Assessment of Arabian Gulf Seaweeds from Kuwait as Sources of Nutritionally Important Polyunsaturated Fatty Acids (PUFAs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Sample Collection
2.2. Fatty Acid Extraction and Methyl Ester Preparation
2.3. Gas Chromatographic (GC) Analysis
2.4. Nutritional Indices
2.5. Statistical Analysis
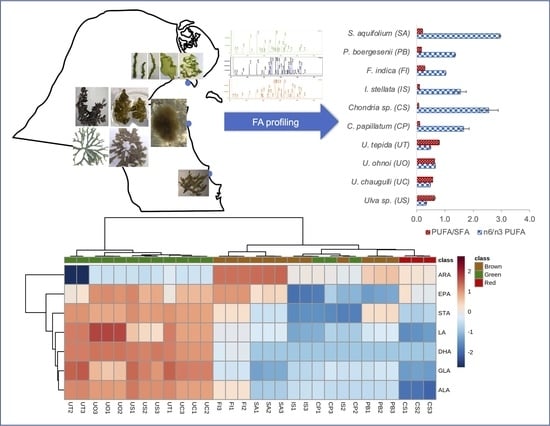
3. Results and Discussion
3.1. Fatty Acid Composition
3.1.1. Chlorophyta
3.1.2. Rhodophyta
3.1.3. Ochrophyta
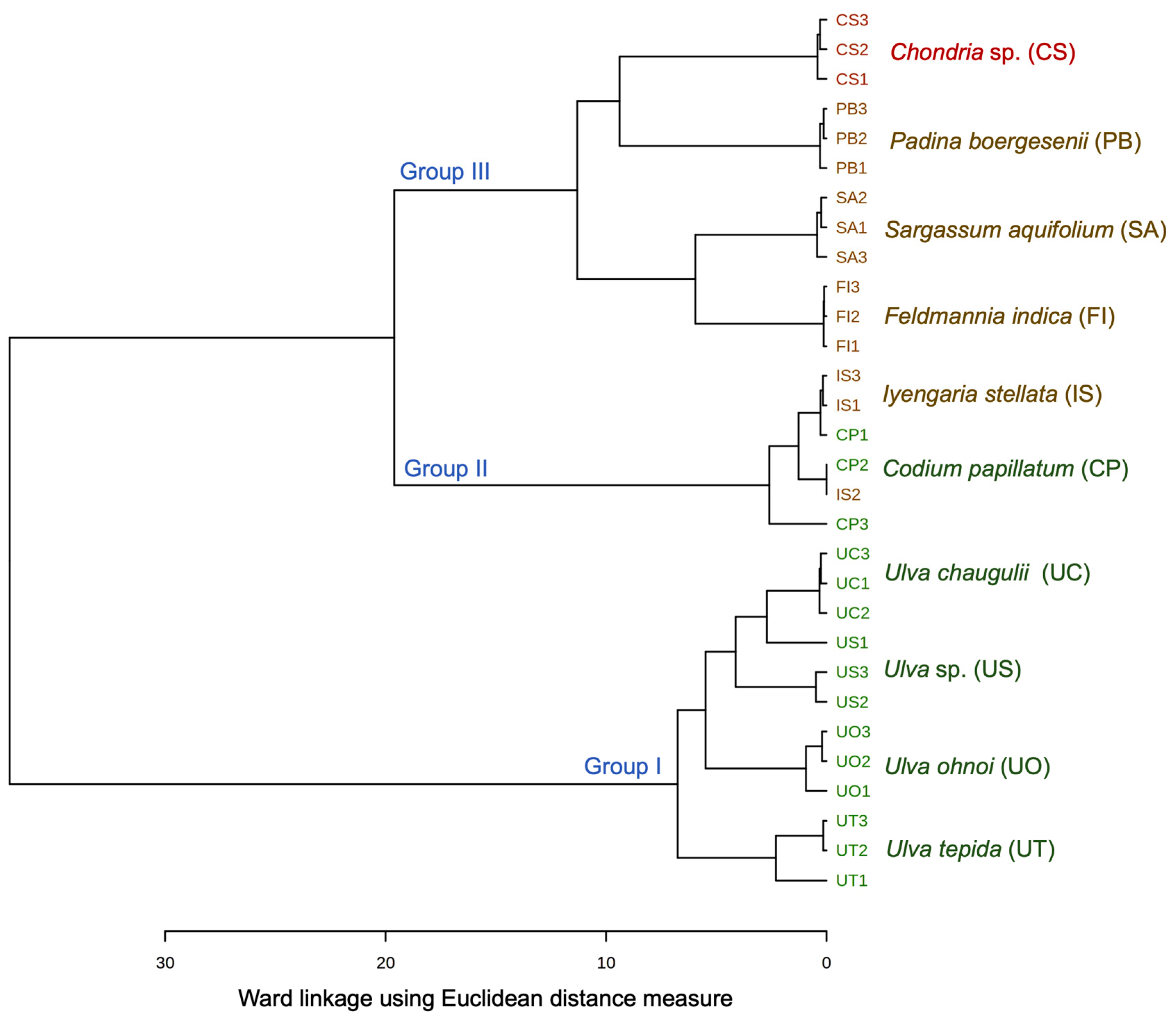
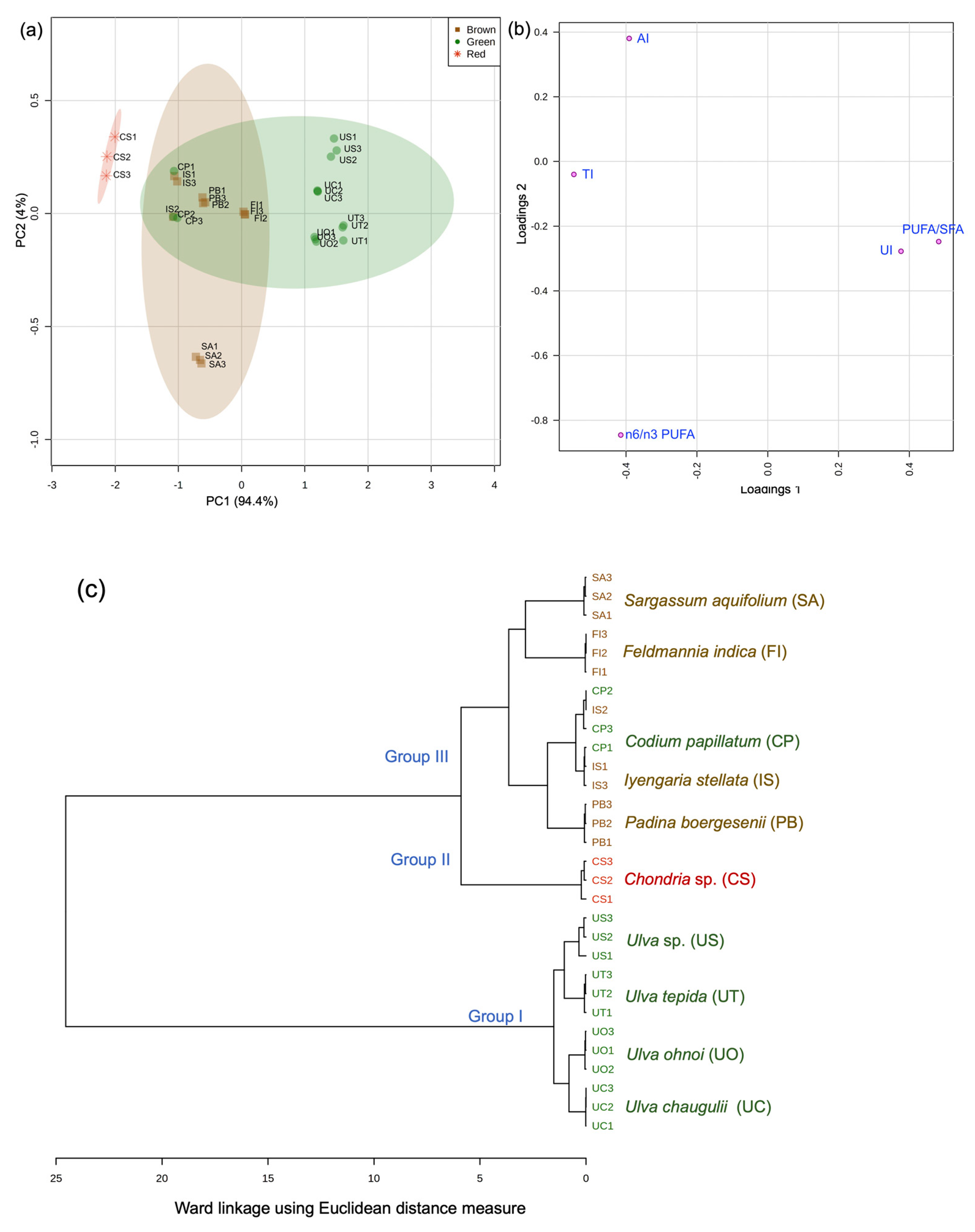
3.2. Fatty Acid Chemotaxonomy
3.3. Nutritional Assessment for Seaweed Valorization
4. Final Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. FAO Globefish 2018, 124, 1. Available online: http://www.fao.org/publications/card/en/c/CA1121EN (accessed on 12 April 2021).
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Kraft, L.G.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.P. Biochemical study of attached macroalgae from the Madeira Archipelago and beach-cast macroalgae from the Canary Islands: Multivariate analysis to determine bioresource potential. Bot. Mar. 2020, 63, 283–298. [Google Scholar] [CrossRef]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Goodyear, D. A New Leaf: Seaweed Could Be a Miracle Food–If We Can Figure out How to Make it Taste Good. Available online: http://www.newyorker.com/magazine/2015/11/02/a-new-leaf (accessed on 6 May 2021).
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Kemmler, W.; Von Stengel, S.; Bebenek, M.; Engelke, K.; Hentschke, C.; Kalender, W. Exercise and fractures in postmenopausal women: 12-year results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporosis Int. 2012, 23, 1276. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Dellatorre, F.G.; Avaro, M.G.; Commendatore, M.G.; Arce, L.; de Vivar, M.E.D. The macroalgal ensemble of Golfo Nuevo (Patagonia, Argentina) as a potential source of valuable fatty acids for nutritional and nutraceutical purposes. Algal Res. 2020, 45, 101726. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.; Mantri, V.A.; Reddy, C.