Effects of Low Doses of L-Carnitine Tartrate and Lipid Multi-Particulate Formulated Creatine Monohydrate on Muscle Protein Synthesis in Myoblasts and Bioavailability in Humans and Rodents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Muscle Cell Culture Experiments
2.3. Protein Synthesis and Phosphorylation
2.4. Bioavailability of LMP-Formulation Creatine in Rodents
2.5. Effect of LMP-Formulation on L-Carnitine and Creatine Bioavailability in Human Subjects
2.6. Serum L-Carnitine and Creatine Analysis
2.7. Statistical Analysis
3. Results and Discussion
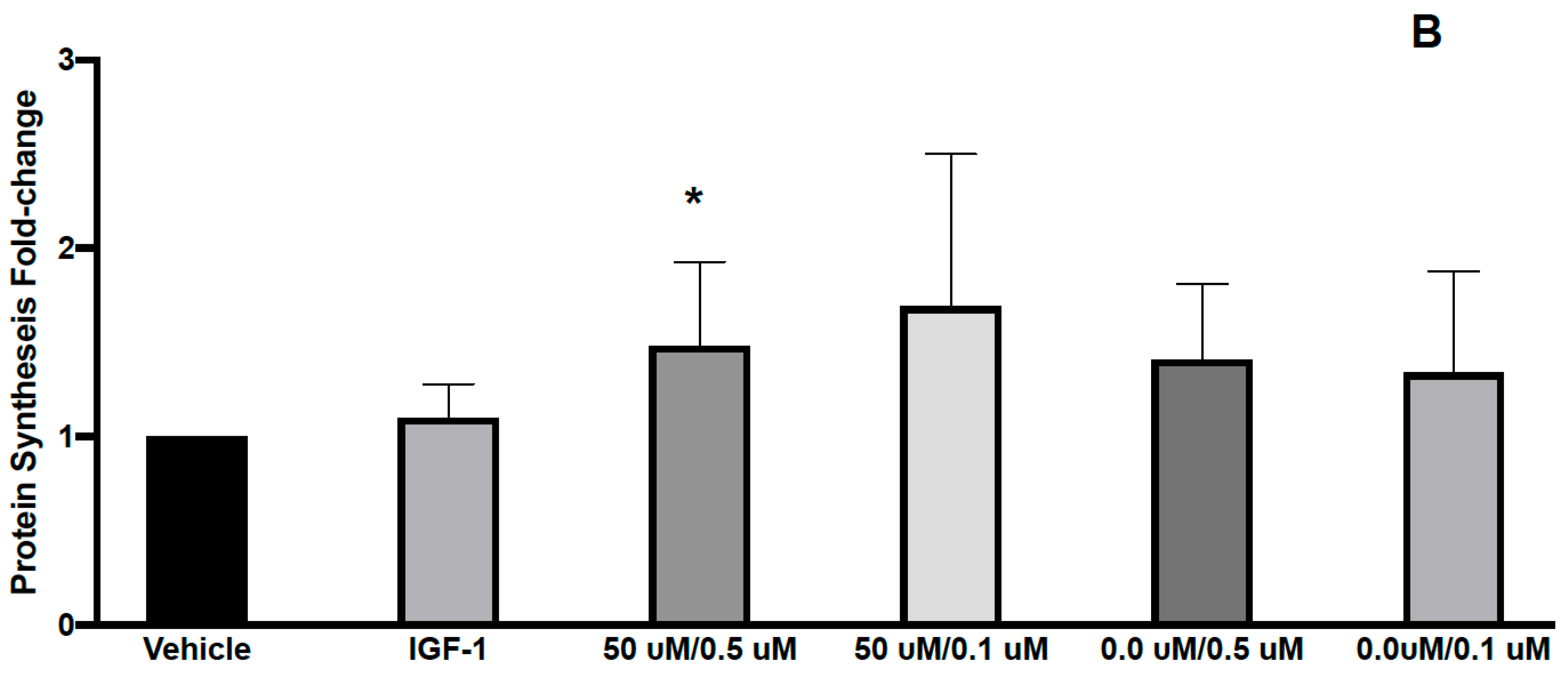
3.1. Effects of L-Carnitine and Creatine on Muscle Protein Synthesis and Anabolic Signaling in Human Primary Myoblasts
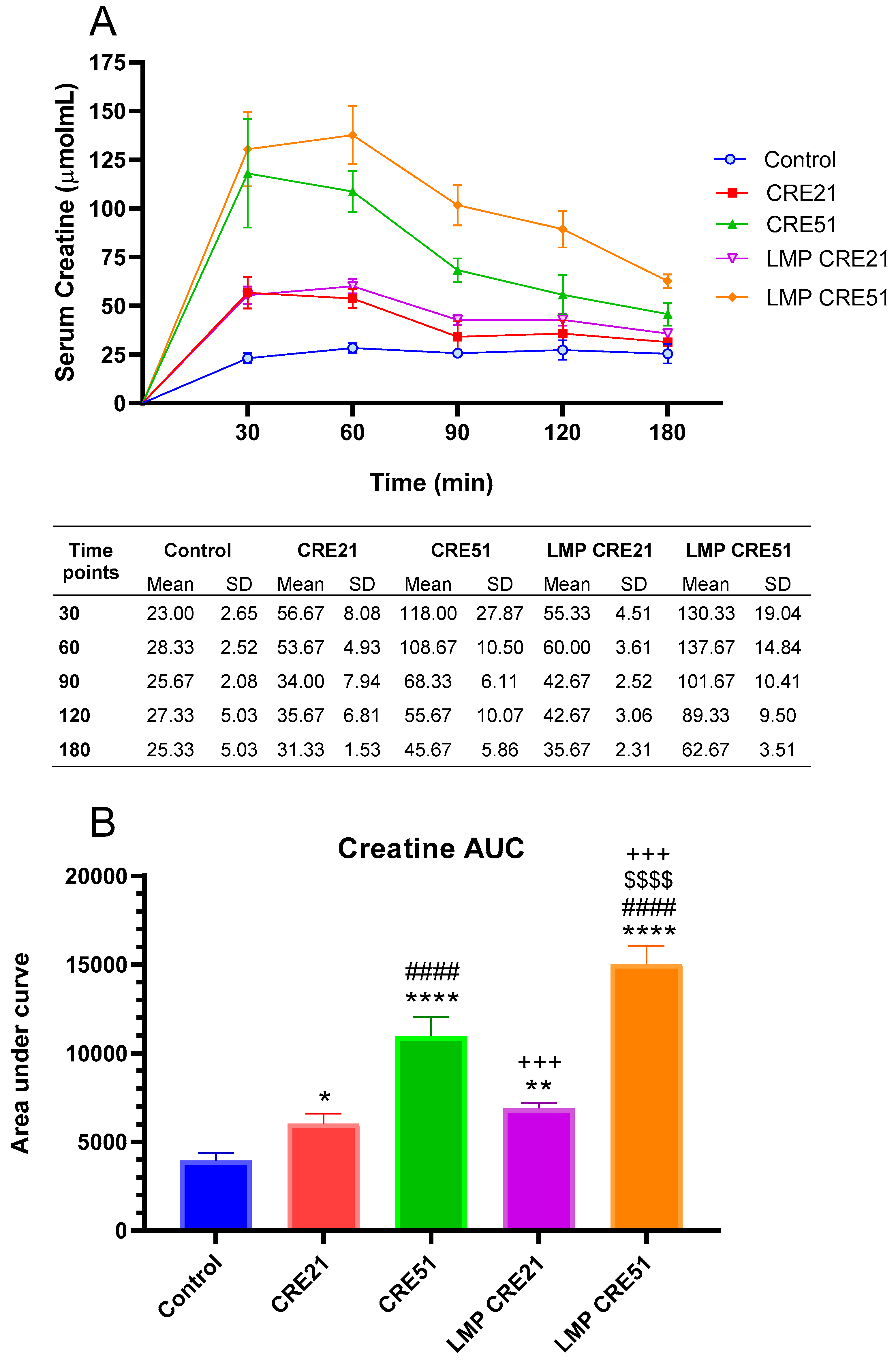
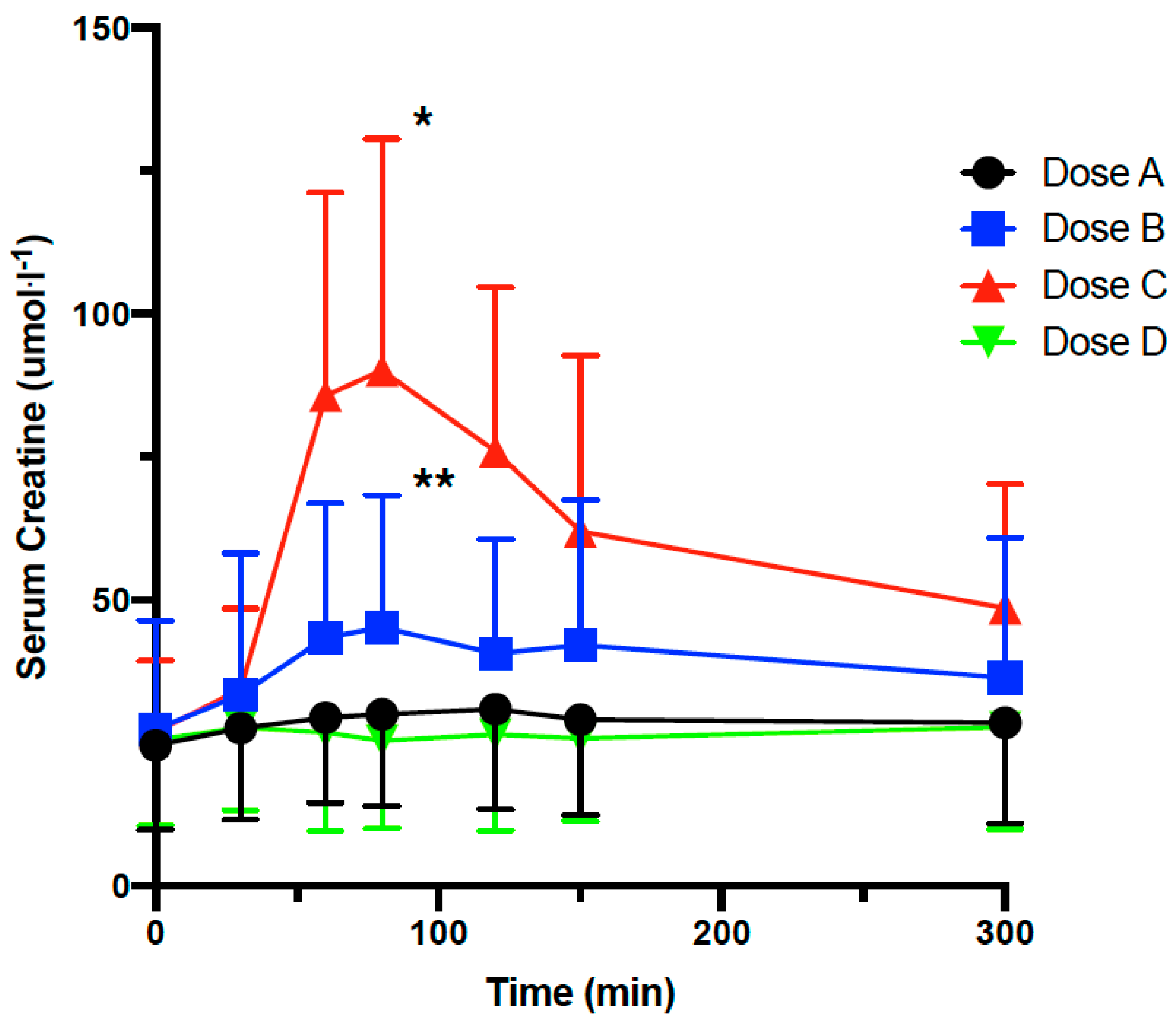
3.2. Effect of Lipid Multi-Particulate Formulated Creatine on Serum Bioavailability in Rodents
