, B.L.; María Angela, O.-A. Influência Do Resíduo Líquido Do Sisal (Agave sisalana, Perrine) Sobre a Alimentação Larvar. Empres. Bras. Pesqui. Agropecuária 2009, 5–7. [Google Scholar]
- Domingues, L.F.; Botura, M.B.; da Cruz, A.C.F.G.; Yuki, C.C.; da Silva, G.D.; Costa, M.S.; Murphy, G.; Moreira, E.L.T.; de Meneses, Í.D.S.; de Almeida, M.; et al. Evaluation of Anthelmintic Activity of Liquid Waste of Agave sisalana (Sisal) in Goats. Rev. Bras. Parasitol. Vet. 2010, 19, 270–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botura, M.B.; dos Santos, J.D.G.; da Silva, G.D.; de Lima, H.G.; de Oliveira, J.V.A.; de Almeida, M.A.O.; Batatinha, M.J.M.; Branco, A. In Vitro Ovicidal and Larvicidal Activity of Agave sisalana Perr. (Sisal) on Gastrointestinal Nematodes of Goats. Vet. Parasitol. 2013, 192, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.D.G.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Chemicals from Agave Sisalana Biomass: Isolation and Identification. Int. J. Mol. Sci. 2015, 16, 8761–8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddhapura, S.; Maharshi, A.; Thaker, V. Varietal Difference in Antifungal Activity of Some Species of Agave. Arch. Phytopathol. Plant Prot. 2011, 44, 135–141. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Antifungal Activity of Agave americana Leaf Extract against Alternaria Brassicae, Causal Agent of Alternaria Blight of Indian Mustard (Brassica juncea). Arch. Phytopathol. Plant Prot. 2009, 42, 370–375. [Google Scholar] [CrossRef]
- Kassankogno, A.; Ouedrogo, I.; Tiendrebeogo, A.; Ouedraogo, L.; Sankara, P. In Vitro Evaluation of the Effect of Aqueous Extracts of Agave sisalana and Cymbopogon citratus on Mycelial Growth and Conidia Production of Pyricularia oryzae, Causal Agent of Rice Blast. J. Appl. Biosci. 2015, 89, 8272. [Google Scholar] [CrossRef]
- Castillo Reyes, F.; Hernández Castillo, F.D.; Julio Alberto, C.C.; Raúl, R.H.; Cristóbal Noé, A. In Vitro Antifungal Activity of Polyphenols-Rich Plant Extracts against Phytophthora cinnamomi Rands. African J. Agric. Res. 2015, 10, 4554–4560. [Google Scholar] [CrossRef]
- Sharma, B.K. Antifungal Properties of Biocontrol Agents and Plants Extracts against Causal Fungi of Yellows and Rhizomes Rot of Ginger. J. Biol. Control 1998, 12, 77–80. [Google Scholar] [CrossRef]
- González-Álvarez, M.; Moreno-Limón, S.; Salcedo-Martínez, S.M.; Pérez-Rodríguez, E.C. Evaluación in Vitro de La Actividad Antifúngica de Extractos de Agave (Agave scabra, Salm Dyck) Sobre Hongos Postcosecha. Int. J. Exp. Bot. 2015, 84, 427–434. [Google Scholar]
- Maazoun, A.M.; Hamdane, A.M.; Mediouni, J.; Jemâa, B.; Marzouki, N. Saponin Content of Agave americana (L.) Leaf Extract and Its Antifungal Attributes against Phytopathogenic Fungi. Int. J. Agric. Biosci. 2019, 8, 106–111. [Google Scholar]
- Adavigowda Deepak, S.; Oros, G.; Syagadadu Giriyanna, S.; Nandinin Pratap, S.; Huntrike Shekar, S.; Sheena, S. Antisporulant Activity of Leaf Extracts of Indian Plants against Sclerospora graminicola Causing Downy Mildew Disease of Pearl Millet. Am. J. Agric. Biol. Sci. 2004, 38, 31–39. [Google Scholar] [CrossRef]
