Tetrandrine Suppresses Human Brain Glioblastoma GBM 8401/luc2 Cell-Xenografted Subcutaneous Tumors in Nude Mice In Vivo
Abstract
:1. Introduction
2. Results
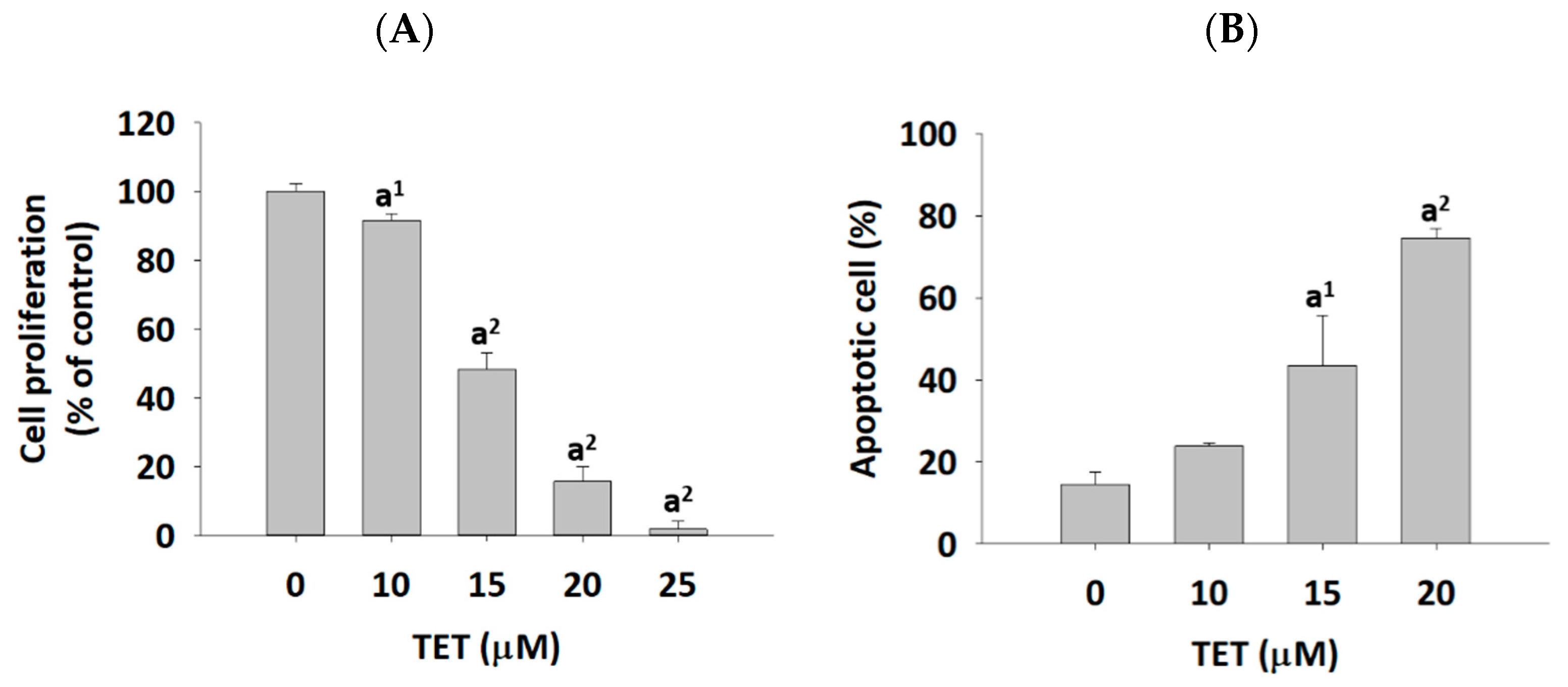
2.1. The Effects of TET on Cell Viability and Apoptosis in GBM 8401/luc2 Cells
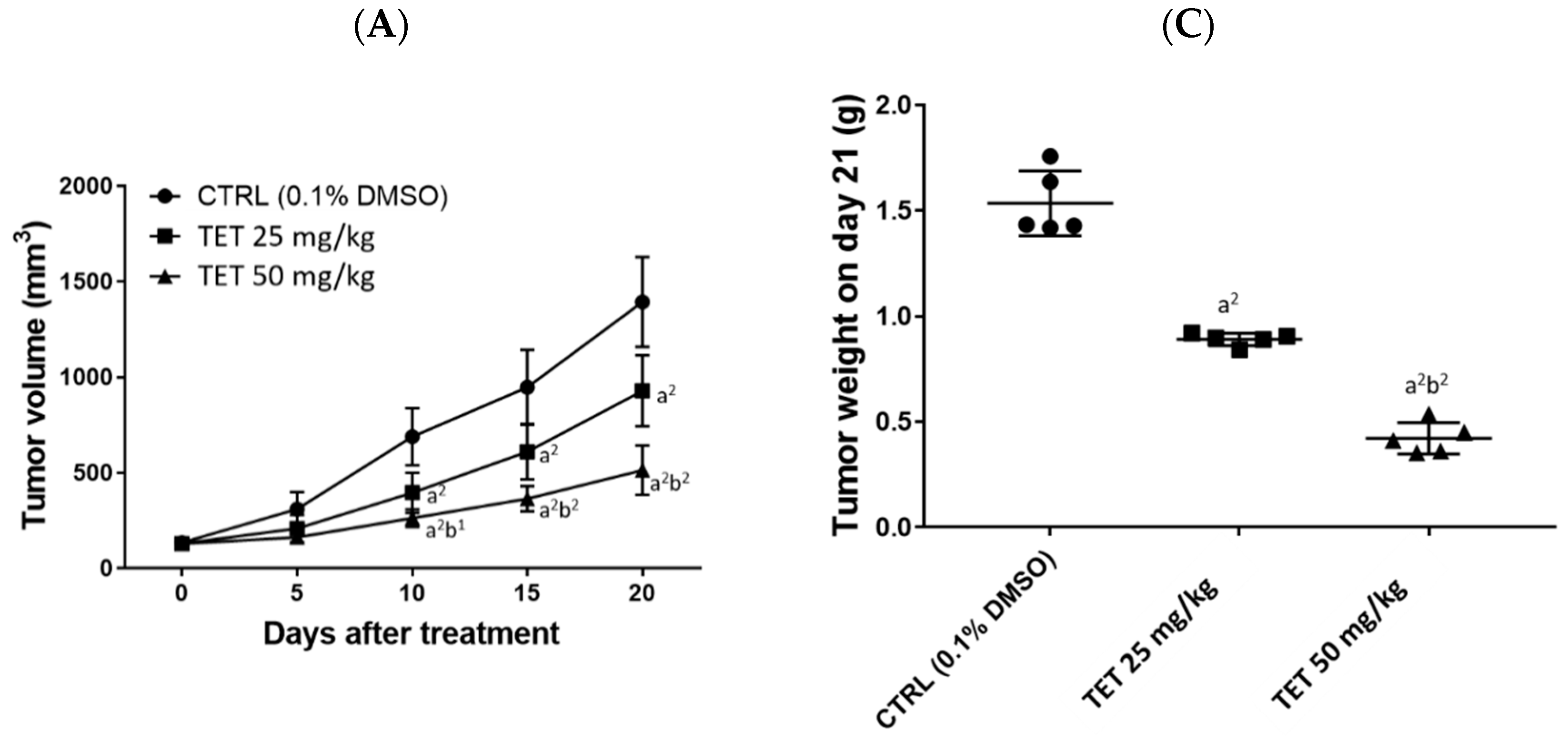
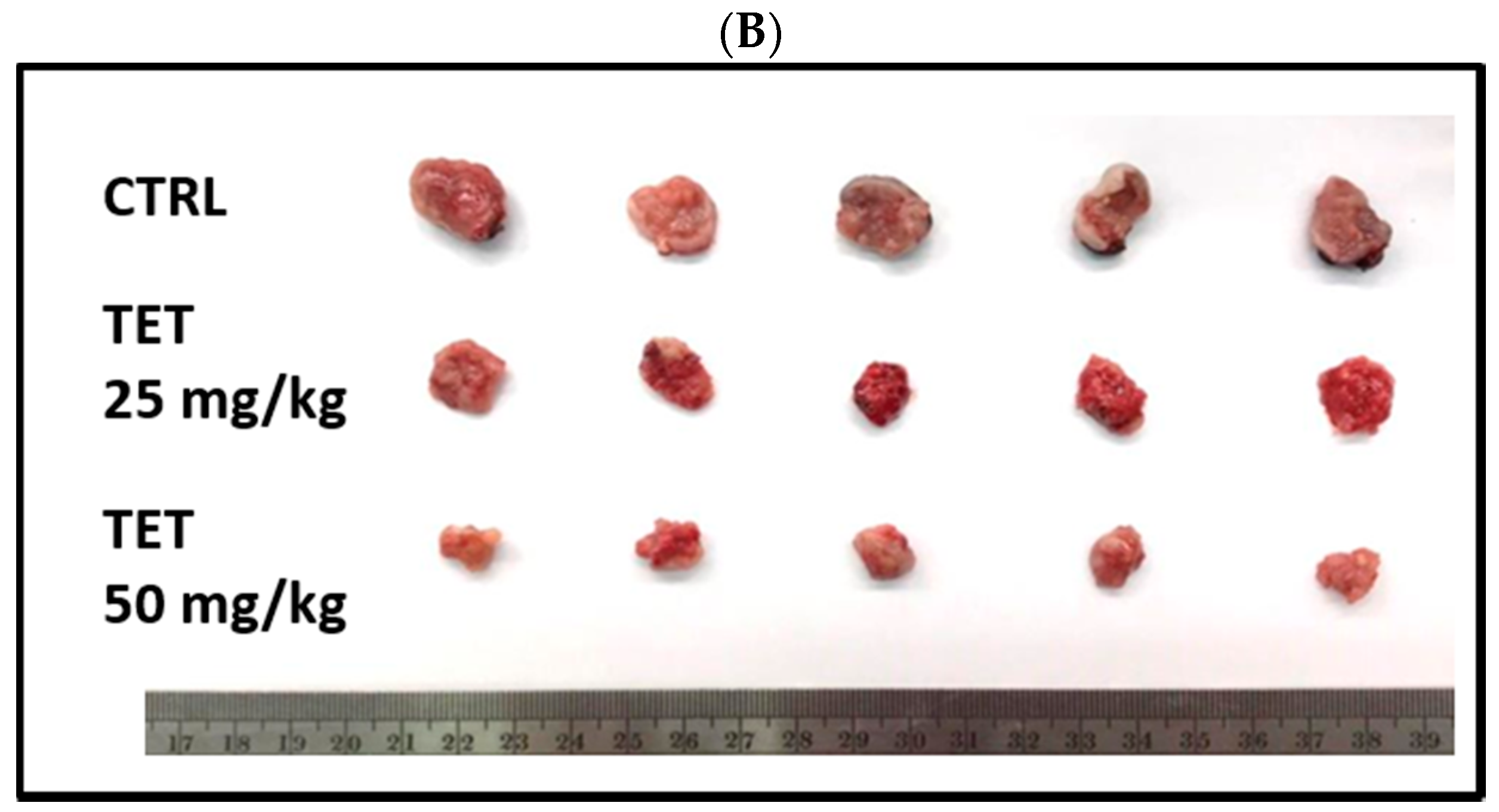
2.2. TET Affected Body Weights and Glioblastoma Tumor Growth
2.3. TET Significantly Reduced the Signal Intensity of Luc2 from the Subcutaneous Glioblastoma-Bearing Mice
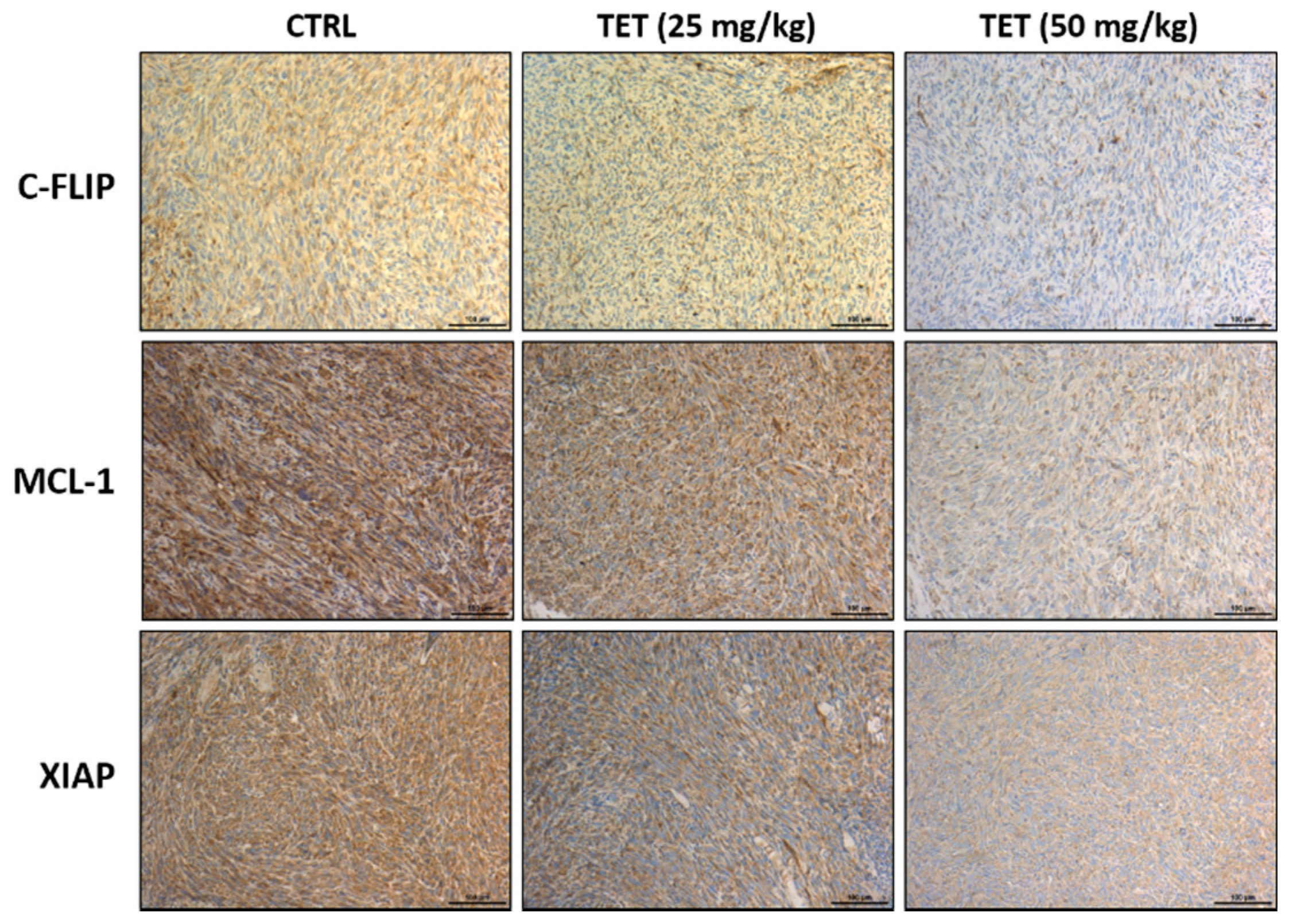
2.4. TET Affected Anti-Apoptosis and Pro-Apoptosis Factors in Glioblastoma-Bearing Mice
2.5. TET Treatment Affected Acute or Decreased Toxicity of Glioblastoma-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture of Human Glioblastoma GBM8401 Cells
4.3. Cell Transfection and Stable Clone Selection in GBM 8401 Cells
4.4. Cell Viability Assay
4.5. Cell Apoptosis Assay
4.6. Xenograft GBM 8401-Cell-Bearing Animal Model
4.7. Treatment and Physical Tumor Growth Validation of the Animal Model
4.8. In Vivo Bioluminescent Imaging (BLI) of Tumors
4.9. Pathology of Kidney, Liver, and Spleen
4.10. Immunohistochemistry Staining
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; Germaine-Smith, C.S.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro-Oncol. 2015, 17, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.; Idema, A.J.; Wesseling, P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007, 114, 443–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar]
- Bhagya, N.; Chandrashekar, K.R. Tetrandrine-A molecule of wide bioactivity. Phytochemistry 2016, 125, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, T.; Qian, X.; Liu, G.; Yu, L.; Ding, Y. Anticancer effect of tetrandrine on primary cancer cells isolated from ascites and pleural fluids. Cancer Lett. 2008, 268, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, X.; Li, W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016, 7, 40800–40815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.