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.A.Z.; Berneira, L.M.; Goulart, N.L.; Mansilla, A.; Astorga-España, M.S.; de Pereira, C.M.P. Rhodophyta, Ochrophyta and Chlorophyta macroalgae from different sub-Antarctic regions (Chile) and their potential for polyunsaturated fatty acids. Braz. J. Bot. 2021, 44, 429–438. [Google Scholar] [CrossRef]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; de Nys, R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. Glob. Chang. Biol. Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Galloway, A.W.; Britton-Simmons, K.H.; Duggins, D.O.; Gabrielson, P.W.; Brett, M.T. Fatty acid signatures differentiate marine macrophytes at ordinal and family ranks. J. Phycol. 2012, 48, 956–965. [Google Scholar] [CrossRef]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Cornish, M.L.; Critchley, A.T.; Mouritsen, O.G. Consumption of seaweeds and the human brain. J. Appl. Phycol. 2017, 29, 2377–2398. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, Y.; Liu, T.; Zhang, L.; Liu, H.; Guan, H. Comparative studies on the characteristic fatty acid profiles of four different Chinese medicinal Sargassum seaweeds by GC-MS and chemometrics. Mar. Drugs 2016, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; da Costa, E.; Melo, T.; Lopes, D.; Pais, A.; Santos, S.A.; Pitarma, B.; Mendes, M.; Abreu, M.H.; Collén, P.N. Polar lipids of commercial Ulva spp. of different origins: Profiling and relevance for seaweed valorization. Foods 2021, 10, 914. [Google Scholar] [CrossRef]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet. 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and site-specific variation in the nutritional quality of temperate seaweed assemblages: Implications for grazing invertebrates and the commercial exploitation of seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Cvitković, D.; Dragović-Uzelac, V.; Dobrinčić, A.; Čož-Rakovac, R.; Balbino, S. The effect of solvent and extraction method on the recovery of lipid fraction from Adriatic Sea macroalgae. Algal Res. 2021, 56, 102291. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O′Doherty, J. Seasonal variation of the proximate composition, mineral content, fatty acid profiles and other phytochemical constituents of selected brown macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Sheppard, C.; Al-Husiani, M.; Al-Jamali, F.; Al-Yamani, F.; Baldwin, R.; Bishop, J.; Benzoni, F.; Dutrieux, E.; Dulvy, N.K.; Durvasula, S.R.V.; et al. Environmental concerns for the future of gulf coral reefs. In Coral Reefs of the Gulf: Adaptation to Climatic Extremes; Riegl, B.M., Purkis, S.J., Eds.; Springer Science and Business Media: New York, NY, USA, 2012. [Google Scholar]
- Heiba, H.I.; Al-Easa, H.S.; Rizk, A.F.M. Fatty acid composition of twelve algae from the coastal zones of Qatar. Plant Foods Hum. Nutr. 1997, 51, 27–34. [Google Scholar] [CrossRef]
- Abomohra, A.E.; El-Naggar, A.H.; Baeshen, A.A. Potential of macroalgae for biodiesel production: Screening and evaluation studies. J. Biosci. Bioeng. 2018, 125, 231–237. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Tecnol. 2012, 49, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef]
- Akbary, P.; Liao, L.M.; Aminikhoei, Z.; Tavabe, K.R.; Hobbi, M.; Erfanifar, E. Sterol and fatty acid profiles of three macroalgal species collected from the Chabahar coasts, southeastern Iran. Aquacult. Int. 2021, 29, 155–165. [Google Scholar] [CrossRef]
- Fariman, G.A.; Shastan, S.J.; Zahedi, M.M. Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J. Appl. Phycol. 2016, 28, 1323–1331. [Google Scholar] [CrossRef]
- Al-Hasan, R.H.; Hantash, F.M.; Radwan, S.S. Enriching marine macroalgae with eicosatetraenoic (arachidonic) and eicosapentaenoic acids by chilling. Appl. Microbiol. Biotechnol. 1991, 35, 530–535. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Preparation of derivatives of fatty acids. In Oily Press Lipid Library Series, Lipid Analysis, 4th ed.; Christie, W.W., Han, X., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 145–158. [Google Scholar]
- Poerschmann, J.; Spijkerman, E.; Langer, U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb. Ecol. 2004, 48, 78–89. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Pirian, K.; Piri, K.; Sohrabipour, J.; Blomster, J. Three species of Ulva (Ulvophyceae) from the Persian Gulf as potential sources of protein, essential amino acids and fatty acids. Phycol. Res. 2018, 66, 149–154. [Google Scholar] [CrossRef]