- Castillo, F.; Hernández, D.; Gallegos, G.; Mendez, M.; Rodríguez, R.; Reyes, A.; Aguilar, C.N. In Vitro Antifungal Activity of Plant Extracts Obtained with Alternative Organic Solvents against Rhizoctonia solani Kühn. Ind. Crops Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Rosas-Taraco, A.; Sanchez, E.; García, S.; Heredia, N.; Bhatnagar, D. Extracts of Agave Americana Inhibit Aflatoxin Production in Aspergillus parasiticus. World Mycotoxin J. 2010, 4, 37–42. [Google Scholar] [CrossRef]
- De Oliveira Sousa, M.J.; de Almeida, F.A.; Leite, M.L.T.; Fonseca, W.L.; Lopes, K.P.; Gomes, C.D.L.; Sampaio, E.G.; da Nobrega Santos, E.; de Oliveira Gondim, A.R. Biocidal Potential of Some Organic By-Products on Sanitary and Physiological Quality of Red and White Fava Beans Seeds. Aust. J. Crop Sci. 2020, 14, 462–468. [Google Scholar] [CrossRef]
- Bolanle Omofunmiloa, O. In vitro Antifungal Effects of Medicinal Plants Extraxt on the Mycelia Growth of Phytophthora Megakarya Causal Agent of Cocoa Blackpod Disease. Pharm. Chem. Sci. 2017, 3, 29–36. [Google Scholar]
- Hong, H.X.; Fu, Y.H.; Xia, L.; Xiao, L.Z.; Hua, N.W.; Hui, L.; Xuan, Z. Antifungal Effects of Sisal Leaf Juice on Lasiodiplodia theobromae, the Causal Agent of Mulberry Root Rot. African J. Biotechnol. 2016, 15, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Hareesh, M.V.; Ganesha Naik, R.; Jayalakshmi, K.; Basavaraj Naik, T.; Pradeep, S. Efficacy of Bioagents, Plant Extracts and Fungicides against Chilli Powdery Mildew Incited by Leveillula taurica (Lev.) Arn. J. Pure Appl. Microbiol. 2016, 10, 3105–3109. [Google Scholar] [CrossRef]
- Naik, V.V.; Bansode, M.S.; Bartakke, S.P. Foliar Application of Agave Cantala Roxb. Leaf Extract Enhances Antioxidative Defense Mechanism in Grape (Vitis Vinifera L.) Leaves Infected with Downy Mildew. World J. Pharm. Res. 2015, 4, 2039–2056. [Google Scholar]
- Maharshi, A.R.; Thaker, V.S. Antifungal Activity of Agave Species from Gujarat, India. Microb. Divers. Biotechnol. Food Secur. 2014, 423–430. [Google Scholar] [CrossRef]
- Naik, V.; Bartakke, S. Effect of Agave cantala Leaf Extract on Downy Mildew of Grape. BIOINFOLET Q. J. Life Sci. 2009, 6, 249–250. [Google Scholar]
- Adavigowda Deepak, S.; Oros, G.; Shekar Shetty, H.; Sheena, S. Antisporulant Activity of Watery Extracts of Plants against Sclerospora Graminicola Causing Downy Mildew Disease of Pearl Millet. Am. J. Agric. 2007, 2, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Koteswara Reddy, G.; Iakshmi Mohana, S.; Ashok Kumar, C.K.; Kumar Satheesh, D.; Lakshmi Srinivas, T. Evaluation of Anti-inflammatory and Antioxidant Activity of Methanolic of Agave cantala Roxb. J. Glob. Trends Pharm. Sci. 2013, 4, 1300–1309. [Google Scholar]
