Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Bone Broth
2.2. Determination of the Nutritional Content of the Bone Broth
2.2.1. Protein
2.2.2. Amino Acids
2.2.3. Minerals
Determination of Hazard Ratio
2.3. Animals
2.4. Experimental Design
2.5. Sample Collection and Processing
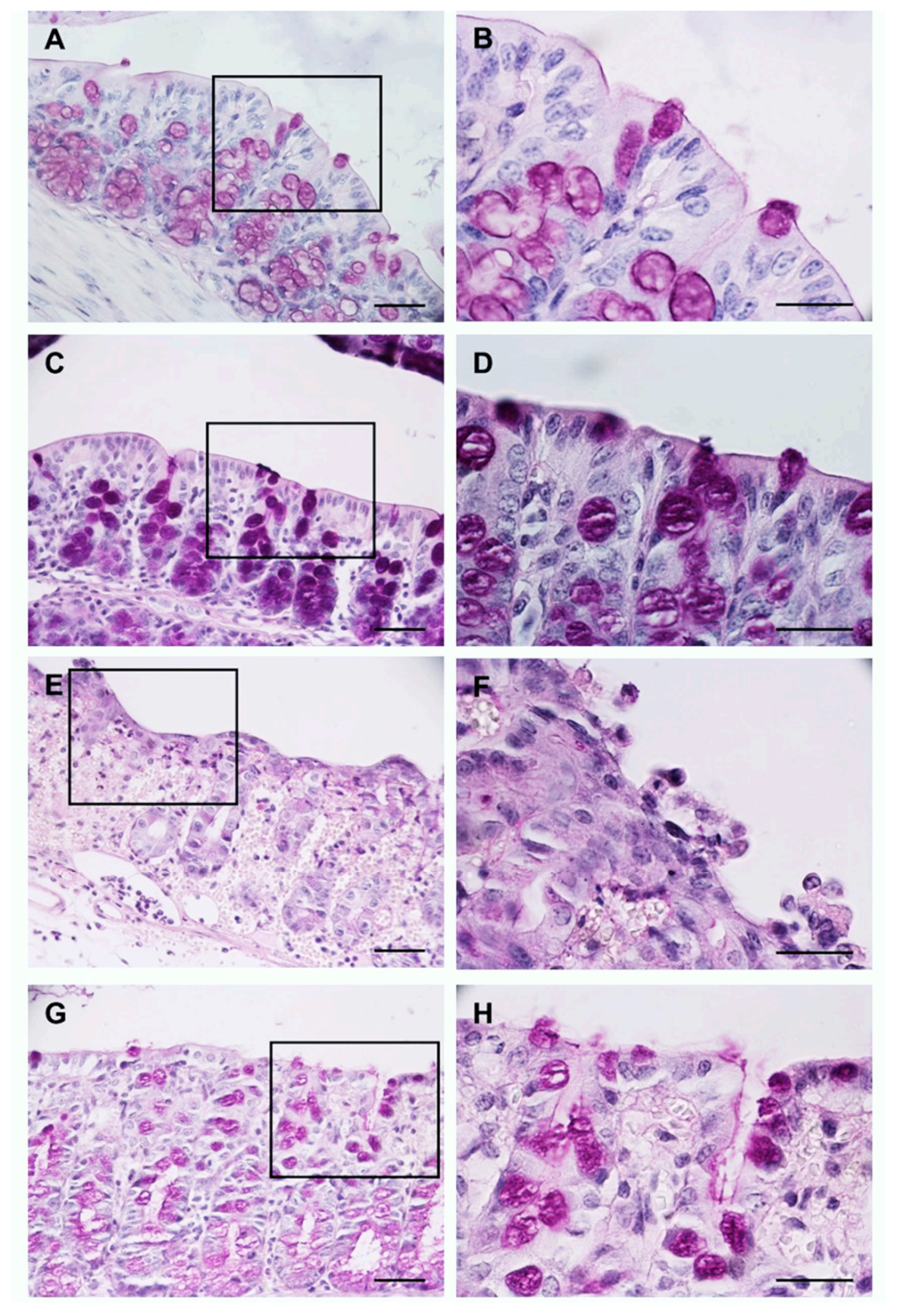
2.6. Histological Analysis
2.7. Cytokine Quantification by qPCR
2.8. Statistic Analysis
3. Results
3.1. Nutritional Content of the Bone Broth
3.1.1. Nutritional Contribution of Amino Acids from Bone Broth
3.1.2. Nutritional Contribution of Minerals from Bone Broth
3.2. The Bone Broth Prevents Histological Damage in the Murine Model of Ulcerative Colitis
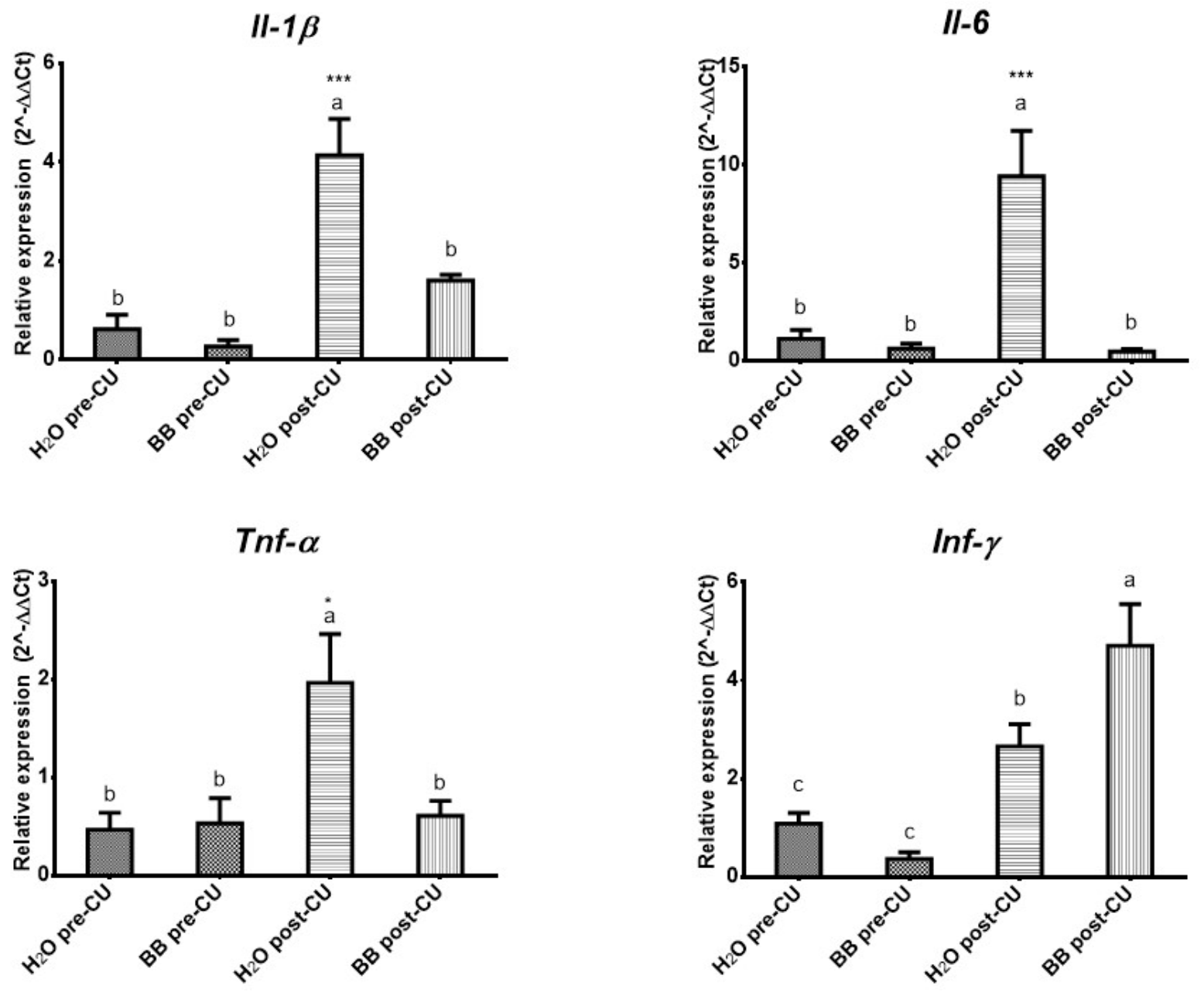
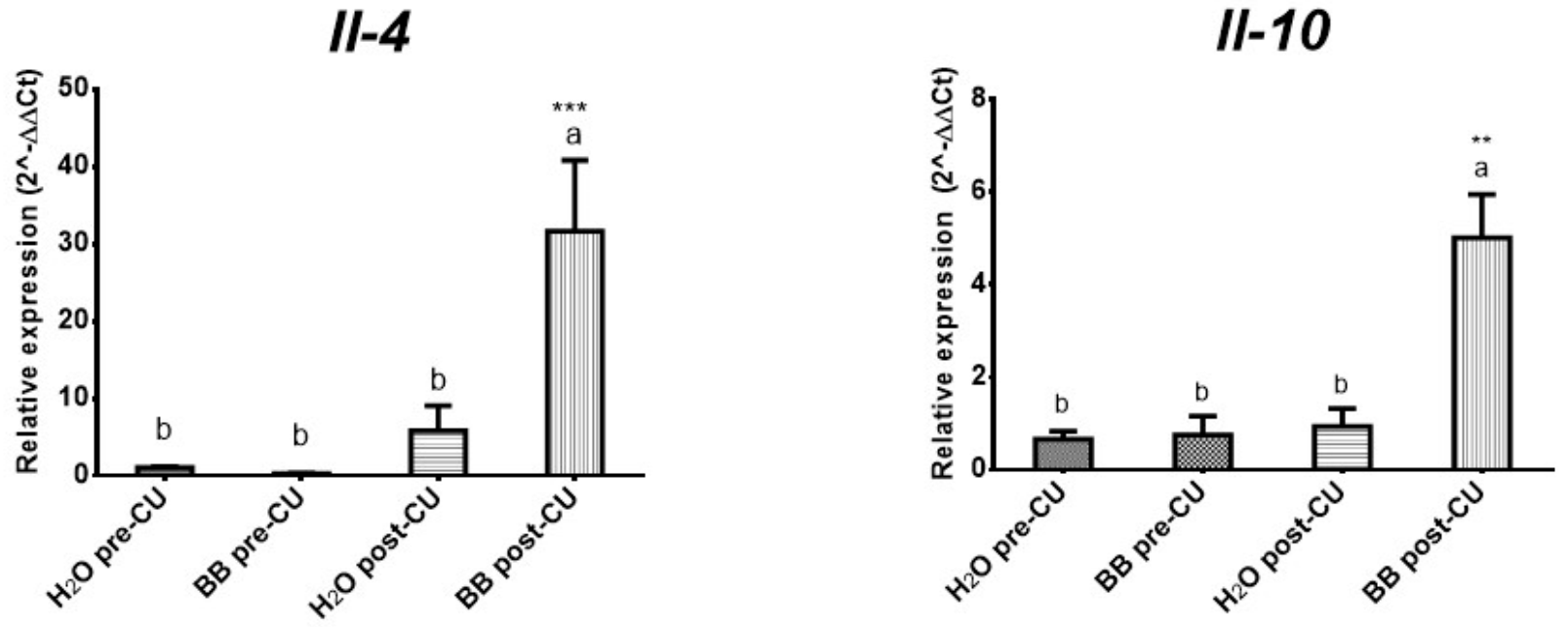
3.3. The Bone Broth Modulates the Expression of Pro-Inflammatory Cytokines in the Murine Model of Ulcerative Colitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chimegee, N.; Dashmaa, D. The daily value of micronutrients in newly produced beef and horse concentrated bone broths. Mong. J. Agric. Sci. 2018, 23, 30–34. [Google Scholar]
- Haskey, N.; Gibson, D. An examination of diet for the maintenance of remission in inflammatory bowel disease. Nutrients 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Rodríguez, M. Evolución y complicaciones de los niños tratados con trasplante de progenitores hematopoyéticos y su relación con el estado nutricional y soporte empleado. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Geerling, B.J.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.J. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am. J. Clin. Nutr. 1998, 67, 919–926. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Liu, J.; Liao, M.; Li, W.; Zou, J.; Han, X.; Kuang, M.; Shen, W.; Li, H. Beneficial effects of fecal microbiota transplantation on ulcerative colitis in mice. Dig. Dis. Sci. 2016, 61, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Cheifetz, A.S. Ulcerative colitis: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2014, 89, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic. Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Filipescu, I.E.; Leonardi, L.; Menchetti, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Piro, F.; Quattrone, A.; Barbato, O.; Brecchia, G. Preventive effects of bovine colostrum supplementation in TNBS-induced colitis in mice. PLoS ONE 2018, 13, e0202929. [Google Scholar] [CrossRef]
- Xiong, J.; Lin, Y.H.; Bi, L.H.; Wang, J.D.; Bai, Y.; Liu, S.D. Effects of interleukin-4 or interleukin-10 gene therapy on trinitrobenzenesulfonic acid-induced murine colitis. BMC Gastroenterol. 2013, 13, 165. [Google Scholar] [CrossRef] [Green Version]
- Lynch, W.D.; Hsu, R. Colitis Ulcerative; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Iacucci, M.; Ghosh, S. Looking beyond symptom relief: Evolution of mucosal healing in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2011, 4, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Papi, C.; Fascì-Spurio, F.; Rogai, F.; Settesoldi, A.; Margagnoni, G.; Annese, V. Mucosal healing in inflammatory bowel disease: Treatment efficacy and predictive factors. Dig. Liver. Dis. 2013, 45, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food Products as Sources of Protein and Amino Acids—The Case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.G.; Choi, H.S.; Choi, Y.S.; Jung, M.O.; Choi, J.S.; Choi, Y.I. Effects of mixed bone and brisket meat on physico-chemical characteristics of shank bone and rib extracts from Hanwoo. Korean J. Food Sci. Anim. Resour. 2016, 36, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.J.; Lee, C.W.; Tsai, W.C.; Chien, Y.C. Essential and toxic metals in animal bone broths. Food Nutr. Res. 2017, 61, 1347478. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, D.; Chae, H.S.; Kim, N.Y.; Jang, A. Nutritional Composition in Bone Extracts from Jeju Crossbred Horses at Different Slaughter Ages. Korean J. Food Sci. Anim. Resour. 