- Pirian, K.; Jeliani, Z.Z.; Arman, M.; Sohrabipour, J.; Yousefzadi, M. Proximate analysis of selected macroalgal species from the Persian Gulf as a nutritional resource. Trop. Life Sci. Res. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef]
- Santos, J.; Guihéneuf, F.; Fleming, G.; Chow, F.; Stengel, D. Temporal stability in lipid classes and fatty acid profiles of three seaweed species from the north-eastern coast of Brazil. Algal Res. 2019, 41, 101572. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Han, L.; Lou, Q. Fatty acids of some algae from the Bohai Sea. Phytochemistry 2002, 59, 157–161. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [Green Version]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef]
- Vaskovsky, V.E.; Khotimchenko, S.V.; Xia, B.; Hefang, L. Polar lipids and fatty acids of some marine macrophytes from the Yellow Sea. Phytochemistry 1996, 42, 1347–1356. [Google Scholar] [CrossRef]
- Yazici, Z.; Aysel, V.; Öksüz, E.; Köse, A.; Cumali, S.; Güven, K. Fatty acid composition of marine macroalgae from the Black Sea and Dardanelles. Toxicol. Environ. Chem. 2007, 89, 371–379. [Google Scholar] [CrossRef]
- McCauley, J.I.; Meyer, B.J.; Winberg, P.C.; Skropeta, D. Parameters affecting the analytical profile of fatty acids in the macroalgal genus Ulva. Food Chem. 2016, 15, 332–340. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Fatty acids of species in the genus Codium. Bot. Mar. 2003, 46, 456–460. [Google Scholar] [CrossRef]
- Dembitsky, V.; Řezanková, H.; Řezanka, T.; Hanuš, L. Variability of the fatty acids of the marine green algae belonging to the genus Codium. Biochem. Syst. Ecol. 2003, 31, 1125–1145. [Google Scholar] [CrossRef]
- Govenkar, M.; Wahidulla, S. Studies on the fatty acids of the red alga Chondria armata (Kütz.) Okamura. Bot. Mar. 1999, 42, 3–5. [Google Scholar] [CrossRef]
- Khotimchenko, S.; Gusarova, I. Red algae of Peter the Great Bay as a source of arachidonic and eicosapentaenoic acids. Russ. J. Mar. Biol. 2004, 30, 183–187. [Google Scholar] [CrossRef]