- Yang, C.R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.J.; Li, X.C. Antifungal Activity of C-27 Steroidal Saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, E.; Heredia, N.; García, S. Inhibition of Growth and Mycotoxin Production of Aspergillus flavus and Aspergillus parasiticus by Extracts of Agave Species. Int. J. Food Microbiol. 2005, 98, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Bimal, S.; Narayan, S.; Jee, C.; Bimal, D.; Das, P.; Bimal, R. Leishmania Donovani: Assessment of Leishmanicidal Effects of Herbal Extracts Obtained from Plants in the Visceral Leishmaniasis Endemic Area of Bihar, India. Exp. Parasitol. 2011, 127, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.P.; Narayan, S.; Bahadur, S.; Thakur, M.; Pandey, S.N.; Kumar, P.; Misra, P.; Mukherjee, P.K.; Mitra, D.K. Anti-Leishmanial Activity of Agave americana L.– A Traditional Indian Medicinal Plant. Indian J. Tradit. Knowl. 2015, 14, 658–663. [Google Scholar]
- Quintanilla-Licea, R.; Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; Bazaldúa-Rodríguez, A.F.; Ángeles-Hernández, I.K.; Garza-González, J.N.; Hernández-García, M.E. Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia Graveolens and Ruta Chalepensis. Molecules 2014, 19, 21044–21065. [Google Scholar] [CrossRef] [PubMed]
| Species/Tissue | Medicinal Use | References |
|---|---|---|
| A. potatorum Zucc./leaf | Healing and cicatrization of wounds | [18] |
| Infusions to heal respiratory infections | ||
| A. americana L./leaf, roots | Healing and cicatrization of wounds from leaf gel | [19] |
| Syphilis treatment, anesthetic, headache, rheumatic pain, and broken bones | [15] | |
| Treatment of gout disease, infections, and burns | [20] | |
| A. sisalana/leaf | Treatment for tick-borne diseases | [14] |
| Stomach detoxifier and constipation | [21] | |
| Antimicrobial against pathogen biota of the intestines and stomach | [22] | |
| A. sisalana/roots | Antidiarrheic | [23] |
| A. sisalana/leaf A. karattos Linnaeous/leaf | Treatment against meningitis and sciatica | [16] |
| A. angustifolia Haw./leaf, roots, fibers, leaf juice and root | Skin eruptions, kidney disease, and hepatic affections. It has antiparasitic and anti-hemorrhagic qualities | [17] |
| Specie/Tissue | Compound(s) | Biological Activity | Dose | Study Model | References |
|---|---|---|---|---|---|
| Phenolic Compounds | |||||
| A. durangensis Gentry Leaf | Flavonoid glycosides, aglycones, phenolic acids, proanthocyanins | Stimulant expression of enzymes | - | N. crassa ITD-G7 A. niger ITD-G1 | [43] |
| A. lechuguilla Torr. Leaf | Afzlequin-4-β-quercetin; dimeric flavonoids, quercetin, kaempferol | Anticancer | IC50: 6.96 µg/mL | SK-LU-1 cells | [47] |
| A. americana L. Leaf | Flavonoid glycosides of kaempferol, quercetin, isorhamnetin, ellagic acid hexoside | Insecticide Anti-repellent | 10.55 µg/insect RD: 0.055 µg/cm2 | Sytophilus oryzae | [49] |
| A. lechuguilla Leaf | 2,4,6-trinitrophnol, flavonoids, carboxylic acids, 2-aminomethil-propanol | Insecticide | LD50: 1035 mg/mL | Bemisia tabaci | [50] |
| A. pygmaea Gentry A. angustifolia Haw. cv. marginata Leaf | Phenolic acids, flavonoid glycosides, homoisoflavonoids | Anti-inflammatory Anti-ulcerogenic | 200 mg/kg 20 mg/kg | Wistar rats: rat paw stomach | [51] |
| A. americana L. Leaf | Apigenin | Antifungal | IC50: 72.15; 18.83 µM | A. oryzae α-amylase | [52] |