2017, 37, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Monro, J.A.; Leon, R.; Puri, B.K. The risk of lead contamination in bone broth diets. Med. Hypotheses 2013, 80, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.Y.; Yoon, J.Y.; Jeong, H.S.; Joo, N.; Choi, S.Y. Anti-Aging Effects of the Hanwoo Leg Bone, Foot and Tail Infusions (HLI, HFI and HTI) on Skin Fibroblast. Korean J. Food Sci. Anim. Resour. 2016, 36, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Etheridge, R.D.; Pesti, G.M.; Foster, E.H. A comparison of nitrogen values obtained utilizing the Kjeldahl nitrogen and Dumas combustion methodologies (Leco CNS 2000) on samples typical of an animal nutrition analytical laboratory. Anim. Feed Sci.Technol. 1998, 73, 21–28. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Bulbuloglu, E.; Kantarceken, B.; Ciralik, H.; Kurutas, E.B.; Buyukbese, M.A.; Gumusalan, Y. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig. Dis. Sci. 2006, 51, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xia, B.; Zhang, L.; Zhou, F.; Zhang, Y.X.; Ye, M.; Hu, Z.G.; Li, J.; Li, J.; Wang, Z.L.; et al. Matrine improves 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in mice. Pharmacol. Res. 2006, 53, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Nivin-Huerta, J.; Carbajal-Urteaga, P.; Aceituno-Llana, L.; Moras-Rosado, M. Efecto preventivo de la Tabebuia serratifolia (palo de arco) en un modelo murino de colitis aguda inducida con ácido acético en ratones BALB-c machos. CIMEL 2013, 18, 19–22. [Google Scholar]
- Tomasello, G.; Sinagra, E.; Raimondo, D.; Palumbo, V.D.; Puleio, R.; Cottone, M.; Damiani, P.; Traina, G.; Abruzzo, A.; Damiani, F.; et al. Validation of a modified model of TNBS-induced colitis in rats. How to induce a chemical colitis in rats. Acta. Biomed. 2015, 86, 92–96. [Google Scholar]
- Xu, Q.; Ming, Z.; Dart, A.M.; Du, X.J. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin. Exp. Pharmacol. Physiol. 2007, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.R.; Guan, Q.; Ma, Y.; Qing, G.; Bernstein, C.N.; Warrington, R.J.; Peng, Z. The potential protective role of caveolin-1 in intestinal inflammation in TNBS-induced murine colitis. PLoS ONE 2015, 10, e0119004. [Google Scholar] [CrossRef] [Green Version]