- Stefanov, K.; Seizova, K.; Elenkov, I.; Kuleva, L.; Popov, S.; Dimitrova-Konaklieva, S. Lipid composition of the red alga Chondria tenuissima (Good et Wood.) Ag., inhabiting waters with different salinities. Bot. Mar. 1994, 37, 445–448. [Google Scholar] [CrossRef]
- Khotimchenko, S. Fatty acid composition of seven Sargassum species. Phytochemistry 1991, 30, 2639–2641. [Google Scholar] [CrossRef]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Comparative evaluation of fatty acid composition of different Sargassum (Fucales, Phaeophyta) species harvested from temperate and tropical waters. J. Aquat. Food Prod. Technol. 2005, 13, 53–70. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef]
- Silberfeld, T.; Leigh, J.W.; Verbruggen, H.; Cruaud, C.; De Reviers, B.; Rousseau, F. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the “brown algal crown radiation”. Mol. Phylogenet. Evol. 2010, 56, 659–674. [Google Scholar] [CrossRef]
- Colombo, M.L.; Rise, P.; Giavarini, F.; De Angelis, L.; Galli, C.; Bolis, C. Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods Hum. Nutr. 2006, 61, 64–69. [Google Scholar] [CrossRef]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Elagizi, A.; Lavie, C.J.; Marshall, K.; DiNicolantonio, J.J.; O′Keefe, J.H.; Milani, R.V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A comprehensive review. Prog. Cardiovasc. Dis. 2018, 61, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.M.; Nedel, F.; Guimaraes, V.; Da Silva, A.F.; Colepicolo, P.; De Pereira, C.M.; Lund, R.G. Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front. Microbiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Küpper, F.C.; Gaquerel, E.; Cosse, A.; Adas, F.; Peters, A.F.; Müller, D.G.; Kloareg, B.; Salaün, J.P.; Potin, P. Free fatty acids and methyl jasmonate trigger defense reactions in Laminaria digitata. Plant Cell Physiol. 2009, 50, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Department of Health London (UK). Nutritional Aspects of Cardiovascular Disease Report on Health and Social; Subject No. 46; Her Majesty′s Stationery Office: London, UK, 1994; p. 202.
