The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
Metal-Based Copper Nanoparticles
2.2. Experimental Protocol
2.3. Experimental Procedures
2.4. Blood Analysis
2.5. Vascular Reactivity Studies
2.6. Data Analysis and Statistics
3. Results
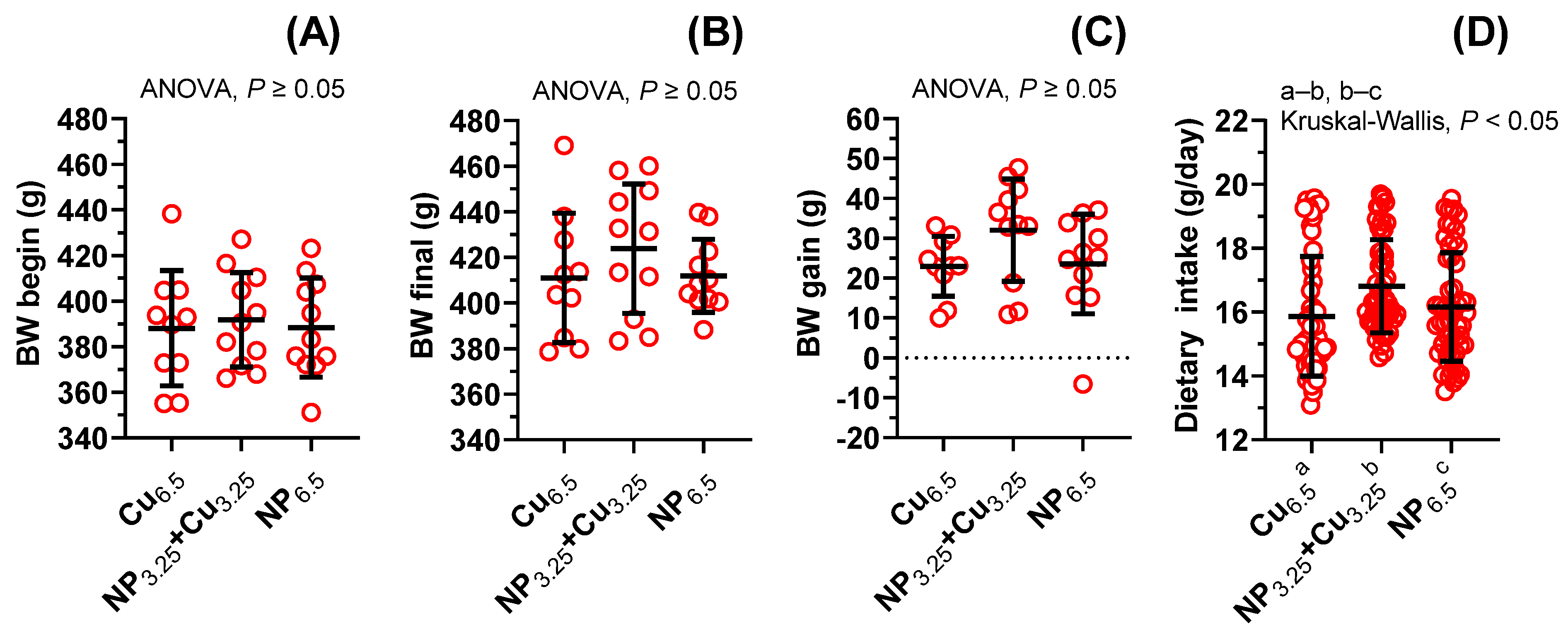
3.1. The General Characterization of WKY Rats
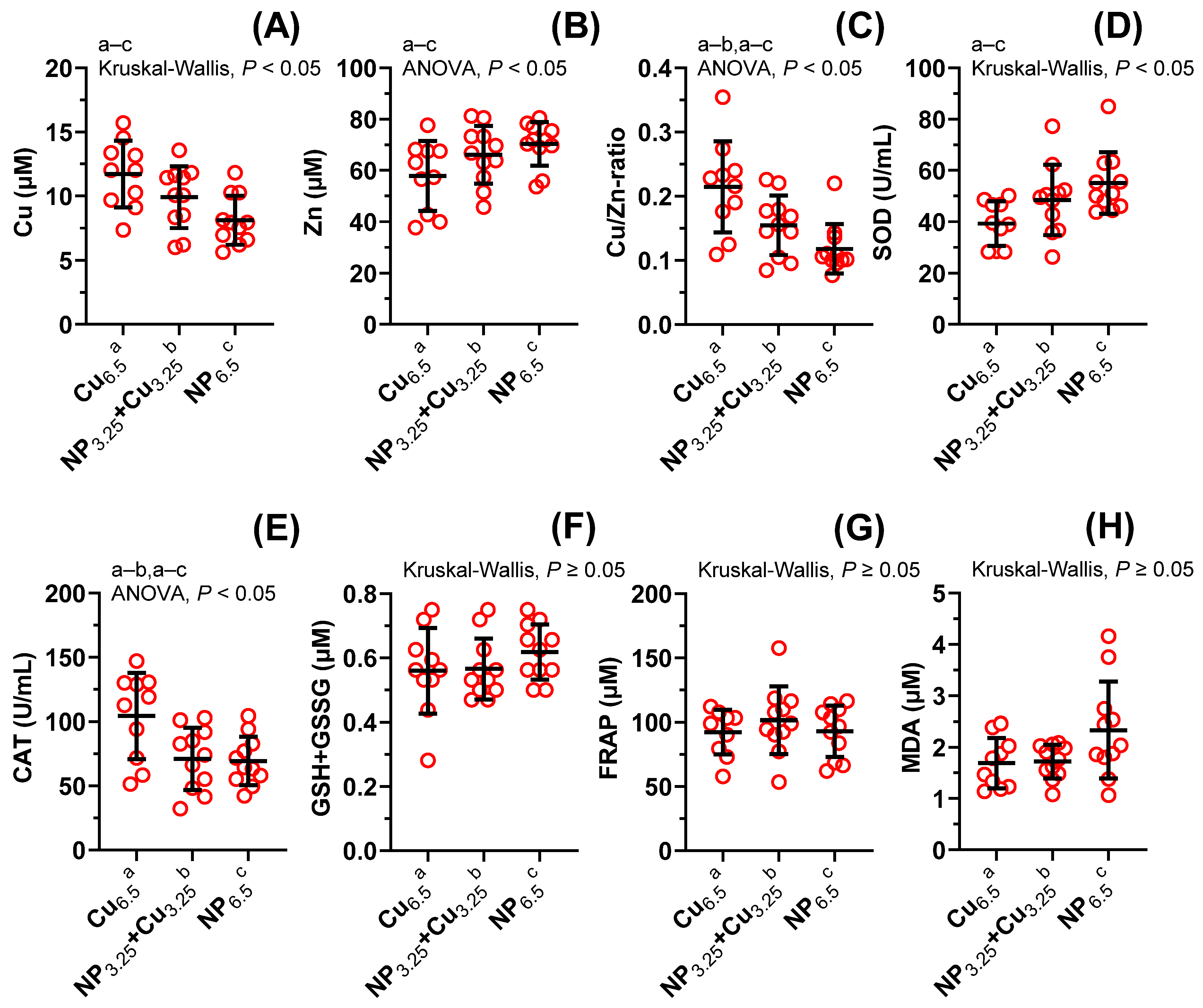
3.2. Biomarkers of Oxidative Stress in the Blood Plasma
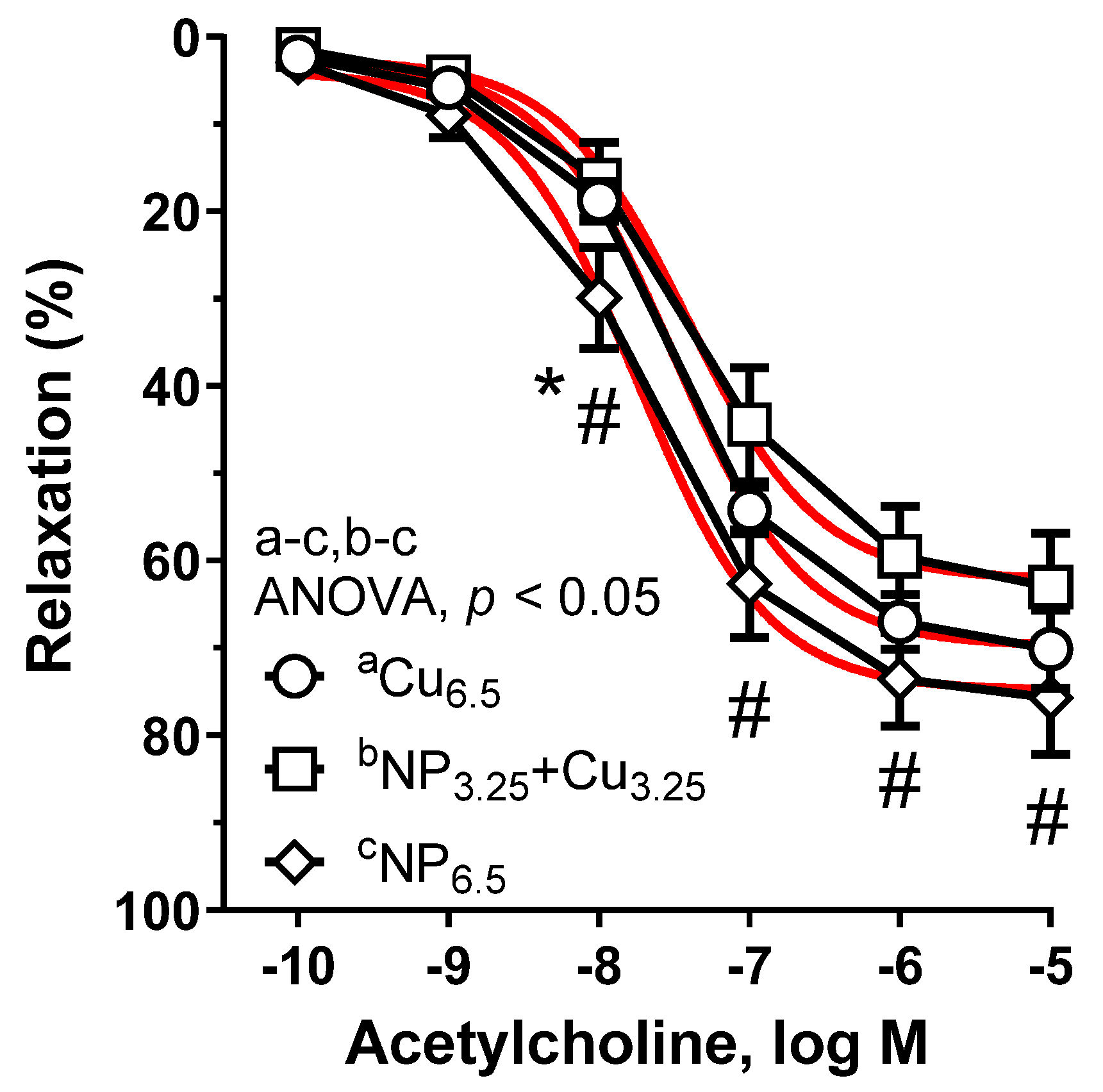
3.3. Vascular Reactivity Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majewski, M.; Lis, B.; Olas, B.; Ognik, K.; Juśkiewicz, J. Dietary supplementation with copper nanoparticles influences the markers of oxidative stress and modulates vasodilation of thoracic arteries in young Wistar rats. PLoS ONE 2020, 15, e0229282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. The interaction between resveratrol and two forms of copper as carbonate and nanoparticles on antioxidant mechanisms and vascular function in Wistar rats. Pharmacol. Rep. 2019, 71, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef] [Green Version]