-T.; Chiang, L.-C.; Lin, Y.-T.; Lin, C.-C. Antiproliferative and Apoptotic Effects of Tetrandrine on Different Human Hepatoma Cell Lines. Am. J. Chin. Med. 2006, 34, 125–135. [Google Scholar] [CrossRef]
- Qiu, W.; Su, M.; Xie, F.; Ai, J.; Ren, Y.; Zhang, J.; Guan, R.; He, W.; Gong, Y.; Guo, Y. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 2014, 5, e1123. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Liu, T.; Mei, L.; Li, J.; Gong, K.; Yu, C.; Li, W. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br. J. Cancer 2013, 109, 342–350. [Google Scholar] [CrossRef]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, Z.; Cui, N.; Yang, B.; Niu, W.; Kuang, H. Tetrandrine inhibits colon carcinoma HT-29 cells growth via the Bcl-2/Caspase 3/PARP pathway and G1/S phase. Biosci. Rep. 2019, 39, BSR20182109. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Shen, H.; Cao, Y.; Fang, Y.; Li, H.; Chen, Q.; Xu, W. Tetrandrine Induces Mitochondria-Mediated Apoptosis in Human Gastric Cancer BGC-823 Cells. PLoS ONE 2013, 8, e76486. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Pei, X. Tetrandrine-Induced Autophagy in MDA-MB-231 Triple-Negative Breast Cancer Cell through the Inhibition of PI3K/AKT/mTOR Signaling. Evid.-Based Complementary Altern. Med. 2019, 2019, 7517431. [Google Scholar] [CrossRef] [Green Version]
- Juan, T.; Liu, K.; Kuo, C.; Yang, M.; Chu, Y.; Yang, J.; Wu, P.; Huang, Y.; Lai, K.; Chung, J. Tetrandrine suppresses adhesion, migration and invasion of human colon cancer SW620 cells via inhibition of nuclear factor-κB, matrix metalloproteinase-2 and matrix metalloproteinase-9 signaling pathways. Oncol. Lett. 2018, 15, 7716–7724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.-C.; Tseng, S.