- Susanto, E.; Fahmi, A.S.; Hosokawa, M.; Miyashita, K. Variation in lipid components from 15 species of tropical and temperate seaweeds. Mar. Drugs 2019, 17, 630. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.A., Jr. Lipids and membrane function in green algae. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1302, 17–45. [Google Scholar] [CrossRef]
- WHO; FAO. Joint Consultation. Fats and oils in human nutrition. Nutr. Rev. 1995, 53, 202–205. [Google Scholar]
- Al-Adilah, H.; Al-Bader, D.A.; Elktob, M.; Kosma, I.; Kumari, P.; Küpper, F.C. Trace element concentrations in seaweeds of the Arabian Gulf identified by morphology and DNA barcodes. Bot. Mar. 2021, 64, 327–338. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Lage, S.; Aluwini, D.F.; Rebours, C.; Brurberg, M.B.; Nitschke, U.; Gentili, F.G. Chemical profiling of the Arctic Sea lettuce Ulva lactuca (Chlorophyta) mass-cultivated on land under controlled conditions for food applications. Food Chem. 2021, 341, 127999. [Google Scholar] [CrossRef]
| S. No. | Species | Abbreviations (*) | Phylogenetic Affinity | Herbarium Code | Date | Location | Coordinates | Offshore Seawater Surface Temperature (°C) |
|---|---|---|---|---|---|---|---|---|
| Chlorophyta | ||||||||
| 1 | Ulva sp. | US | Ulvaceae, Ulvales, Ulvophyceae | Doh010221-1 | 01/02/2021 | Ras Ushairij | 29°23′00.7″ N 47°49′50.9″ E | 14.8–15.0 |
| 2 | Ulva chaugulii M.G.Kavale and M.A.Kazi | UC | Ulvaceae, Ulvales, Ulvophyceae | Doh010221-2 | 01/02/2021 | Ras Ushairij | 29°23′00.7″ N 47°49′50.9″ E | 14.8–15.0 |
| 3 | Ulva tepida Masakiyo and S.Shimada | UT | Ulvaceae, Ulvales, Ulvophyceae | Doh010221-5 | 01/02/2021 | Ras Ushairij | 29°23′00.7″ N 47°49′50.9″ E | 14.8–15.0 |
| 4 | Ulva ohnoi M.Hiraoka and S.Shimada | UO | Ulvaceae, Ulvales, Ulvophyceae | Doh010221-3 | 01/02/2021 | Ras Ushairij | 29°23′00.7″ N 47°49′50.9″ E | 14.8–15.0 |
| 5 | Codium papillatum C.K.Tseng and W.J. Gilbert | CP | Codiaceae, Bryopsidales, Ulvophyceae | ABUH030618-2 | 3/06/2018 | Abu Al Hasaniya | 29°12′19.4″ N 48°06′41.5″ E | 30.2–30.3 |
| Rhodophyta | ||||||||