- Galhardi, C.M.; Diniz, Y.S.; Faine, L.A.; Rodrigues, H.G.; Burneiko, R.C.M.; Ribas, B.O.; Novelli, E.L. Toxicity of copper intake: Lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem. Toxicol. 2004, 42, 2053–2060. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. The toxic effects of monosodium glutamate (MSG)—The involvement of nitric oxide, prostanoids and potassium channels in the reactivity of thoracic arteries in MSG-obese rats. Toxicol. Appl. Pharmacol. 2018, 359, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–20. [Google Scholar] [PubMed]
- Majewski, M.; Ognik, K.; Thoene, M.; Rawicka, A.; Juśkiewicz, J. Resveratrol modulates the blood plasma levels of Cu and Zn, the antioxidant status and the vascular response of thoracic arteries in copper deficient Wistar rats. Toxicol. Appl. Pharmacol. 2020, 390, 114877. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kasica, N.; Jakimiuk, A.; Podlasz, P. Toxicity and cardiac effects of acute exposure to tryptophan metabolites on the kynurenine pathway in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharmacol. 2018, 341, 16–29. [Google Scholar] [CrossRef]
- Luo, J.; Hao, S.; Zhao, L.; Shi, F.; Ye, G.; He, C.; Lin, J.; Zhang, W.; Liang, H.; Wang, X.; et al. Oral exposure of pregnant rats to copper nanoparticles caused nutritional imbalance and liver dysfunction in fetus. Ecotoxicol. Environ. Saf. 2020, 206, 111206. [Google Scholar] [CrossRef]
- Jankowski, J.; Ognik, K.; Kozłowski, K.; Stępniowska, A.; Zduńczyk, Z. Effect of different levels and sources of dietary copper, zinc and manganese on the performance and immune and redox status of Turkeys. Animals 2019, 9, 883. [Google Scholar] [CrossRef] [Green Version]
- Ognik, K.; Cholewińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Tutaj, K.; Szlązak, R. The effect of copper nanoparticles and copper (II) salt on redox reactions and epigenetic changes in a rat model. J. Anim. Physiol. Anim. Nutr. Berl. 2019, 103, 675–686. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Jedrejek, D.; Stochmal, A.; Olas, B. The composition and vascular/antioxidant properties of Taraxacum officinale flower water syrup in a normal-fat diet using an obese rat model. J. Ethnopharmacol. 2021, 265, 113393. [Google Scholar] [CrossRef] [PubMed]