| A. americana L. Leaf | p-coumaric acid, puerarin | Antidiabetic | IC50: 98.8 µM, 3.87 µM | Human α-amylase | [53] |
| A. sisalana Fiber | Acylated glycosyl flavonoids, flavonoid glycosides, phenolic acids | Stimulant and growth protection of microgreens | - | Microgreens | [54] |
| Saponins | |||||
| A. salmiana Sap | Kammogenin-pentaglucoside and tetraglycoside, Gentrogenin pentaglucoside | Anticancer | IC50 82.7, 108.4 µg/mL | HT-29 cells | [55] |
| A. americana L. marginata Hort. Leaf | Cantala-saponin-1 | Anti-neuroinflammatory | 5-10 mg/kg | Rat brain | [56] |
| A. salmiana Sap | Kammogenin-glycosides | Anticancer | 50 µg/mL | Hep-G2, CaCo-2 cells | [57] |
| A. marmorata Roezl. Leaf | Smilagenin-diglycoside | Immunomodulatory Anti-inflammatory | IC50: 50 mg/mL, 0.086 mg/mL | RAW 264.7 cells | [58] |
| A. sisalana Perrine Leaf juice | Glycosylated steroidal saponins Hecogenin-pentaglycoside | Cytotoxic | IC50: 125 µg/mL | C6 cells | [59] |
| A. sisalana Perrine Leaf juice | Steroidal saponins | Antineoplastic | 50 µg/mL | Vero-cells Human lymphocytes, rats | [60] |
| A. angustifolia var. marginata Leaf | Steroidal-hexaglycosylated saponin | Anti-ulcerogenic | 100 mg/kg | Rat stomach | [61] |
| A. seemanniana Jacobi Leaf | Steroidal saponins | Analgesic Anti-inflammatory Anti-ulcerogenic | 100 mg/kg | Rat stomach | [62] |
| Phytosterols | |||||
| A. tequilana Weber Piña | β-sitosterol-glycoside, stigmasta-3,5-dien-7-one Cicloartenone, cycloartenol | Immunomodulatory Anti-inflammatory | 50 mg/kg | Urine, blood serum, kidney rat | [63] |
| A. angustifolia Haw. Leaf | 3-O-[(6′-O-palmitoyl)-β-d-glucopiranosyl-sitosterol] | Immunomodulatory Anti-inflammatory | 50 mg/kg | Mice ear edema | [64] |
| Species/Tissue | Extract | Targeted Organism | Affected Organism | Biological Activity | LD50 (mg/mL) | MI (%) | t | References |
|---|---|---|---|---|---|---|---|---|
| A. americana L. Leaf | M | Sitophilus oryzae (L.) | Triticum durum Desf. | Insecticide Repellent | 8.99 µg/cm2 0.055 µg/cm2 | 50 | 24 h | [49] |
| A. lechuguilla Torr. Leaf | HA | Bemisia tabaci | Phaseolus vulgaris | Insecticide | 1035 mg/L | - | 72 h | [50] |
| A. sisalana Perrine Leaf | HA | Dactylopius opuntiae | Opuntia ficus-indica | Insecticide | 17, 46 | 51, 97 | 10 Days | [77] |
| A. sisalana Perrine Leaf juice | A | Aedes aegyptii | - | Ovicide Cytotoxic | 6 | 73.8 | 24 h | [78] |
| A. sisalana Perrine Leaf | Hxn | Callosobruchus maculatus | Phaseolus spp. | Insecticide Ovicide | 100 | 100 | 24–96 h | [80] |
| A. americana var. Marginata Trel. Leaf | A | Brevicoryne brassicae | Brassica oleracea L. var. acephala | Insecticide | 0.750 | >70 | 3 h | [81] |
| A. americana Leaf | A | Aphis gossypii | Gossypium hirsitum L. | Insecticide | 25, 50 | 70.3, 92.5 | 24, 36 h | [82] |
| A. americana L. Leaf | A | Olygonichus ilicis | Coffea arabica L. Coffea canephora | Insecticide | 4% (v/v) | 100 | 72 h | [84] |
| A. angustifolia Haw. Leaf | A | Tetranychus urticae | Fragaria L. Fragaria | Insecticide | 10% (v/v) | 88 | 5 Days | [85] |
| A. tequilana Weber Leaf juice | Hxn | Bemisia tabaci | Solanum lycopersicum | Insecticide | 20–40 | 90 | 24 h | [86] |