| 6 | Chondria sp. C. Agardh | CS | Rhodomelaceae Ceramiales, Florideophyceae | BNA260518-1 | 26/05/2018 | Bnaider Beach | 28°47′01.5″ N 48°17′50.6″ E | 27.8–27.9 |
| Ochrophyta | ||||||||
| 7 | Iyengaria stellata (Børgesen) Børgesen | IS | Scytosiphonaceae, Ectocarpales, Phaeophyceae | ABUH060618-1 | 27/05/2018 | Abu Al Hasaniya | 29°12′19.4″ N 48°06′41.5″ E | 30.2–30.3 |
| 8 | Feldmannia indica (Sonder) Womersley and A. Bailey | FI | Acinetosporaceae Ectocarpales, Phaeophyceae | ABUH030618-1 | 3/06/2018 | Abu Al Hasaniya | 29°12′19.4″ N 48°06′41.5″ E | 30.2–30.3 |
| 9 | Padina boergesenii Allender and Kraft | PT | Dictyotaceae, Dictyotales, Phaeophyceae | ABUH270518-1 | 27/05/2018 | Abu Al Hasaniya | 29°12′19.4″ N 48°06′41.5″ E | 30.2–30.3 |
| 10 | Sargassum aquifolium (Turner) C. Agardh | SA | Sargassaceae, Fucales, Phaeophyceae | ABUH270518-2 | 27/05/2018 | Abu Al Hasaniya | 29°12′19.4″ N 48°06′41.5″ E | 30.2–30.3 |
| Fatty Acids | Ulva sp. | Ulva chaugulii | Ulva ohnoi | Ulva tepida | Codium papillatum | Chondria sp. | Iyengaria stellata | Feldmannia indica | Padina boergesenii | Sargassum aquifolium |
|---|---|---|---|---|---|---|---|---|---|---|
| 10:0 | nd | nd | nd | nd | 0.3 ± 0.01 c | 0.5 ± 0.1 a | 0.3 ± 0.01 c | 0.4 ± 0.003 b | 0.3 ± 0.03 c | 0.3 ± 0.02 c |
| 12:0 | 0.2 ± 0.1 f | 0.4 ± 0.02 de | 1.6 ± 0.2 b | 1.2 ± 0.1 c | 1.9 ± 0.01 a | 0.5 ± 0.01 d | 1.9 ± 0.04 a | 0.2 ± 0.02 f | 1.3 ± 0.1 c | 0.4 ± 0.1 de |
| 13:0 | 0.1 ± 0.05 b | nd | nd | nd | 0.01 ± 0.001 c | 0.2 ± 0.02 a | 0.01 ± 0.001 c | 0.01 ± 0.001 c | 0.04 ± 0.04 c | nd |
| 14:0 | 1.3 ± 0.11 g | 1.7 ± 0.02 fg | 2.5 ± 0.04 e | 2.1 ± 0.05 ef | 3.3 ± 0.07 d | 11.7 ± 0.42 a | 3.2 ± 0.06 d | 7.6 ± 0.04 b | 7.5 ± 0.05 b | 5.6 ± 0.13 c |
| 15:0 | 0.2 ± 0.04 e | 0.2 ± 0.01 e | 0.3 ± 0.001 d | 0.7 ± 0.02 ab | 0.1 ± 0.01 f | 0.8 ± 0.04 a | 0.1 ± 0.02 f | 0.5 ± 0.001 c | 0.7 ± 0.01 ab | 0.4 ± 0.03 d |
| 16:0 | 43.5 ± 5.0 de | 44.6 ± 0.2 cde | 41.3 ± 0.4 e | 35.7 ± 0.6 f | 53.9 ± 0.6 b | 63.9 ± 0.5 a | 53.7 ± 0.8 b | 49.0 ± 0.2 bcd | 49.2 ± 0.3 bc | 49.1 ± 0.5 bc |
| 17:0 | 0.1 ± 0.003 c | 0.2 ± 0.05 bc | 0.2 ± 0.1 bc | 0.1 ± 0.003 c | 0.2 ± 0.02 bc | 0.4 ± 0.02 b | 0.2 ± 0.01 bc | 0.3 ± 0.002 bc | 5.9 ± 0.2 a | 0.1 ± 0.05 bc |