- Żary-Sikorska, E.; Fotschki, B.; Jurgoński, A.; Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Majewski, M.; Ognik, K.; Juśkiewicz, J. Protective effects of a strawberry ellagitannin-rich extract against pro-oxidative and pro-inflammatory dysfunctions induced by a high-fat diet in a rat model. Molecules 2020, 25, 5874. [Google Scholar] [CrossRef]
- Majewski, M.; Kucharczyk, E.; Kaliszan, R.; Markuszewski, M.; Fotschki, B.; Juskiewicz, J.; Borkowska-Sztachańska, M.; Ognik, K. The characterization of ground raspberry seeds and the physiological response to supplementation in hypertensive and normotensive rats. Nutrients 2020, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lepczyńska, M.; Dzika, E.; Grzegorzewski, W.; Markiewicz, W.; Mendel, M.; Chłopecka, M. Evaluation of the time stability of aortic rings in young wistar rats during an eight-hour incubation period. J. Elementol. 2019, 24, 677–686. [Google Scholar] [CrossRef]
- Majewski, M.; Ognik, K.; Zdunczyk, P.; Juskiewicz, J. Effect of dietary copper nanoparticles versus one copper (II) salt: Analysis of vasoreactivity in a rat model. Pharmacol. Rep. 2017, 69, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles enhance vascular contraction induced by prostaglandin F2-alpha and decrease the blood plasma cu-zn ratio in wistar rats. J. Elementol. 