-H. Tetrandrine suppresses tumor growth and angiogenesis of gliomas in rats. Int. J. Cancer 2009, 124, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.; Zhen, Y.; Cui, J.; Hu, G.; Lin, Y. Antitumor activity of tetrandrine citrate in human glioma U87 cells in vitro and in vivo. Oncol. Rep. 2019, 42, 2345–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagya, N.; Chandrashekar, K.R. Tetrandrine and cancer—An overview on the molecular approach. Biomed. Pharmacother. 2018, 97, 624–632. [Google Scholar]
- Chen, B.; Sun, Q.; Wang, X.; Gao, F.; Dai, Y.; Yin, Y.; Ding, J.; Gao, C.; Cheng, J.; Li, J.; et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int. J. Nanomed. 2008, 3, 277–286. [Google Scholar]
- Pang, Z.; Feng, L.; Hua, R.; Chen, J.; Gao, H.; Pan, S.; Jiang, X.; Zhang, P. Lactoferrin-Conjugated Biodegradable Polymersome Holding Doxorubicin and Tetrandrine for Chemotherapy of Glioma Rats. Mol. Pharm. 2010, 7, 1995–2005. [Google Scholar] [CrossRef]
- Li, X.-T.; Tang, W.; Jiang, Y.; Wang, X.-M.; Wang, Y.-H.; Cheng, L.; Meng, X.-S. Multifunctional targeting vinorelbine plus tetrandrine liposomes for treating brain glioma along with eliminating glioma stem cells. Oncotarget 2016, 7, 24604–24622. [Google Scholar] [CrossRef] [Green Version]
- Lien, J.-C.; Lin, M.-W.; Chang, S.-J.; Lai, K.-C.; Huang, A.-C.; Yu, F.-S.; Chung, J.-G. Tetrandrine induces programmed cell death in human oral cancer CAL 27 cells through the reactive oxygen species production and caspase-dependent pathways and associated with beclin-1-induced cell autophagy. Environ. Toxicol. 2017, 32, 329–343. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Peng, S.-F.; Lin, M.-L.; Kuo, C.-L.; Lu, K.-W.; Liao, C.-L.; Ma, Y.-S.; Chueh, F.-S.; Liu, K.-C.; Yu, F.-S.; et al. Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway. Molecules 2016, 21, 1353. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.C.; Lin, Y.J.; Hsiao, Y.T.; Lin, M.L.; Yang, J.L.; Huang, Y.P.; Chu, Y.L.; Chung, J.G. Tetrandrine induces apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells by endoplasmic reticulum stress and Ca2+/calpain pathways. Anticancer Res. 2017, 37, 6107–6118. [Google Scholar]