3.3. Effect of Lipid Multi-Particulate Formulated L-Carnitine and Creatine on Serum Bioavailability in Humans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hesketh, S.J.; Stansfield, B.N.; Stead, A.C.; Burniston, J.G. The application of proteomics in muscle exercise physiology. Expert Rev. Proteom. 2020, 17, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Kraemer, W.J.; Rubin, M.R.; Gómez, A.L.; Ratamess, N.A.; Gaynor, P. L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, D.A.; Fielding, R.A. Exercise as a Countermeasure for Sarcopenia. In Sarcopenia-Age-Related Muscle Wasting and Weakness: Mechanisms and Treatments; Springer: Berlin/Heidelberg, Germany, 2011; pp. 333–371. [Google Scholar]
- Anton, S.D.; Woods, A.J.; Ashizawa, T.; Barb, D.; Buford, T.W.; Carter, C.S.; Clark, D.J.; Cohen, R.A.; Corbett, D.B.; Cruz-Almeida, Y.; et al. Successful aging: Advancing the science of physical independence in older adults. Ageing Res. Rev. 2015, 24, 304–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Meschi, T.; Lauretani, F.; Felis, G.; Franchi, F.; Pedrolli, C.; Barichella, M.; Benati, G.; Di Nuzzo, S.; Ceda, G.P.; et al. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients 2016, 8, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearlman, J.P.; Fielding, R.A. Creatine monohydrate as a therapeutic aid in muscular dystrophy. Nutr. Rev. 2006, 64, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L. Creatine and its application as an ergogenic aid. Int. J. Sport Nutr. 1995, 5, S100–S110. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Casey, A.; Short, A.H.; Harris, R.; Soderlund, K.; Hultman, E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin. Sci. 1993, 84, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Greenhaff, P.L. The nutrtional biochemistry of creatine. J. Nutr. Biochem. 1997, 8, 610–618. [Google Scholar] [CrossRef]
- Jager, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.A.; THornberger, A. Measuring protein synthesis with SUnSET: A valid alternative to traditional techniques? Exerc. Sport Sci. Rev. 2013, 41, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D.; Wolf, A.; Frolich, J.C. Simplified HPLC method for urinary and circulating creatinine. Clin. Chem. 2004, 50, 201–203. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.F.; Sharer, J.D. Methods for Quantitative Creatinine Determination. Curr. Protoc. Hum. Genet. 2017, 93, A.3O.1–A.3O.7. [Google Scholar] [CrossRef]
- Sowell, J.; Fuqua, M.; Wood, T. Quantification of total and free carnitine in human plasma by hydrophilic interaction liquid chromatography tandem mass spectrometry. J. Chromatogr. Sci. 2011, 49, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Pojednic, R.M.; Ceglia, L.; Olsson, K.; Gustafsson, T.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif. Tissue Int. 2015, 96, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Legros, V.; Jeannin, P.; Burlaud-Gaillard, J.; Chaze, T.; Gianetto, Q.G.; Butler-Browne, G.; Mouly, V.; Zoladek, J.; Afonso, P.V.; Gonzàlez, M.-N.; et al. Differentiation-dependent susceptibility of human muscle cells to Zika virus infection. PLoS Negl. Trop. Dis. 2020, 14, e0008282. [Google Scholar] [CrossRef]
- Harding, C.P.; Vargis, E. Muscle Atrophy Marker Expression Differs between Rotary Cell Culture System and Animal Studies. Biomed. Res. Int. 2019, 2019, 2042808. [Google Scholar] [CrossRef]