2019, 24, 911–922. [Google Scholar] [CrossRef]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. The antioxidant status, lipid profile, and modulation of vascular function by fish oil supplementation in nano-copper and copper carbonate fed Wistar rats. J. Funct. Foods 2020, 64, 103595. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewińska, E.; Tutaj, K.; Cendrowska-Pinkosz, M.; Dworzański, W.; Dworzańska, A.; Juśkiewicz, J. The effect of the source and dosage of dietary Cu on redox status in rat tissues. J. Anim. Physiol. Anim. Nutr. Berl. 2020, 104, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Cendrowska-Pinkosz, M.; Krauze, M.; Juśkiewicz, J.; Ognik, K. The effect of the use of copper carbonate and copper nanoparticles in the diet of rats on the level of β-amyloid and acetylcholinesterase in selected organs. J. Trace Elem. Med. Biol. 2021, 67, 126777. [Google Scholar] [CrossRef] [PubMed]
- Logvinov, S.V.; Naryzhnaya, N.V.; Kurbatov, B.K.; Gorbunov, A.S.; Birulina, Y.G.; Maslov, L.L.; Oeltgen, P.R. High carbohydrate high fat diet causes arterial hypertension and histological changes in the aortic wall in aged rats: The involvement of connective tissue growth factors and fibronectin. Exp. Gerontol. 2021, 154, 111543. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; DelFavero, J.; Ungvari, A.; Papp, M.; Tarantini, A.; Price, N.; de Cabo, R.; Tarantini, S. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res. Rev. 2020, 64, 101189. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, G.S.; Scarmeas, N. Dietary interventions in mild cognitive impairment and dementia. Dialogues Clin. Neurosci. 2019, 21, 69–82. [Google Scholar] [CrossRef] [PubMed]
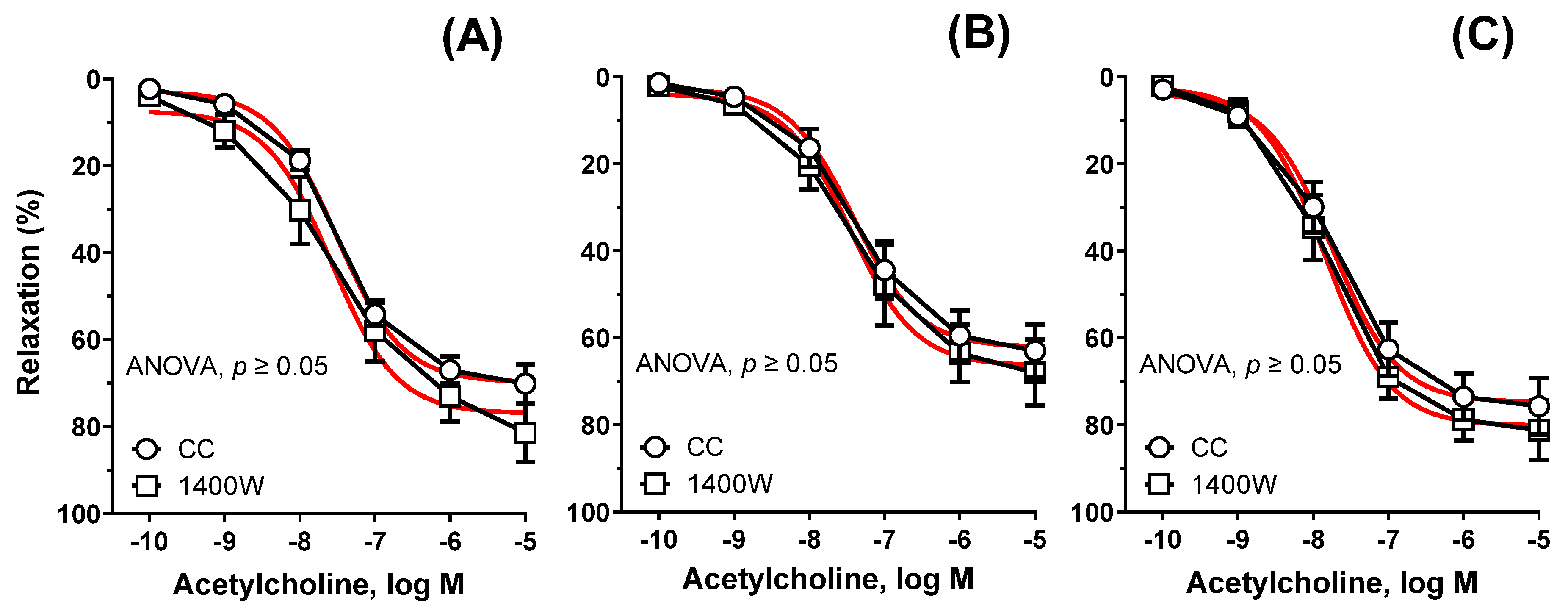
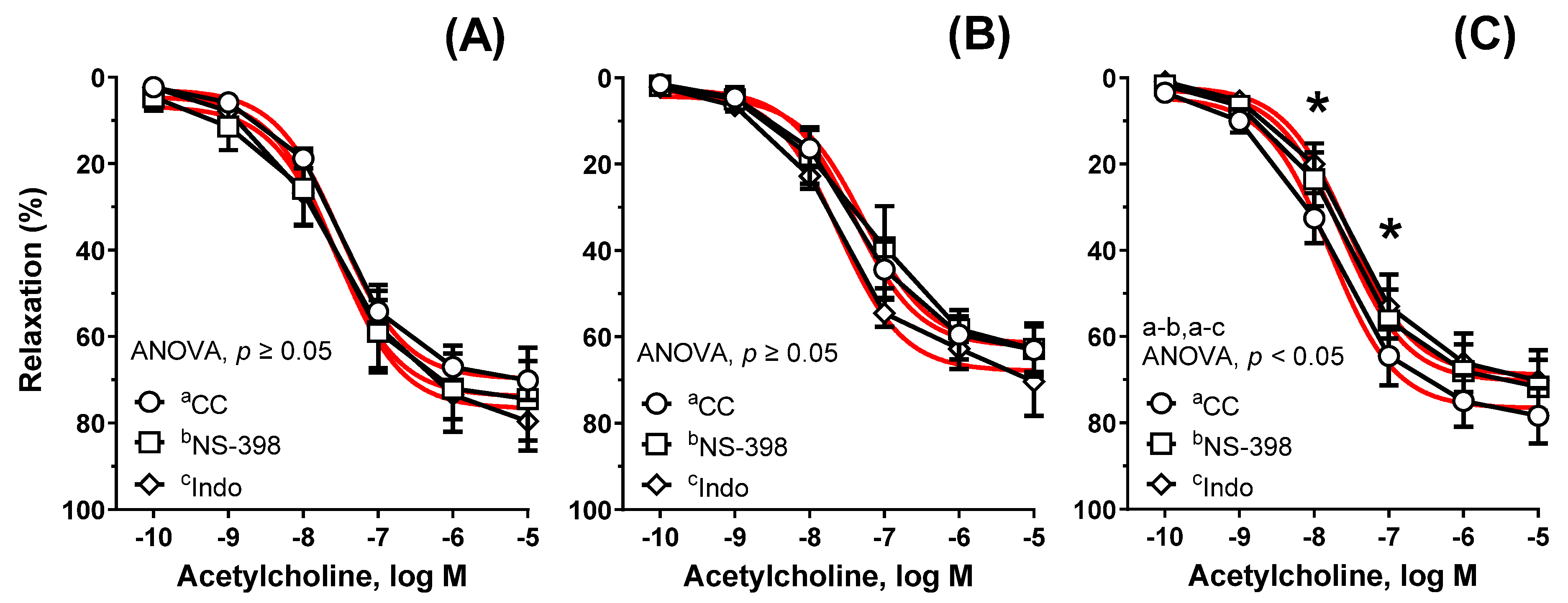
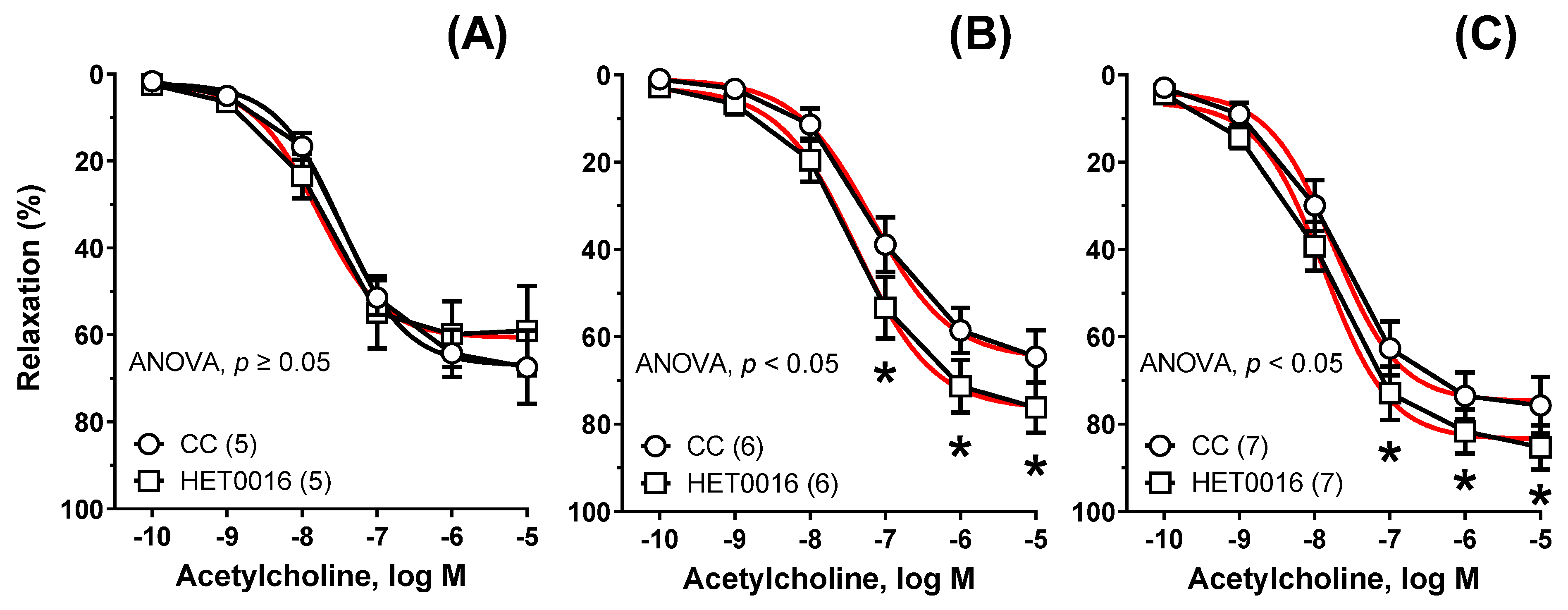
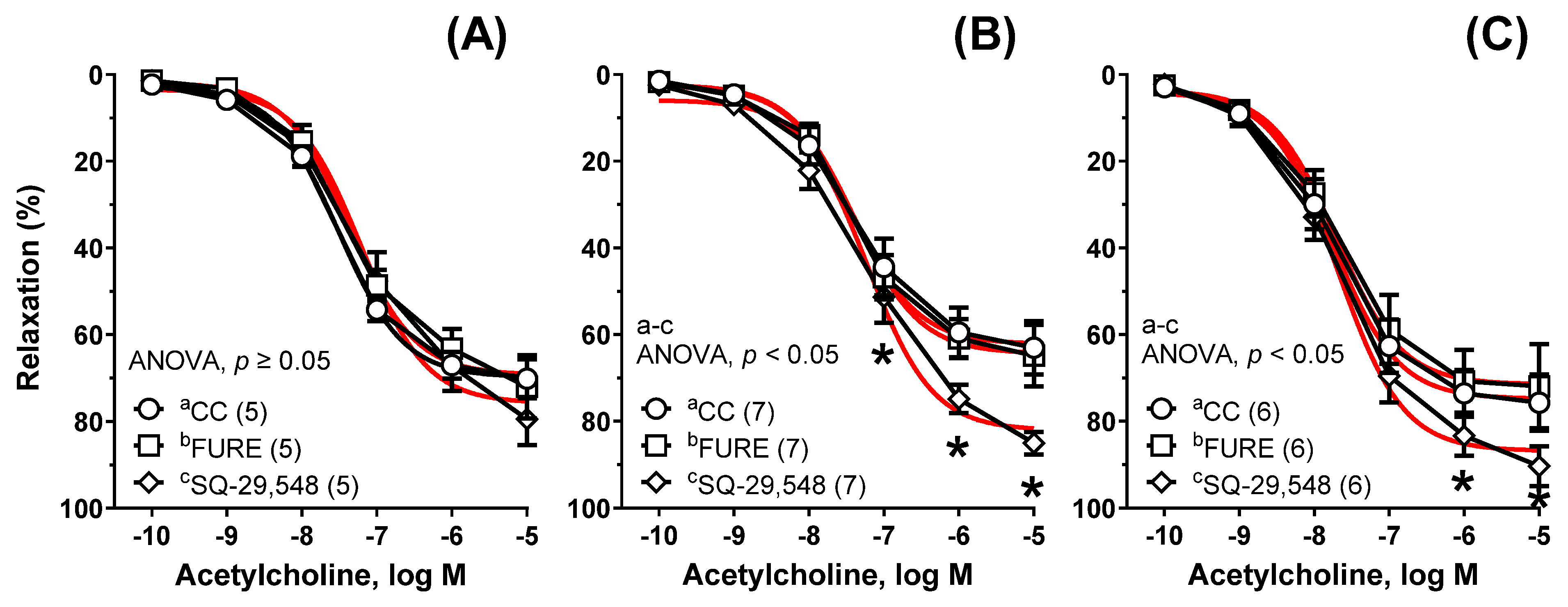
| Group | Cu6.5 | NP3.25 + Cu3.25 | NP6.5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Emax (%) | pEC50 | AUC | n | Emax (%) | pEC50 | AUC | n | Emax (%) | pEC50 | AUC | |
| Control conditions | 11 | 69.76 | 7.509 | 182.0 | 11 | 62.19 | 7.413 | 157.0 | 11 | 76.80 $ | 7.748 | 214.3 $ |
| ±SEM | 1.899 | 0.087 | 12.73 | 3.460 | 0.172 | 25.01 | 3.294 | 0.147 | 25.10 | |||
