Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. Preparation and Analysis of Fatty Acids in PSO
2.2. Partial Purification and Polyphenolic Quantification of RA-RF
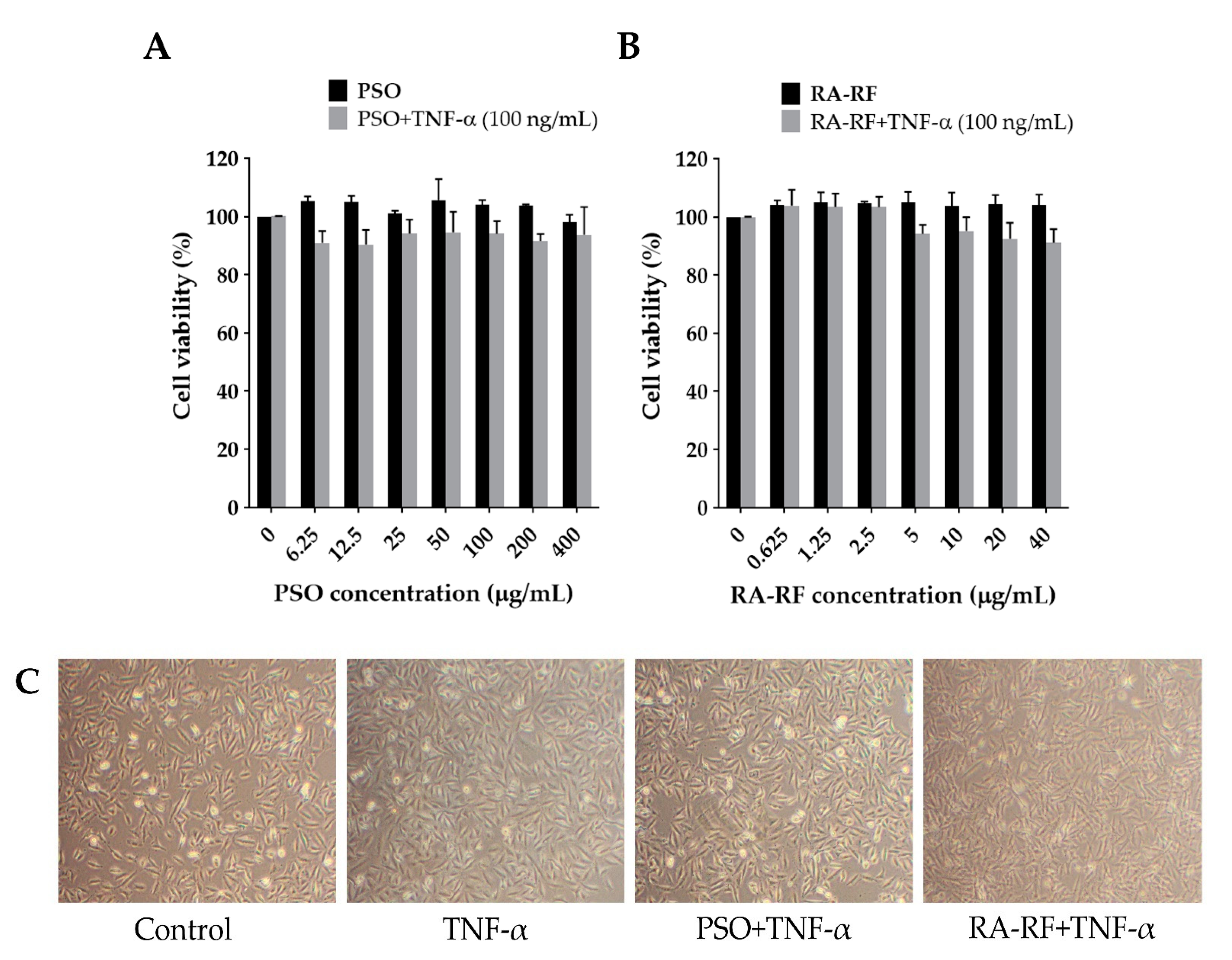
2.3. Effect of PSO and RA-RF on Cell Viability and Morphology
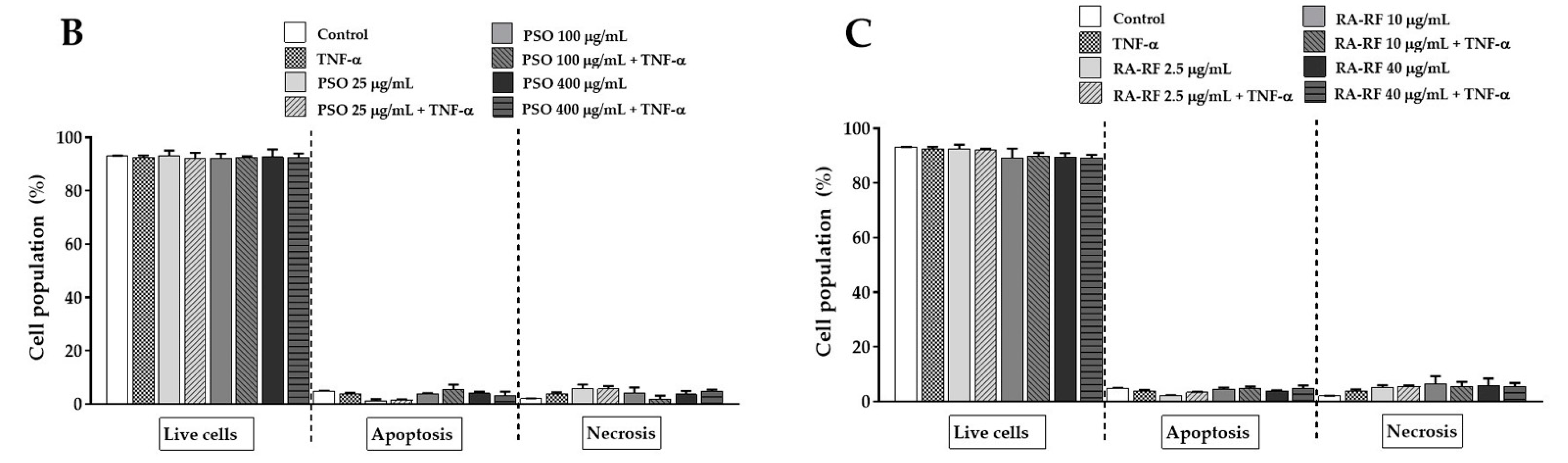
2.4. Effect of PSO and RA-RF on TNF-α-Induced A549 Cells Apoptosis
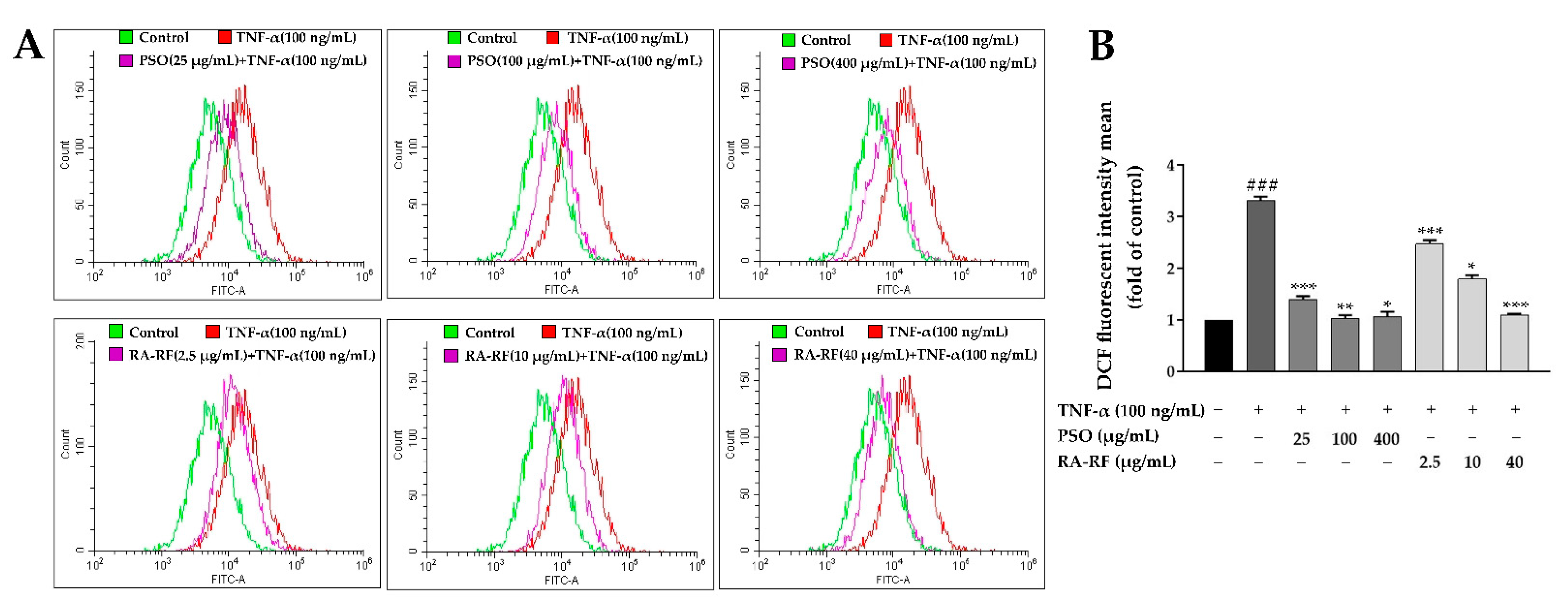
2.5. Effect of PSO and RA-RF on Intracellular ROS Production
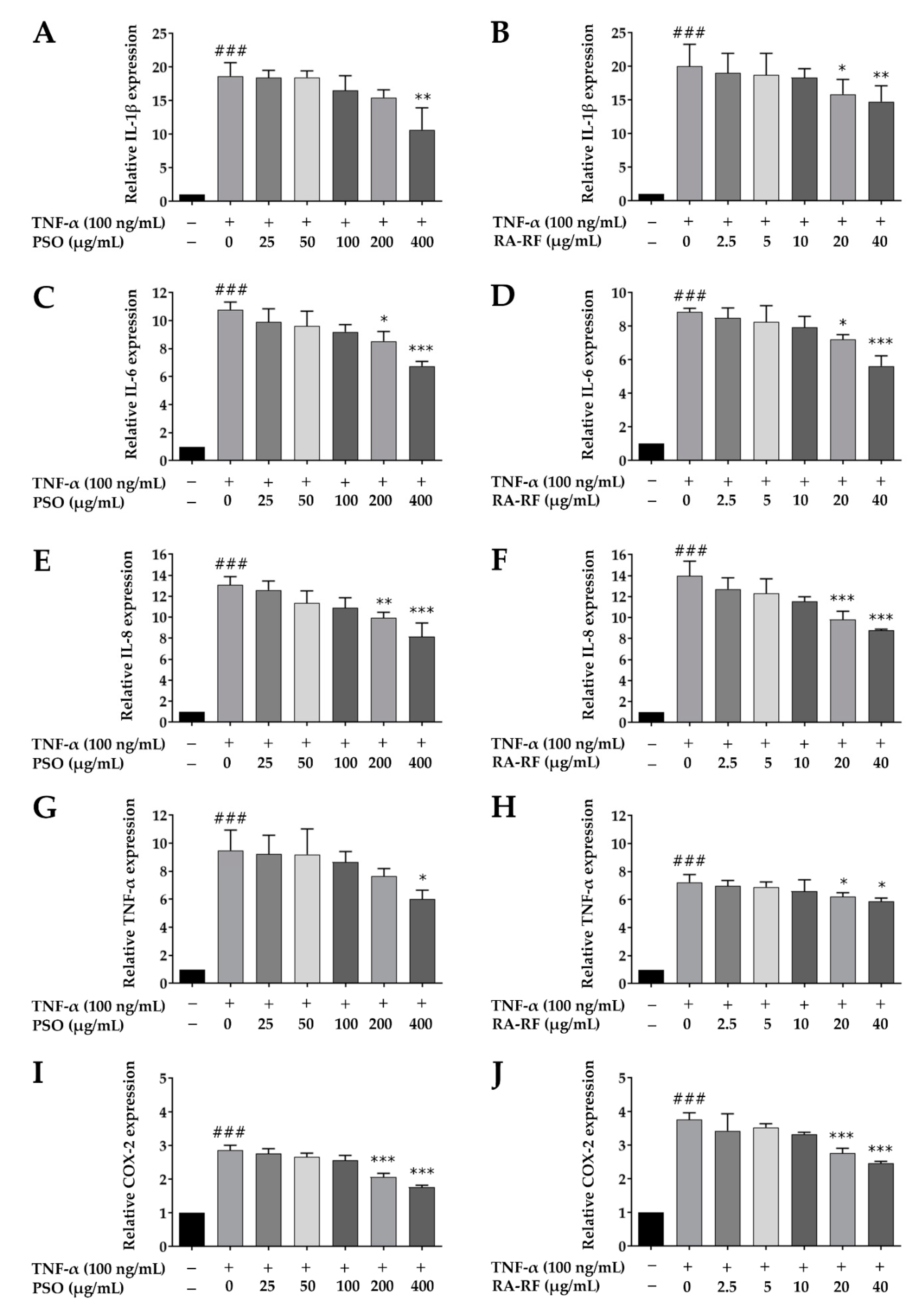
2.6. Downregulation of IL-1β, IL-6, IL-8, TNF-α, and COX-2 mRNA Expression in A549 Cells Treated with PSO and RA-RF
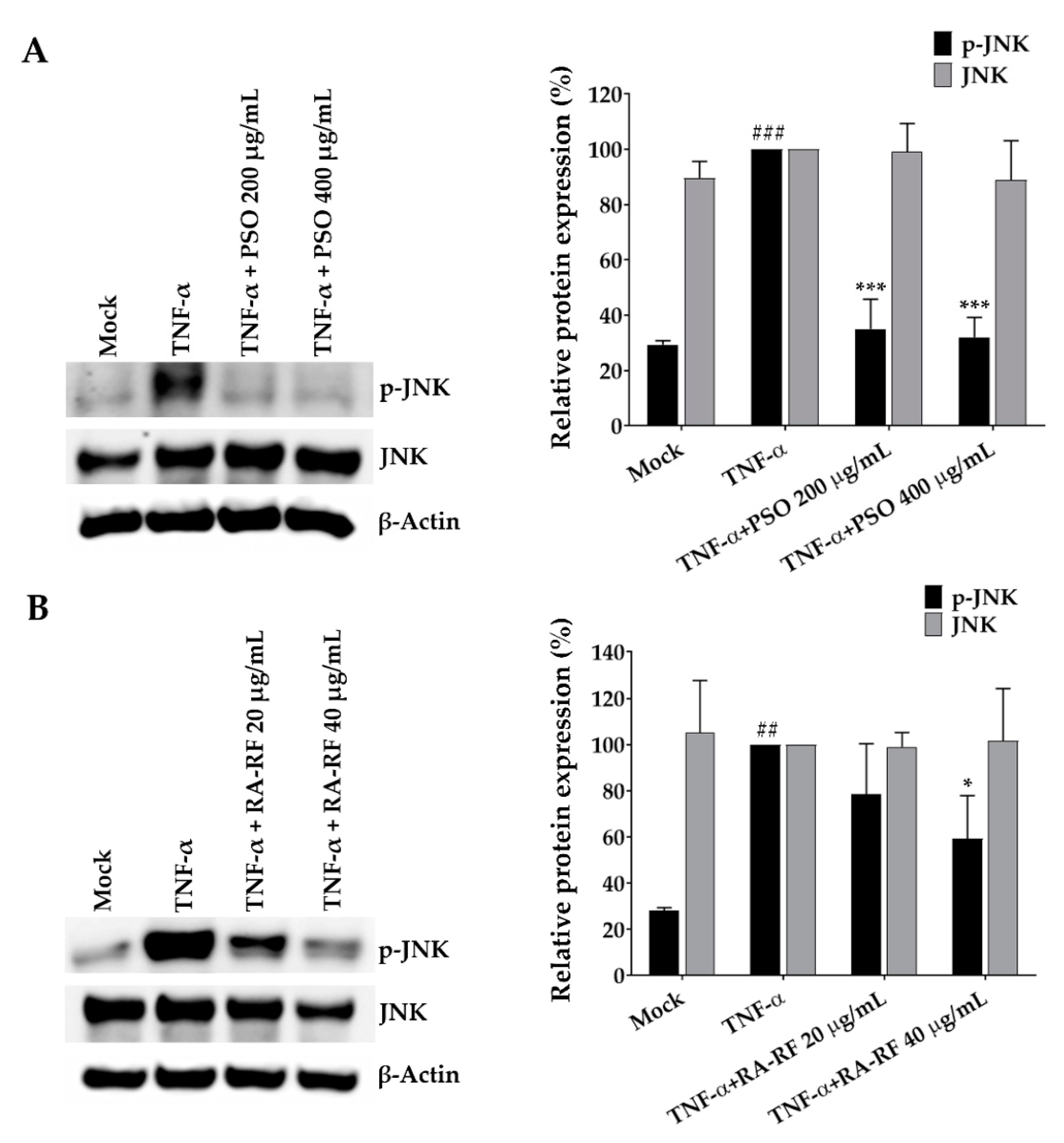
2.7. Suppression Effect of PSO and RA-RF on JNK Phosphorylation
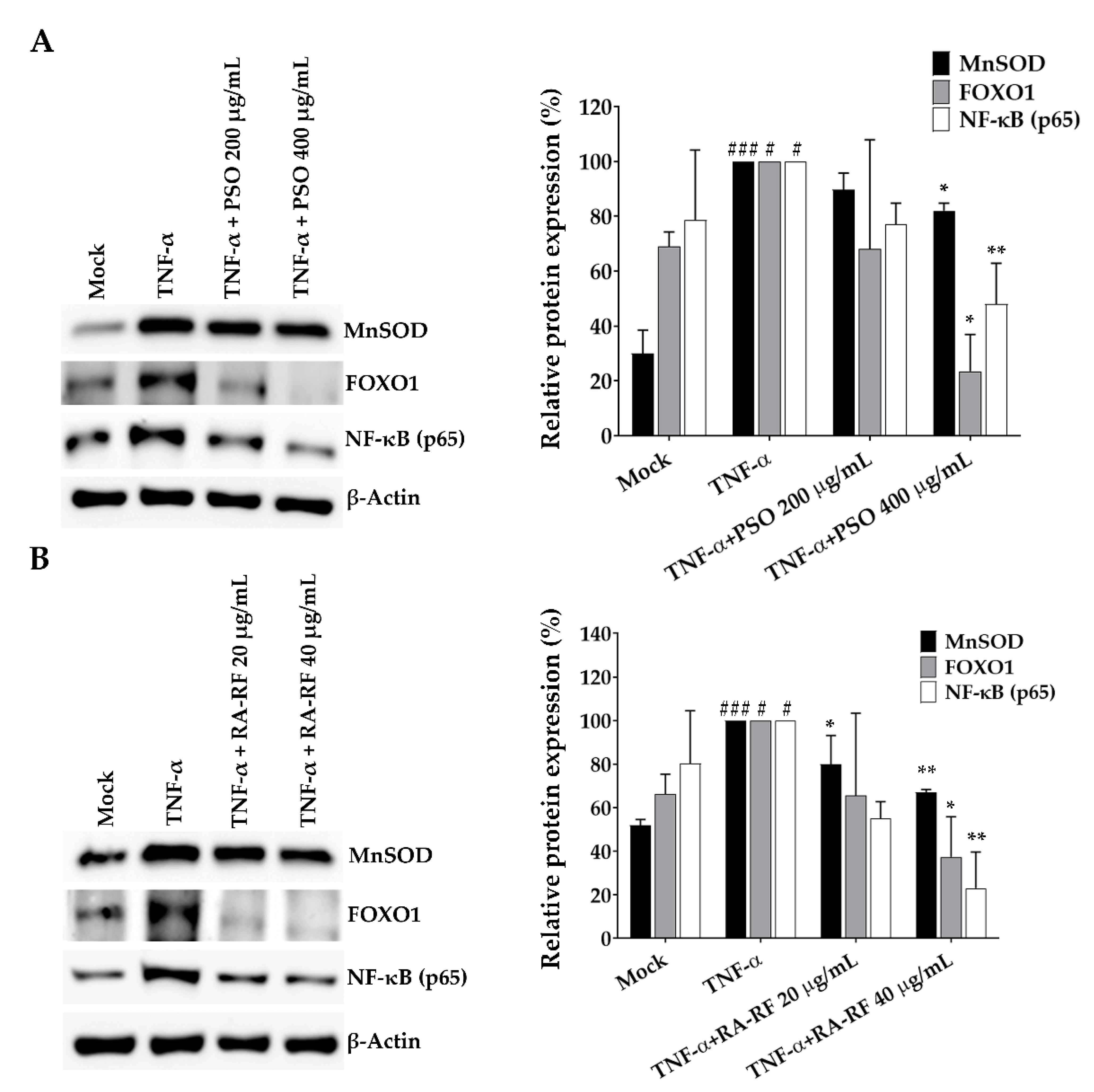
2.8. Expression of NF-κB, FOXO1 and MnSOD in A549 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of PSO and RA-RF
4.2. Determination of Total Phenolic Content
4.3. Determination of Total Flavonoid Content
4.4. Determination of RA Content
4.5. Cell Culture
4.6. Reagents and Antibodies
4.7. Cell Viability Assay and Morphological Assessment
4.8. Annexin V-FITC/PI Staining for Apoptosis Quantification Using Flow Cytometry
4.9. Effect of PSO and RA-RF on Intracellular ROS Production
4.10. Effect of PSO and RA-RF on IL-1β, IL-6, IL-8, TNF-α, and COX-2 mRNA Expression
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahmed, H.M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [Green Version]
- Kongkeaw, S.; Riebroy, S.; Chaijan, M. Comparative studies on chemical composition, phenolic compounds and antioxidant activities of brown and white perilla (Perilla frutescens) seeds. Chiang Mai J. Sci. 2015, 42, 896–906. [Google Scholar]
- Dhyani, A.; Chopra, R.; Garg, M. A review on nutritional value, functional properties and pharmacological application of perilla (Perilla frutescens L.). Biomed. Pharmacol. J. 2019, 12, 649–660. [Google Scholar] [CrossRef]