- Louis, M.; Van Beneden, R.; Dehoux, M.; Thissen, J.P.; Francaux, M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C(2)C(12) cells. FEBS Lett. 2004, 557, 243–247. [Google Scholar] [CrossRef] [Green Version]
- McCall, W.; Persky, A.M. Pharmacokinetics of creatine. Subcell. Biochem. 2007, 46, 261–273. [Google Scholar] [PubMed]
- Hijikata, Y.; Katsuno, M.; Suzuki, K.; Hashizume, A.; Araki, A.; Yamada, S.; Inagaki, T.; Iida, M.; Noda, S.; Nakanishi, H.; et al. Impaired muscle uptake of creatine in spinal and bulbar muscular atrophy. Ann. Clin. Transl. Neurol. 2016, 3, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Clarkson, P.M.; Price, T.B.; Miles, M.P. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol. Scand. 2002, 174, 57–65. [Google Scholar] [CrossRef]
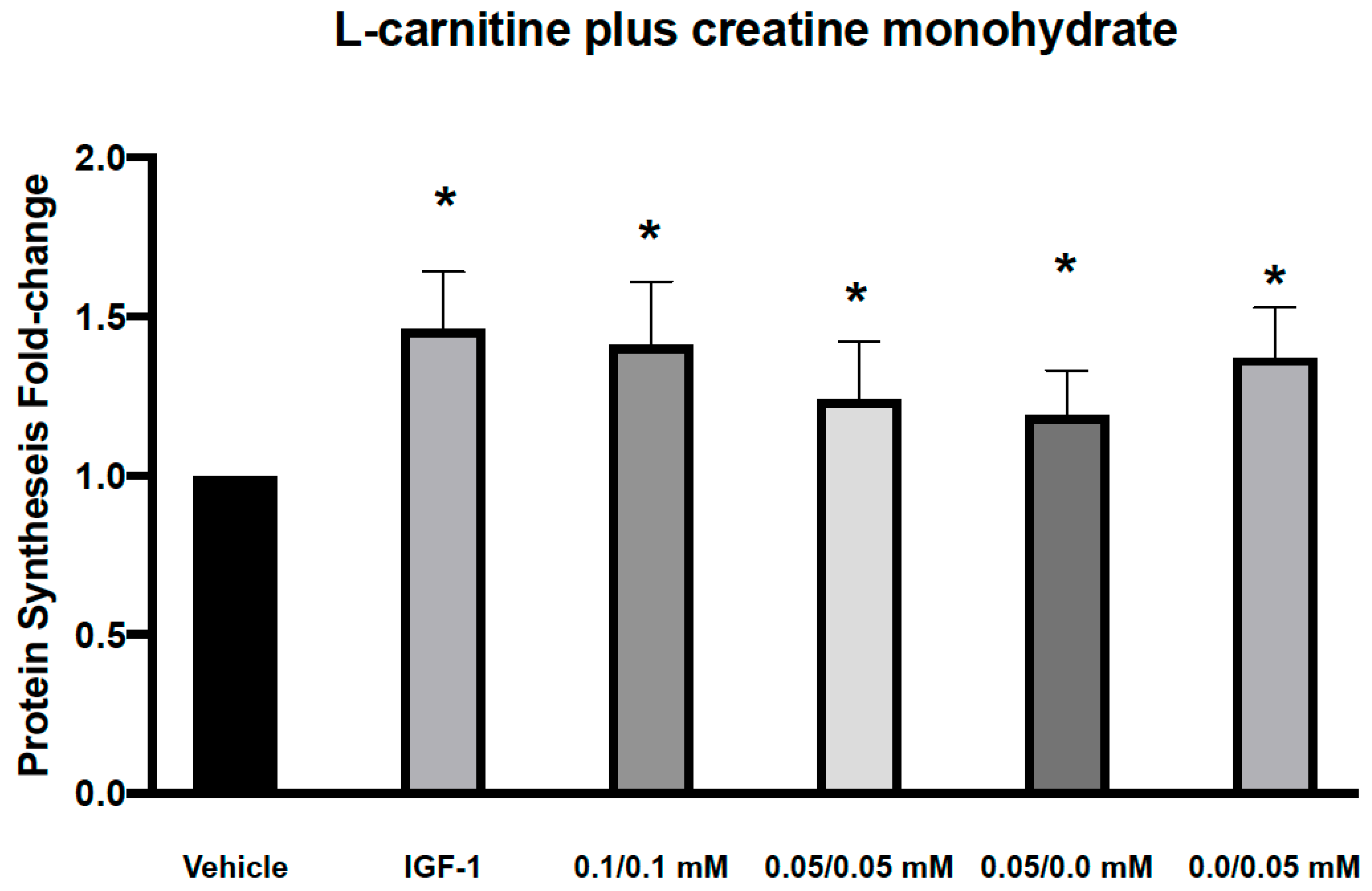
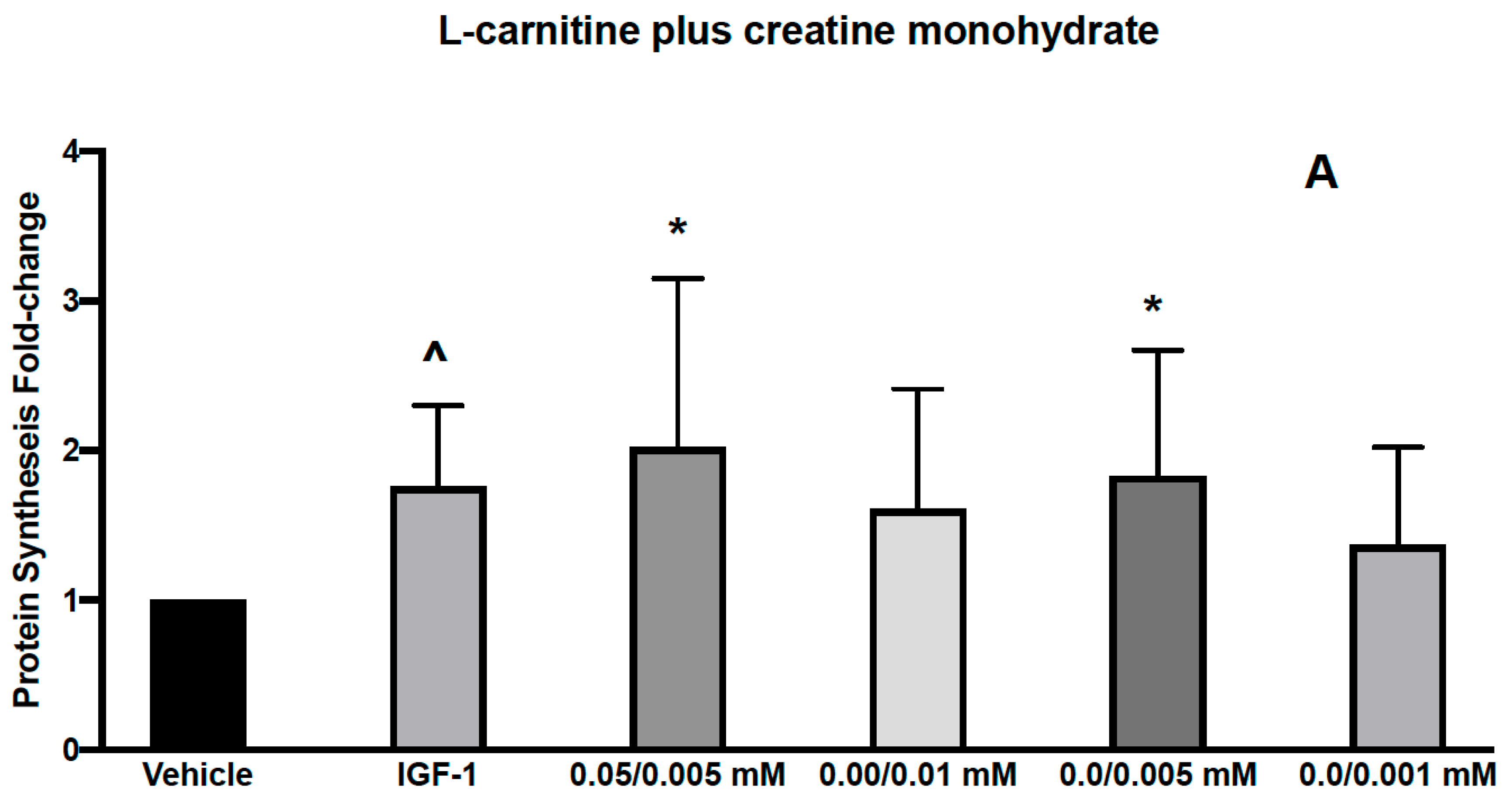
| IGF-1 | 0.05/0.005 mM | 0.0/0.01 mM | 0.0/0.005 mM | 0.0/0.001 mM | ||
|---|---|---|---|---|---|---|