| A. sisalana Perrine Leaf juice | A | Tetranycuhs urticae | Gossypium hirsitum L. | Insecticide | - | 100 | 35–65 Days | [87] |
| A. attenuata Leaf | A | Dyuraphis noxia | Triticum spp. | Insecticide | 0.838 | 98.75 | 24 h | [88] |
| A. filifera Whole plant | A | Biomphalaria alexandrina | Humans | Molluscicide Ovicide | 42.30 85.62 | 40.5 | 6 weeks | [89] |
| A. celsii Leaf | M | Biomphalaria alexandrina | Humans | Molluscicide | 40, 73 | - | 24 h | [90] |
| A. lophantha Leaf | B | Biomphalaria alexandrina | Humans | Molluscicide | 17, 100 | 100 | 48 h | [91] |
| A. attenuata Leaf | A | Bulinus africanus | Humans | Molluscicide | 65 | - | 24 h | [92] |
| A. legrelliana Jacobi, A. americana marginata L. Leaf juice | A | Praticolella griseola (Pfeiffer) | Humans Animals Crops | Molluscicide | 50% mL/L | 82.5 77.5 | 7 Days | [93] |
| A. americana var. expansa Leaf | A | Melanoides tuberculata (Thiaridae) | Native mollusks Humans | Molluscicide | 1.01 mL/L | 65 | 24 h | [94] |
| A. americana Leaf | B | Indoplanorbis exustus Lymnaea luteola Gyraulus convexiusculus | Humans Animals | Molluscicide Ovicide | 21.9, 18.5, 16 16.4, 15.2, 7.5 | - | 24 h | [95] |
| A. sisalana Perrine Leaf | A, B, E | Pomacea canaliculata L. | Aegle marmelos | Molluscicide | 35.3 g/L, 93.3, 298.6 | - | 72 h | [96] |
| A. sisalana A. attenuata Leaf | E | Biomphalaria alexandrina | Humans | Molluscicide | 82, 101 | - | 72 h | [97] |
| A. sisalana Perrine Leaf juice | A | A. aegypti | Humans | Larvicide | 4.5, 6.5 | 100 | 12, 24 h | [98] |
| A. sisalana Perrine Leaf | A, M | A. aegypti C. quinquefasciatus A. stephensi | Humans | Larvicide | 86, 76, 75 82, 220, 36 | 100 | 24 h | [99] |
| A. sisalana Leaf | A | A. aegypti C. quinquefasciatus | Humans | Larvicide | 100 | 100 | 3–4 Days | [100] |
| A. cantala (Haw.) Roxb. Ex Salm-Dyck Leaf | Acn | Plutella xylostella (L.) | Brassica oleracea | Larvicide | 60 | 35.77 | 12 h | [101] |
| A. sisalana Perrine Leaf juice | A | Radopholus similis | Musa x paradisiaca | Nematicide | 5, 25% (v/v) | 90.1, 99.2 | 24, 48 h | [102] |
| A. sislana Perrine Leaf juice | A | Meloidogyne javanica | Solanum lycopersicum | Nematicide | 20% (v/v) | 100 | 48 h | [103] |
| A. sisalana Perrine Leaf juice | A | Scutellomena bradys | Dioscorea esculenta | Nematicide | 20–40% (v/v) | 100 | [104] | |
| A. angustifolia Haw. Leaf | Acn | Sitophilus zeamais Motschulsky | Zea mays L. | Insecticide | 5% (v/v) | 34 | 72 h | [105] |
| A. angustifolia marginata Leaf | A | A. aegypti C. quinquefasciatus A. stephensi | Humans | Larvicide | 28.27 µg/mL 100 µg/mL | 100 | 12 h | [106] |
| Species/Tissue | Extract | Target Organism | Affected Crop | Biological Activity | LD50/MIC | IG (%) | t | References |
|---|---|---|---|---|---|---|---|---|
| A. montana Villareal A. marginata A. americana L. A. ferox K. Koch Leaf | AM | Postia placenta | - | Antifungal | - | 69.31 b | 7 days | [116] |
| A. americana L. Leaf | M | Alternaria brassicae | Brassica juncea | Antifungal | 40 µg/mL | - | 4 days | [117] |
| A. sisalana Perrine Leaf | A | Pyricularia oryzae | Oryza sativa L. | Antifungal | 3% (v/v) | 100 a | 10 days | [118] |