- Um, S.; Bhandari, S.R.; Kim, N.; Yang, T.; Lee, J.K.; Lee, Y. Characterization of lipophilic nutraceutical compounds in seeds and leaves of Perilla frutescens. Hortic. Sci. Technol. 2013, 31, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Zamani Ghaleshahi, A.; Ezzatpanah, H.; Rajabzadeh, G.; Ghavami, M. Comparison and analysis characteristics of flax, perilla and basil seed oils cultivated in Iran. J. Food Sci. Technol. 2020, 57, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- Zhang, H.X.; Guan, J.; Tian, Y.H.; Su, G.Y.; Zhao, Y.Q. Acute and sub-chronic 90-day oral toxicity study of Perilla seed oil in rodents and Beagle dogs. Regul. Toxicol. Pharmacol. RTP 2019, 103, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kaewwongse, M.; Sriraksa, N.; Chandeying, V.; Vanittanakom, P.; Suttajit, M. Continuous supplementation of perilla seed oil is safe and facilitates the learning and memory in adult rats. Naresuan Phayao J. 2019, 12, 4–12. [Google Scholar]
- Hashimoto, M.; Matsuzaki, K.; Kato, S.; Hossain, S.; Ohno, M.; Shido, O. Twelve-month studies on perilla oil intake in japanese adults-Possible supplement for mental health. Foods 2020, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Kamalashiran, C.; Sriyakul, K.; Pattaraarchachai, J.; Muengtaweepongsa, S. Outcomes of perilla seed oil as an additional neuroprotective therapy in patients with mild to moderate dementia: A randomized control trial. Curr. Alzheimer Res. 2019, 16, 146–155. [Google Scholar] [CrossRef]
- Wei, M.; Xiong, P.; Zhang, L.; Fei, M.; Chen, A.; Li, F. Perilla oil and exercise decrease expressions of tumor necrosis factor-α, plasminogen activator inhibitor-1 and highly sensitive C-reactive protein in patients with hyperlipidemia. J. Tradit. Chin. Med. 2013, 33, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-X.; Tian, Y.-H.; Guan, J.; Xie, Q.-M.; Zhao, Y.-Q. The anti-tussive, anti-inflammatory effects and sub-chronic toxicological evaluation of perilla seed oil. J. Sci. Food Agric. 2021, 101, 1419–1427. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yoon, K.Y. Functional properties and biological activities of perilla seed meal protein hydrolysates obtained by using different proteolytic enzymes. Food Sci. Biotechnol. 2020, 29, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Liceaga, A.M.; Yoon, K.Y. Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Sci. Nutr. 2019, 7, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Fu, Q. Optimization of ultrasound-assisted extraction process of perilla seed meal proteins. Food Sci. Biotechnol. 2012, 21, 1701–1706. [Google Scholar] [CrossRef]