| +1400 W | 5 | 74.95 | 7.581 | 205.9 | 5 | 66.40 | 7.454 | 173.2 | 5 | 80.15 $ | 7.839 | 232.1 $ |
| ±SEM | 4.092 | 0.183 | 21.09 | 4.326 | 0.209 | 25.66 | 3.212 | 0.131 | 18.19 | |||
| +HET0016 | 5 | 60.64 | 7.795 | 175.1 | 6 | 76.50 *# | 7.386 | 190.3 * | 7 | 88.44 *#$ | 7.874 $ | 252.9 *#$ |
| ±SEM | 4.180 | 0.227 | 22.66 | 4.714 | 0.165 | 19.81 | 2.919 | 0.120 | 20.99 | |||
| +SQ-29,548 | 5 | 75.77 | 7.210 | 176.2 | 7 | 82.08 * | 7.222 | 199.0 * | 6 | 86.82 *# | 7.683 #$ | 241.4 *# |
| ±SEM | 3.725 | 0.139 | 17.27 | 2.855 | 0.102 | 15.66 | 2.929 | 0.112 | 17.28 | |||
| +FURE | 5 | 69.25 | 7.360 | 167.1 | 7 | 64.34 | 7.387 | 159.7 | 6 | 71.47 | 7.711 | 202.2 |
| ±SEM | 2.888 | 0.124 | 12.77 | 3.030 | 0.143 | 16.53 | 4.186 | 0.195 | 27.60 | |||
| +NS-398 | 5 | 73.82 | 7.582 | 207.5 | 5 | 61.61 # | 7.277 | 152.7 # | 5 | 70.36 | 7.624 $ | 190.9 * |
| ±SEM | 5.746 | 0.267 | 29.95 | 4.113 | 0.200 | 20.22 | 3.652 | 0.170 | 24.58 | |||
| +INDO | 5 | 76.61 | 7.564 | 206.6 | 5 | 67.88 | 7.618 | 182.8 | 5 | 68.88 | 7.540 | 179.8 * |