- Yu, F.S.; Yu, C.S.; Chen, J.C.; Yang, J.L.; Lu, H.F.; Chang, S.J.; Lin, M.W.; Chung, J.G. Tetrandrine induces apoptosis via caspase-8, -9, and -3 and poly (ADP ribose) polymerase dependent pathways and autophagy through beclin-1/ LC3-I, II signaling pathways in human oral cancer HSC-3 cells. Environ. Toxicol. 2016, 31, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jia, X.; Zhang, Y.; Dong, Y.; Lei, Y.; Tan, X.; Williamson, R.A.; Wang, A.; Zhang, D.; Ma, J. Tetrandrine induces apoptosis in human neuroblastoma through regulating the Hippo/YAP signaling pathway. Biochem. Biophys. Res. Commun. 2019, 513, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, H.; Kuo, C.; Way, T.; Lien, J.; Chueh, F.; Lin, Y.; Chung, J. Tetrandrine inhibits human brain glioblastoma multiforme GBM 8401 cancer cell migration and invasion in vitro. Environ. Toxicol. 2019, 34, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Lin, J.-J.; Lin, C.-C.; Lien, J.-C.; Peng, S.-F.; Fan, M.-J.; Hsu, F.-T.; Chung, J.-G. Benzyl isothiocyanate inhibits human brain glioblastoma multiforme GBM 8401 cell xenograft tumor in nude mice in vivo. Environ. Toxicol. 2018, 33, 1097–1104. [Google Scholar] [CrossRef]
- Pore, S.K.; Hahm, E.-R.; Latoche, J.D.; Anderson, C.J.; Shuai, Y.; Singh, S.V. Prevention of breast cancer-induced osteolytic bone resorption by benzyl isothiocyanate. Carcinogenesis 2018, 39, 134–145. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef]