- Paradee, N.; Howes, M.-J.R.; Utama-ang, N.; Chaikitwattna, A.; Hider, R.C.; Srichairatanakool, S. A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human hepatoma (HuH7) cells. Phytother. Res. 2019, 33, 2064–2074. [Google Scholar] [CrossRef]
- Paradee, N.; Utamaang, N.; Uthaipibull, C.; Porter, J.B.; Garbowski, M.W.; Srichairatanakool, S. Extracts of Thai Perilla frutescens nutlets attenuate tumour necrosis factor-α-activated generation of microparticles, ICAM-1 and IL-6 in human endothelial cells. Biosci. Rep. 2020, 40, BSR20192110. [Google Scholar] [CrossRef]
- Tang, W.; Sun, B.; Zhao, Y. Preparative separation and purification of rosmarinic acid from perilla seed meal via combined column chromatography. J. Chromatogr. B 2014, 947–948, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.; Zhang, Z.; Xia, Y.; Li, X.; Cui, L.; Chen, T. Optimization of ultrasound-assisted extraction of procyanidins from perilla seed hull and their antioxidant activities in vitro. Food Sci. Technol. 2019, 39, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Guo, G.; Beckley, N.; Zhang, Y.; Yang, X.; Sharma, M.; Habib, A.A. Tumor necrosis factor in lung cancer: Complex roles in biology and resistance to treatment. Neoplasia 2021, 23, 189–196. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Hou, J.; Zhu, B.; Min, Z.; Zhang, M.; Song, D.; Cheng, Y.; Wang, X. Targeting roles of inflammatory microenvironment in lung cancer and metastasis. Cancer Metastasis Rev. 2015, 34, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, H.; Xie, Y.; Cao, Z.; Lin, X.; Wang, Y. FoxO1 overexpression ameliorates TNF-α-induced oxidative damage and promotes osteogenesis of human periodontal ligament stem cells via antioxidant defense activation. Stem. Cells Int. 2019, 2019, 2120453. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Cao, X.; Liu, L.; Cui, Y.; Li, X.; Quan, M.; Ren, K.; Chen, A.; Xu, C.; Qiu, Y.; et al. Genistein inhibits lung cancer cell stem-like characteristics by modulating MnSOD and FoxM1 expression. Oncol. Lett. 2020, 20, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-M.; Wu, T.-C.; Shieh, S.-H.; Wu, Y.-H.; Li, M.-C.; Sheu, G.-T.; Cheng, Y.-W.; Chen, C.-Y.; Lee, H. MnSOD promotes tumor invasion via upregulation of FoxM1–MMP2 axis and related with poor survival and relapse in lung adenocarcinomas. Mol. Cancer Res. 2013, 11, 261. [Google Scholar] [CrossRef] [Green Version]