- Bolant, B.; Calvo, M.A.; Cejalvo, D.; Gimeno, L.O.; Gimeno, L.; Lloris, J.M. La eutanasia en los animales de laboratorio. Centro de investigación. Hospital General Universitario de Valencia. Res. Surg. 1990, 5, 45–56. [Google Scholar]
- Feldman, D.B.; Gupta, B.N. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab. Anim. Sci. 1976, 26, 218–221. [Google Scholar]
- Adeyeye, E.I. Bone marrow: A source of nutritionally valuable fats as typified in the femur of ram and bull. OJACR 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sandanger, T.M.; Brustad, M. Level of selected nutrients in meat, liver, tallow and bone marrow from semi-domesticated reindeer (Rangifer t. tarandus L.). Int. J. Circumpolar Health 2012, 71, 17997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, R.O.; Bueno, K.; Campos, N.; López, D.; Wyatt, C.J.; Ortega, M.I. Contenido total y disponibilidad in vitro de hierro y zinc en alimentos de mayor consumo en Sonora y Oaxaca, México. Arch. Latinoam. Nutr. 2005, 55, 187–193. [Google Scholar]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and signaling pathways of amino acids in intestinal inflammation. BioMed. Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Bao, X.; Feng, Z.; Yao, J.; Li, T.; Yin, Y. Roles of dietary amino acids and their metabolites in pathogenesis of inflammatory bowel disease. Mediat. Inflamm. 2017, 2017, 6869259. [Google Scholar] [CrossRef]
- Farshid, A.A.; Tamaddonfard, E.; Belasius, M.S.; Hamzeh-Gooshchi, N. Histopathological comparison of the effects of histidine and ketotifen in a rat model of colitis. Bull. Vet. Inst. Pulawy 2009, 53, 795–800. [Google Scholar]
- García-Hernández, M.; Guerrero-Ramírez, G.; Castro-Corona, M.D.L.Á.; Medina-de-la-Garza, C.E. Inmunomoduladores como terapia adyuvante en la enfermedad infecciosa. Med. Univ. 2009, 11, 247–259. [Google Scholar]