- Florentin, A.; Arama, E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J. Cell Biol. 2012, 196, 513–527. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.W.; O’Neill, A.; Brannigan, A.E.; Coffey, R.; Marshall, J.C.; Brady, H.R.; Fitzpatrick, J.M. Regulation of Fas antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett. 1999, 453, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Tseng, B. Why target apoptosis in cancer treatment? Mol. Cancer Ther. 2003, 2, 573–580. [Google Scholar] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safa, A. c-FLIP, a master anti-apoptotic regulator. Exp. Oncol. 2012, 34, 176–184. [Google Scholar] [PubMed]
- Chen, L.; Fletcher, S. Mcl-1 inhibitors: A patent review. Expert Opin. Ther. Pat. 2016, 27, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Desplanques, G.; Giuliani, N.; Delsignore, R.; Rizzoli, V.; Bataille, R.; Barillé-Nion, S. Impact of XIAP protein levels on the survival of myeloma cells. Haematologica 2009, 94, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ren, H.; Yue, P.; Chen, M.; Khuri, F.R.; Sun, S.Y. The novel Akt inhibitor API-1 induces c-FLIP degradation and synergizes with TRAIL to augment apoptosis independent of Akt inhibition. Cancer Prev. Res. 2012, 5, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xia, L.; Gabrilove, J.; Waxman, S.; Jing, Y. Downregulation of Mcl-1 through GSK-3β activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia 2013, 27, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.-Z.; Chapman, J.; Reynolds, S.H. Proteasome inhibitors induce apoptosis in human lung cancer cells through a positive feedback mechanism and the subsequent Mcl-1 protein cleavage. Oncogene 2009, 28, 3775–3786. [Google Scholar] [CrossRef] [Green Version]
- Macho, A.; Hirsch, T.; Marzo, I.; Marchetti, P.; Dallaporta, B.; Susin, S.A.; Zamzami, N.; Kroemer, G. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J. Immunol. 1997, 158, 4612–4619. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Vellanki, S.H.; Grabrucker, A.; Liebau, S.; Proepper, C.; Eramo, A.; Braun, V.; Boeckers, T.; Debatin, K.M.; Fulda, S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia 2009, 11, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Peter, G.; Ursula, M.; Erich, G. Chemosensitivity of human malignant glioma: Modulation by p53 gene transfer. J. Neurooncol. 1998, 39, 19–32. [Google Scholar]