- Toh, Y.; Kuninaka, S.; Oshiro, T.; Ikeda, Y.; Nakashima, H.; Baba, H.; Kohnoe, S.; Okamura, T.; Mori, M.; Sugimachi, K. Overexpression of manganese superoxide dismutase mRNA may correlate with aggressiveness in gastric and colorectal adenocarcinomas. Int. J. Oncol. 2000, 17, 107–119. [Google Scholar] [CrossRef]
- Rankantha, A.; Chitapanarux, I.; Pongnikorn, D.; Prasitwattanaseree, S.; Bunyatisai, W.; Sripan, P.; Traisathit, P. Risk patterns of lung cancer mortality in northern Thailand. BMC Public Health 2018, 18, 1138. [Google Scholar] [CrossRef]
- Chang, J.T.; Jeon, J.; Sriplung, H.; Yeesoonsang, S.; Bilheem, S.; Rozek, L.; Chitapanarux, I.; Pongnikorn, D.; Daoprasert, K.; Vatanasapt, P.; et al. Temporal Trends and Geographic Patterns of Lung Cancer Incidence by Histology in Thailand, 1990 to 2014. J. Glob. Oncol. 2018, 4, 1–29. [Google Scholar] [CrossRef]
- Lee, G.; Walser, T.C.; Dubinett, S.M. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr. Opin. Pulm. Med. 2009, 15, 303–307. [Google Scholar] [CrossRef]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014, 816, 1–23. [Google Scholar] [CrossRef]
- Ohri, C.M.; Shikotra, A.; Green, R.H.; Waller, D.A.; Bradding, P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer 2010, 10, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooppachai, C.; Limtrakul, P.; Yodkeeree, S. Dicentrine potentiates TNF-α-induced apoptosis and suppresses invasion of A549 lung adenocarcinoma cells via modulation of NF-κB and AP-1 activation. Molecules 2019, 24, 4100. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Babu, P.V.; Si, H.; Nallasamy, P.; Zhu, H.; Zhen, W.; Misra, H.P.; Li, Y.; Liu, D. Genistein inhibits TNF-α-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int. J. Cardiol. 2013, 168, 2637–2645. [Google Scholar] [CrossRef] [Green Version]
- Chumphukam, O.; Pintha, K.; Khanaree, C.; Chewonarin, T.; Chaiwangyen, W.; Tantipaiboonwong, P.; Suttajit, M.; Khantamat, O. Potential anti-mutagenicity, antioxidant, and anti-inflammatory capacities of the extract from perilla seed meal. J. Food Biochem. 2018, 42, e12556. [Google Scholar] [CrossRef]