- Trinchieri, G. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J. Exp. Med. 2001, 194, F53–F57. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Iwabuchi, K.; Onoé, K. Co-operative action of interleukin-10 and interferon-gamma to regulate dendritic cell functions. Immunology 2009, 127, 345–353. [Google Scholar] [CrossRef]
- Lindsay, J.; Van Montfrans, C.; Brennan, F.; Van Deventer, S.; Drillenburg, P.; Hodgson, H.; Te-Velde, A.; Pena- Rodriguez, M.S. IL-10 gene therapy prevents TNBS-induced colitis. Gene. Ther. 2002, 9, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Stevceva, L.; Pavli, P.; Husband, A.; Ramsay, A.; Doe, W.F. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes. Immun. 2001, 2, 309–316. [Google Scholar] [CrossRef] [Green Version]

| Gen | Genebank ID | Primers F (Forward), R (Reverse) |
|---|---|---|
| IL-1β | NM_008361.4 | F: 5’ GGT ACA TCA GCA CCT CAC AA-3’ R: 5’ TTA GAA ACA GTC CAG CCC ATAC-3’ |
| IL-4 | NM_021283.2 | F: 5’ TTG AGA GAG ATC ATC GGC ATT T-3’ R: 5’ CTC ACT CTC TGT GGT GTT CTT C-3’ |
| IL-6 | NM_031168.2 | F: 5’ CTT CCA TCC AGT TGC CTT CT-3’ R: 5′ CTC CGA CTT GTG AAG TGG TAT AG-3′ |
| IL-10 | NM_010548.2 | F: 5’ TTG AAT TCC CTG GGT GAG AAG-3’ R: 5’ TCC ACT GCC TTG CTC TTA TTT-3’ |
| INF-γ | NM_008337.4 | F: 5’ CTC TTC CTC ATG GCT GTT TCT-3’ R: 5’ TTC TTC CAC ATC TAT GCC ACT T-3’ |
| GPD1 | NM_010271.3 | F: 5’ CCT ACT GCT GAC CTT TCT TCT C-3’ R: 5’ GCC CTG AGG ACG ATA AAC TAT AA-3’ |
| TNF-a | NM_013693.3 | F: 5’ TTG TCT ACT CCC AGG TTC TCT-3’ R: 5’ GAG GTT GAC TTT CTC CTG GTA TG-3’ |
| Nutritional Content | mg/100 mL |
|---|---|
| Protein | 248.5181 |
| AA | 232.8671 |
| Minerals | 25.0176 |
| AA | mg/100 mL |
|---|---|
| Asp | 16.7499 |
| Glu | 50.1499 |
| Ser | 6.8265 |
| Gly | 15.0183 |
| Ala | 2.5974 |
| Pro | 3.3966 |
| Cys | 3.4632 |
| Tyr | 5.2614 |
| Lys | 15.0849 |
| His | 43.9893 |
| Thr | 14.7519 |
| Arg | 17.2827 |
| Val | 14.1858 |
| Met | 9.2907 |
| Ile | 3.0969 |
| Leu | 4.6620 |
| Phe | 7.0596 |
| Total AA | 232.8671 |
| EAA | 129.4039 |
| NEAA | 103.4632 |
| Minerals | mg/100 mL | Recommended Daily Intake (mg) | HR |
|---|---|---|---|
| Ca | 6.4160 | 1000 | 0.0064 |
| Mg | 1.8460 | 400 | 0.0046 |
| P | 2.0370 | 1000 | 0.0020 |