| p-AKT | 1.65 (0.50) p < 0.05 | CARN/Cr | 1.46 (0.40) p < 0.05 | 1.13 (0.31) | 1.26 (0.31) | 1.06 (0.28) |
| p-RPS6 | 1.39 (0.41) p < 0.05 | 1.41 (0.53) p < 0.10 | 1.07 (0.32) | 1.31 (0.44) | 1.14 (0.46) | |
| CARN/Cr | 50 μM/0.5 μM | 50 μM/0.1 μM | 0.0 μM/0.5 μM | 0.0 μM/0.1 μM | ||
| p-AKT | 1.37 (0.28) p < 0.05 | 1.08 (0.41) | 1.16 (0.40) | 1.20 (0.47) | 1.28 (0.31) | |
| p-RPS6 | 0.84 (0.27) | 0.87 (0.25) | 0.87 (0.22) | 0.93 (0.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fielding, R.A.; Rivas, D.; Grosicki, G.J.; Ezzyat, Y.; Ceglia, L.; Price, L.L.; Orhan, C.; Sahin, K.; Fowler, K.; White, T.; et al. Effects of Low Doses of L-Carnitine Tartrate and Lipid Multi-Particulate Formulated Creatine Monohydrate on Muscle Protein Synthesis in Myoblasts and Bioavailability in Humans and Rodents. Nutrients 2021, 13, 3985. https://doi.org/10.3390/nu13113985
Fielding RA, Rivas D, Grosicki GJ, Ezzyat Y, Ceglia L, Price LL, Orhan C, Sahin K, Fowler K, White T, et al. Effects of Low Doses of L-Carnitine Tartrate and Lipid Multi-Particulate Formulated Creatine Monohydrate on Muscle Protein Synthesis in Myoblasts and Bioavailability in Humans and Rodents. Nutrients. 2021; 13(11):3985. https://doi.org/10.3390/nu13113985
Chicago/Turabian StyleFielding, Roger A., Donato Rivas, Gregory J. Grosicki, Yassine Ezzyat, Lisa Ceglia, Lori Lyn Price, Cemal Orhan, Kazim Sahin, Kelli Fowler, Tyler White, and et al. 2021. "Effects of Low Doses of L-Carnitine Tartrate and Lipid Multi-Particulate Formulated Creatine Monohydrate on Muscle Protein Synthesis in Myoblasts and Bioavailability in Humans and Rodents" Nutrients 13, no. 11: 3985. https://doi.org/10.3390/nu13113985
APA StyleFielding, R. A., Rivas, D., Grosicki, G. J., Ezzyat, Y., Ceglia, L., Price, L. L., Orhan, C., Sahin, K., Fowler, K., White, T., Durkee, S., Kritsch, K., & Bellamine, A. (2021). Effects of Low Doses of L-Carnitine Tartrate and Lipid Multi-Particulate Formulated Creatine Monohydrate on Muscle Protein Synthesis in Myoblasts and Bioavailability in Humans and Rodents. Nutrients, 13(11), 3985. https://doi.org/10.3390/nu13113985