- Mungmai, L.; Preedalikit, W.; Pintha, K.; Tantipaiboonwong, P.; Aunsri, N. Collagenase and melanogenesis inhibitory effects of Perilla frutescens pomace extract and its efficacy in topical cosmetic formulations. Cosmetics 2020, 7, 69. [Google Scholar] [CrossRef]
- Suttajit, M.; Khanaree, C.; Tantipaiboonwong, P.; Pintha, K. Omega-3, omega-6 fatty acids and nutrients of Nga-mon seeds in Northern Thailand. Naresuan Phayao J. 2015, 8, 80–86. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essers, M.A.; Weijzen, S.; de Vries-Smits, A.M.; Saarloos, I.; de Ruiter, N.D.; Bos, J.L.; Burgering, B.M.T. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot Essent Fat. Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Candela, C.G.; López, L.B.; Kohen, V.L. Importance of a balanced Omega 6/Omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Arbex, A.K.; Bizarro, V.R.; Santos, J.C.S.; Araújo, L.M.M.; Jesus, A.L.C.; Fernandes, M.S.A.; Salles, M.M.; Rocha, D.R.T.W.; Marcadenti, A. The impact of the essential fatty acids (EFA) in human health. Open J. Endocr. Metab. Dis. 2015, 5, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the Omega-6/Omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sakakibara, J.; Nagatsu, A.; Sekiya, K. Inhibitors of arachidonate lipoxygenase from defatted perilla seed. J. Agric. Food Chem. 1998, 46, 862–865. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, L.; Yin, P.; Shi, L.; Zhang, J.; Liu, Y.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Jun, H.; Kim, B.; Song, G.; Kim, Y. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014, 148, 367–372. [Google Scholar] [CrossRef]
- Hong, E.; Park, K.H.; Kim, G. Phenolic-enriched fractions from Perilla frutescens var. acuta: Determinating rosmarinic acid and antioxidant activity. J. Food Biochem. 2011, 35, 1637–1645. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Song, B.R.; Kim, J.E.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Lee, H.S.; Lee, C.Y.; Kim, B.-H.; et al. Therapeutic effects of cold-pressed perilla oil mainly consisting of linolenic acid, oleic acid and linoleic acid on UV-induced photoaging in NHDF cells and SKH-1 hairless mice. Molecules 2020, 25, 989. [Google Scholar] [CrossRef] [Green Version]