| ±SEM | 4.008 | 0.173 | 21.78 | 3.088 | 0.158 | 13.54 | 3.772 | 0.174 | 22.93 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, M.; Juśkiewicz, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Socha, K.; Cholewińska, E.; Ognik, K. The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats. Nutrients 2021, 13, 3793. https://doi.org/10.3390/nu13113793
Majewski M, Juśkiewicz J, Krajewska-Włodarczyk M, Gromadziński L, Socha K, Cholewińska E, Ognik K. The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats. Nutrients. 2021; 13(11):3793. https://doi.org/10.3390/nu13113793
Chicago/Turabian StyleMajewski, Michał, Jerzy Juśkiewicz, Magdalena Krajewska-Włodarczyk, Leszek Gromadziński, Katarzyna Socha, Ewelina Cholewińska, and Katarzyna Ognik. 2021. "The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats" Nutrients 13, no. 11: 3793. https://doi.org/10.3390/nu13113793
APA StyleMajewski, M., Juśkiewicz, J., Krajewska-Włodarczyk, M., Gromadziński, L., Socha, K., Cholewińska, E., & Ognik, K. (2021). The Role of 20-HETE, COX, Thromboxane Receptors, and Blood Plasma Antioxidant Status in Vascular Relaxation of Copper-Nanoparticle-Fed WKY Rats. Nutrients, 13(11), 3793. https://doi.org/10.3390/nu13113793