- Chu, C.-W.; Ko, H.-J.; Chou, C.-H.; Cheng, T.-S.; Cheng, H.-W.; Liang, Y.-H.; Lai, Y.-L.; Lin, C.-Y.; Wang, C.; Loh, J.-K.; et al. Thioridazine Enhances P62-Mediated Autophagy and Apoptosis Through Wnt/β-Catenin Signaling Pathway in Glioma Cells. Int. J. Mol. Sci. 2019, 20, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.S.; Shih, Y.L.; Lu, T.J.; Lee, C.H.; Hsueh, S.C.; Chou, Y.C.; Lu, H.F.; Liao, N.C.; Chung, J.G. Benzyl isothiocyanate (BITC) induces apoptosis of GBM 8401 human brain glioblastoma multiforms cells via activation of caspase-8/Bid and the reactive oxygen species-dependent mitochondrial pathway. Environ. Toxicol. 2016, 31, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.-C.; Li, M.-H.; Chung, J.G.; Liu, Y.-C.; Wu, J.-Y.; Hsu, F.-T.; Wang, H.-E. Apoptosis induction and AKT/NF-κB inactivation are associated with regroafenib-inhibited tumor progression in non-small cell lung cancer in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109032. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-C.; Yang, H.-H.; Chiang, S.-C.; Chou, Y.-X.; Yang, H.-T. Auricularia polytricha aqueous extract supplementation decreases hepatic lipid accumulation and improves antioxidative status in animal model of nonalcoholic fatty liver. BioMedicine 2014, 4, 12. [Google Scholar] [CrossRef]
- Weng, M.-C.; Wang, M.-H.; Tsai, J.-J.; Kuo, Y.-C.; Liu, Y.-C.; Hsu, F.-T.; Wang, H.-E. Regorafenib inhibits tumor progression through suppression of ERK/NF-κB activation in hepatocellular carcinoma bearing mice. Biosci. Rep. 2018, 38, 3. [Google Scholar] [CrossRef] [Green Version]





| Compares Group | TET 25 mg/kg | TET 50 mg/kg |
|---|---|---|
| Day 5 | ||
| Vs. Control | 0.120 | 0.014 |