- Phromnoi, K.; Suttajit, M.; Saenjum, C.; Limtrakul, P.D. Inhibitory effect of a rosmarinic acid-enriched fraction prepared from Nga-Mon (Perilla frutescens) seed meal on osteoclastogenesis through the RANK signaling pathway. Antioxidants 2021, 10, 307. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Chang, H.; Chen, C.; Lin, J. Dietary perilla oil inhibits proinflammatory cytokine production in the bronchoalveolar lavage fluid of ovalbumin-challenged mice. Lipids 2008, 43, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic acid enriched fraction from Perilla frutescens leaves strongly protects indomethacin-induced gastric ulcer in rats. BioMed Res. Int. 2019, 2019, 9514703. [Google Scholar] [CrossRef] [Green Version]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, R.F.; Brenner, D.A. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G583–G589. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Kim, J.E.; Choi, H.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Seo, S.; Yang, S.Y.; An, B.-S.; Lee, H.S.; et al. α-linolenic acid-enriched cold-pressed perilla oil suppress high-fat diet-induced hepatic steatosis through amelioration of the ER stress-mediated autophagy. Molecules 2020, 25, 2662. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xu, Y.; Wen, X.; Nie, H.; Hu, T.; Yang, X.; Chu, X.; Yang, J.; Deng, X.; He, J. Rosmarinic acid attenuates airway inflammation and hyperresponsiveness in a murine model of asthma. Molecules 2016, 21, 769. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.K.; Chauhan, A.S.; Zhuang, L.; Gan, B. FoxO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018, 50, 65–76. [Google Scholar] [CrossRef]
- Ito, Y.; Daitoku, H.; Fukamizu, A. Foxo1 increases pro-inflammatory gene expression by inducing C/EBPbeta in TNF-alpha-treated adipocytes. Biochem. Biophys. Res. Commun. 2009, 378, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Coudriet, G.M.; Hyun Kim, D.; Lu, Y.; Perdomo, G.; Qu, S.; Slusher, S.; Tse, H.M.; Piganelli, J.; Giannoukakis, N.; et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes 2009, 58, 2624–2633. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. BioMed Res. Int. 2014, 925350. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Pogrebniak, H.W.; Prewitt, T.W.; Matthews, W.A.; Pass, H.I. Tumor necrosis factor-alpha alters response of lung cancer cells to oxidative stress. J. Thorac. Cardiovasc. Surg. 1991, 102, 904–907. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, C.-C.; Li, T.-K.; Wang, P.-H.; Liu, L.-R.; Chang, F.-Y.; Wang, Y.-C.; Yu, Y.-H.; Lin, S.-P.; Mersmann, H.J.; et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J. Nutr. Biochem. 2012, 23, 1609–1616. [Google Scholar] [CrossRef]