| Na | 12.5840 | 2000 | 0.0063 |
| K | 1.9610 | 3800 | 0.0005 |
| Fe | 0.0430 | 18 | 0.0024 |
| Cu | 0.0310 | 1.7 | 0.0182 |
| Zn | 0.0970 | 14 | 0.0069 |
| Mn | 0.0022 | 5.5 | 0.0004 |
| Co | 0.0004 | 1.8 | 0.0002 |
| Total | 25.0176 | 8241 | 0.0030 |
| Cytokines | H2O Pre-UC | BB Pre-UC | H2O Post-UC | BB Post-UC |
|---|---|---|---|---|
| IL-1β | 0.62 0.30 | 0.27 0.13 | 4.14 0.73 | 1.61 0.12 |
| IL-6 | 1.14 0.45 | 0.64 0.25 | 9.43 2.32 | 0.50 0.12 |
| TNF-α | 0.47 0.17 | 0.53 0.26 | 1.96 0.50 | 0.61 0.14 |
| INF-γ | 1.08 0.22 | 0.37 0.14 | 2.66 0.45 | 4.71 0.85 |
| IL-4 | 1.04 0.13 | 0.26 0.05 | 5.85 3.20 | 31.67 9.15 |
| IL-10 | 0.67 0.17 | 0.76 0.41 | 0.94 0.39 | 5.01 9.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mar-Solís, L.M.; Soto-Domínguez, A.; Rodríguez-Tovar, L.E.; Rodríguez-Rocha, H.; García-García, A.; Aguirre-Arzola, V.E.; Zamora-Ávila, D.E.; Garza-Arredondo, A.J.; Castillo-Velázquez, U. Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis. Medicina 2021, 57, 1138. https://doi.org/10.3390/medicina57111138
Mar-Solís LM, Soto-Domínguez A, Rodríguez-Tovar LE, Rodríguez-Rocha H, García-García A, Aguirre-Arzola VE, Zamora-Ávila DE, Garza-Arredondo AJ, Castillo-Velázquez U. Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis. Medicina. 2021; 57(11):1138. https://doi.org/10.3390/medicina57111138
Chicago/Turabian StyleMar-Solís, Laura M., Adolfo Soto-Domínguez, Luis E. Rodríguez-Tovar, Humberto Rodríguez-Rocha, Aracely García-García, Víctor E. Aguirre-Arzola, Diana E. Zamora-Ávila, Aime J. Garza-Arredondo, and Uziel Castillo-Velázquez. 2021. "Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis" Medicina 57, no. 11: 1138. https://doi.org/10.3390/medicina57111138
APA StyleMar-Solís, L. M., Soto-Domínguez, A., Rodríguez-Tovar, L. E., Rodríguez-Rocha, H., García-García, A., Aguirre-Arzola, V. E., Zamora-Ávila, D. E., Garza-Arredondo, A. J., & Castillo-Velázquez, U. (2021). Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis. Medicina, 57(11), 1138. https://doi.org/10.3390/medicina57111138