| A. lechuguilla Torr. Leaf | A L | Phytophthora cinnamomi | Persea americana Mill. | Antifungal | 28.87 23.07 mg/mL | 60 a | - | [119] |
| A. americana L. Leaf | A | Fusarium oxysporum Pythium aphanidermatum | Zingiber officinale Rosc. | Antifungal | - | 67.98 a 76.75 a | 96 h | [120] |
| A. scabra, Salm Dyck Leaf | HA | Botrytis cinerea Penicillium sp. | - | Antifungal | - | 90 c 86.6 c | 7 days | [121] |
| A. americana L. Leaf | M | Penicillium digitatum Sclerotium rolfsii | - | Antifungal | 11.86 21.97 µg/well | 87.73 a 80.81 a | 9 days | [122] |
| A. americana L. Leaf | M | Sclerospora graminicola | Cenchrus americanus | Antisporulant | - | - | 12–14 h | [123] |
| A. lechuguilla Torr. Leaf | L C | Rhizoctonia solani | - | Antifungal | 1.70 × 104 a 6.72 × 103 a mg/L | 14.1 c | - | [124] |
| A. americana L. Leaf | A | Aspergillus parasiticus | Cereal crops | Reduce aflatoxin production | - | - | 7 days | [125] |
|
- Leaf | E | Penicillium sp. Penicillium variable Fusarium verticillioides | Phaseolus lunatus L. | Antifungal | - | 85 c 92.5 c 100 c | 7 days | [126] |
| A. americana L. Leaf | A | Phytophthora megakarya | Theobroma cacao L. | Antifungal | 60 mg/mL | 54.33 a | 7 days | [127] |
| A. sisalana Perrine Leaf juice | A | Lasiodiplodia theobromae | - | Antifungal | 75.60 a | 3 days | [128] | |
| A. americana L. Leaf | - | Leveillula taurica (Lev.) Arn | Capsicum annum L. | Weak antifungal | - | - | 10 days | [129] |
| A. cantala (Haw.) Roxb. Ex Salm-Dyck Leaf | A | Peronospora farinosa | Vinis vinifera L. | Fungal protection | - | - | 10 days | [130] |
| A. marginata A. americana L. A. ferox K. Koch Leaf | AM | Macrophomina phaseolina | - | Antifungal | - | 64.75 b 61.69 b 59.39 b | 7 days | [131] |
| A. cantala (Haw.) Roxb. Ex Salm-Dyck Leaf | A | Plasmopara viticola | Vinis vinifera L. | Fungal protection | - | - | 10 days | [132] |
| A. americana L. Leaf | A | Sclerospora graminicola | Cenchrus americanus | Antisporulant | - | - | 12–14 h | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermúdez-Bazán, M.; Castillo-Herrera, G.A.; Urias-Silvas, J.E.; Escobedo-Reyes, A.; Estarrón-Espinosa, M. Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential. Molecules 2021, 26, 6789. https://doi.org/10.3390/molecules26226789
Bermúdez-Bazán M, Castillo-Herrera GA, Urias-Silvas JE, Escobedo-Reyes A, Estarrón-Espinosa M. Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential. Molecules. 2021; 26(22):6789. https://doi.org/10.3390/molecules26226789
Chicago/Turabian StyleBermúdez-Bazán, Misael, Gustavo Adolfo Castillo-Herrera, Judith Esmeralda Urias-Silvas, Antonio Escobedo-Reyes, and Mirna Estarrón-Espinosa. 2021. "Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential" Molecules 26, no. 22: 6789. https://doi.org/10.3390/molecules26226789
APA StyleBermúdez-Bazán, M., Castillo-Herrera, G. A., Urias-Silvas, J. E., Escobedo-Reyes, A., & Estarrón-Espinosa, M. (2021). Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential. Molecules, 26(22), 6789. https://doi.org/10.3390/molecules26226789