- Zajdel, A.; Paduszyński, P.; Gruchlik, A.; Głogowska-Ligus, J.; Wilczok, A.; Dzierzewicz, A. Polyunsaturated fatty acids alter expression of genes encoding antioxidant enzymes in A549 cells exposed to doxorubicin. Acta Pol. Pharm.-Drug Res. 2010, 67, 696–700. [Google Scholar]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a double-edge sword for cancer and other age-related diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Lee, J.; Song, Y.O. Perilla oil rich in α-linolenic acid suppresses hepatic SREBPs and NF-κB expression in hypercholesterolemia-induced apolipoprotein E knockout mice. Food Sci. Biotechnol. 2012, 21, 807–813. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.; Kim, K. Perilla oil alleviates high-fat diet-induced inflammation in the colon of mice by suppressing nuclear factor-kappa B activation. J. Med. Food 2020, 23, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharm. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, P.; Wang, X. Lipids in Health and Disease; Springer Science & Business Media: New York, NY, USA, 2008; Volume 49. [Google Scholar]
- So, Y.; Lee, S.Y.; Han, A.-R.; Kim, J.-B.; Jeong, H.G.; Jin, C.H. Rosmarinic acid methyl ester inhibits LPS-induced NO production via suppression of MyD88- dependent and -independent pathways and induction of HO-1 in RAW 264.7 cells. Molecules 2016, 21, 1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Method 996.06: Fats (total, saturated, and monounsaturated in foods). Hydrolytic Extraction. Gas Chromatographic Method. In Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2001. [Google Scholar]
- Pintha, K.; Tantipaiboonwong, P.; Yodkeeree, S.; Chaiwangyen, W.; Chumphukam, O.; Khantamat, O.; Khanaree, C.; Kangwan, N.; Thongchuai, B.; Suttajit, M.; et al. Thai perilla (Perilla frutescens) leaf extract inhibits human breast cancer invasion and migration. Maejo Int. J. Sci. 2018, 12, 112–123. [Google Scholar]