| Vs. TET 25 mg/kg | -- | 0.640 |
| Day 10 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | 0.030 |
| Day 15 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | <0.0001 |
| Day 20 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | <0.0001 |
| Compares Group | TET 25 mg/kg | TET 50 mg/kg |
|---|---|---|
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | <0.0001 |
| Compares Group | TET 25 mg/kg | TET 50 mg/kg |
|---|---|---|
| Day 7 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | --. | 0.05 |
| Day 14 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | <0.0001 |
| Day 21 | ||
| Vs. Control | <0.0001 | <0.0001 |
| Vs. TET 25 mg/kg | -- | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-L.; Ma, Y.-S.; Hsia, T.-C.; Chou, Y.-C.; Lien, J.-C.; Peng, S.-F.; Kuo, C.-L.; Hsu, F.-T. Tetrandrine Suppresses Human Brain Glioblastoma GBM 8401/luc2 Cell-Xenografted Subcutaneous Tumors in Nude Mice In Vivo. Molecules 2021, 26, 7105. https://doi.org/10.3390/molecules26237105
Liao C-L, Ma Y-S, Hsia T-C, Chou Y-C, Lien J-C, Peng S-F, Kuo C-L, Hsu F-T. Tetrandrine Suppresses Human Brain Glioblastoma GBM 8401/luc2 Cell-Xenografted Subcutaneous Tumors in Nude Mice In Vivo. Molecules. 2021; 26(23):7105. https://doi.org/10.3390/molecules26237105
Chicago/Turabian StyleLiao, Ching-Lung, Yi-Shih Ma, Te-Chun Hsia, Yu-Cheng Chou, Jin-Cherng Lien, Shu-Fen Peng, Chao-Lin Kuo, and Fei-Ting Hsu. 2021. "Tetrandrine Suppresses Human Brain Glioblastoma GBM 8401/luc2 Cell-Xenografted Subcutaneous Tumors in Nude Mice In Vivo" Molecules 26, no. 23: 7105. https://doi.org/10.3390/molecules26237105
APA StyleLiao, C.-L., Ma, Y.-S., Hsia, T.-C., Chou, Y.-C., Lien, J.-C., Peng, S.-F., Kuo, C.-L., & Hsu, F.-T. (2021). Tetrandrine Suppresses Human Brain Glioblastoma GBM 8401/luc2 Cell-Xenografted Subcutaneous Tumors in Nude Mice In Vivo. Molecules, 26(23), 7105. https://doi.org/10.3390/molecules26237105