- Khaw-on, P.; Pompimon, W.; Banjerdpongchai, R. Goniothalamin induces necroptosis and anoikis in human invasive breast cancer MDA-MB-231 cells. Int. J. Mol. Sci. 2019, 20, 3953. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kaowinn, S.; Yawut, N.; Koh, S.S.; Chung, Y.-H. Cancer upregulated gene (CUG)2 elevates YAP1 expression, leading to enhancement of epithelial-mesenchymal transition in human lung cancer cells. Biochem. Biophys. Res. Commun. 2019, 511, 122–128. [Google Scholar] [CrossRef] [PubMed]



| Fractions | %Yield | TPC | TFC | RA Content |
|---|---|---|---|---|
| EtOH | 5.63 ± 0.27 | 84.81 ± 0.85 | 68.33 ± 1.14 | 71.72 ± 0.72 |
| HEX | 13.53 ± 0.85 | 4.74 ± 0.80 | 12.17 ± 1.62 | 54.56 ± 1.63 |
| DCM | 2.76 ± 0.57 | 198.36 ± 1.67 | 88.84 ± 4.88 | 29.42 ± 0.20 |
| EA (RA-RF) | 5.33 ± 0.44 | 248.00 ± 5.14 | 165.21 ± 2.61 | 647.68 ± 53.08 |
| Water | 23.75 ± 1.34 | 27.31 ± 0.11 | 23.56 ± 0.87 | 37.66 ± 0.19 |
| Genes | Forward Sequence | Reverse Sequence |
|---|---|---|
| IL-1β | 5′-AAA CAG ATG AAG TGC TCC TTC CAG G-3′ | 5′-TGG AGA ACA CCA CTT GTT GCT CCA-3′ |
| IL-6 | 5′-ATG AAC TCC TTC TCC ACA AGC-3′ | 5′-GTT TTC TGC CAG TGC CTC TTT G-3′ |
| IL-8 | 5′-AGA TAT TGC ACG GGA GAA-3′ | 5′-GAA ATA AAG GAG AAA CCA-3′ |
| TNF-α | 5′-CCC AGG CAG TCA GAT CAT CTT C-3′ | 5′-AGC TGC CCC TCA GCT TGA-3′ |
| COX-2 | 5′-CCC TTG GGT GTC AAA GGT AA-3′ | 5′-GCC CTC GCT TAT GAT CTG TC-3′ |
| GAPDH | 5′-GAA GGT GAA GGT CGA GTC A-3′ | 5′-GCT CCT GGA AGA TGG TGA T-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. https://doi.org/10.3390/molecules26226757
Tantipaiboonwong P, Chaiwangyen W, Suttajit M, Kangwan N, Kaowinn S, Khanaree C, Punfa W, Pintha K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules. 2021; 26(22):6757. https://doi.org/10.3390/molecules26226757
Chicago/Turabian StyleTantipaiboonwong, Payungsak, Wittaya Chaiwangyen, Maitree Suttajit, Napapan Kangwan, Sirichat Kaowinn, Chakkrit Khanaree, Wanisa Punfa, and Komsak Pintha. 2021. "Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells" Molecules 26, no. 22: 6757. https://doi.org/10.3390/molecules26226757
APA StyleTantipaiboonwong, P., Chaiwangyen, W., Suttajit, M., Kangwan, N., Kaowinn, S., Khanaree, C., Punfa, W., & Pintha, K. (2021). Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules, 26(22), 6757. https://doi.org/10.3390/molecules26226757