| 18:0 | 3.4 ± 2.0 ab | 1.1 ± 0.05 c | 1.7 ± 0.17 bc | 1.3 ± 0.13 bc | 3.2 ± 0.19 abc | 4.5 ± 0.37 a | 3.0 ± 0.2 abc | 1.9 ± 0.03 bc | 2.3 ± 0.1 abc | 3.2 ± 0.01 abc |
| 20:0 | 1.2 ± 0.3 ab | 1.2 ± 0.01 abc | 0.9 ± 0.01 abcd | 1.4 ± 0.03 a | 0.7 ± 0.5 bcd | 0.5 ± 0.01 d | 0.9 ± 0.1 abcd | 0.5 ± 0.001 d | 0.6 ± 0.02 cd | 0.9 ± 0.02 abcd |
| 22:0 | 1.6 ± 0.01 c | 1.7 ± 0.01 c | 4.5 ± 0.1 b | 2.0 ± 0.04 c | 5.6 ± 0.4 a | 0.2 ± 0.04 de | 5.6 ± 0.31 a | 0.5 ± 0.01 de | 0.1 ± 0.0 e | 0.8 ± 0.02 d |
| 24:0 | nd | 0.5 ± 0.004 c | 1.1 ± 0.02 b | 0.02 ± 0.001 de | 2.7 ± 0.4 a | 0.3 ± 0.03 c | 2.4 ± 0.3 a | 0.3 ± 0.02 c | 0.3 ± 0.03 c | 0.4 ± 0.1 c |
| ΣSFAs | 51.6 ± 3.0 e | 51.4 ± 0.11 e | 54.1 ± 0.6 e | 44.5 ± 0.8 f | 72.0 ± 0.9 b | 83.3 ± 0.3 a | 71.3 ± 0.5 b | 61.3 ± 0.1 d | 68.0 ± 0.4 c | 61.2 ± 0.5 d |
| 9c-14:1 | 0.2 ± 0.04 a | nd | nd | nd | 0.02 ± 0.003 c | nd | 0.02 ± 0.003 c | 0.03 ± 0.004 bc | nd | 0.1 ± 0.01 b |
| 10c-15:1 | nd | nd | 0.04 ± 0.003 a | 0.02 ± 0.001 b | nd | nd | nd | nd | nd | nd |
| 9c-16:1 | 7.3 ± 2.2 a | 6.4 ± 0.03 ab | 3.9 ± 0.04 cd | 1.4 ± 0.02 d | 1.6 ± 0.04 d | 5.4 ± 0.1 abc | 1.5 ± 0.1 d | 4.4 ± 0.1 bc | 3.2 ± 0.3 cd | 4.6 ± 0.1 bc |
| 10c-17:1 | 0.3 ± 0.2 bc | 0.7 ± 0.001 a | 0.1 ± 0.003 c | nd | 0.04 ± 0.002 c | nd | 0.04 ± 0.002 c | 0.5 ± 0.003 ab | 0.1 ± 0.02 c | 0.1 ± 0.02 c |
| 9t-18:1 | 0.1 ± 0.06 b | 3.0 ± 0.03 a | 0.7 ± 0.1 b | 3.2 ± 0.1 a | 0.1 ± 0.05 b | nd | 0.1 ± 0.04 b | 0.04 ± 0.0004 b | 0.1 ± 0.002 b | 0.1 ± 0.01 b |
| 9c-18:1 | 5.9 ± 3.0 de | 8.9 ± 0.03 d | 6.1 ± 0.01 de | 15.7 ± 0.1 c | 18.1 ± 0.7 ab | 4.8 ± 0.1 e | 19.4 ± 0.5 ab | 16.0 ± 0.04 bc | 16.8 ± 0.8 abc | 19.8 ± 0.2 a |
| 11c-20:1 | 1.5 ± 1.2 a | 0.1 ± 0.1 b | 0.2 ± 0.002 ab | 0.2 ± 0.1 ab | 0.1 ± 0.03 b | 0.4 ± 0.01 ab | 0.1 ± 0.03 b | 0.03 ± 0.0003 b | 0.1 ± 0.01 b | 0.9 ± 0.03 ab |
| 13c-22:1 | 0.2 ± 0.01 cd | 0.2 ± 0.05 cd | 0.6 ± 0.1 a | 0.2 ± 0.06 cd | 0.02 ± 0.001 e | 0.20 ± 0.01 cd | 0.02 ± 0.001 e | 0.1 ± 0.01 de | 0.2 ± 0.1 cd | 0.4 ± 0.02 b |
| 15c-24:1 | 0.5 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| ΣMUFAs | 15.8 ± 7.0 bc | 19.4 ± 0.1 ab | 11.5 ± 0.9 b | 20.7 ± 0.2 ab | 20.0 ± 0.7 ab | 10.8 ± 0.2 c | 21.2 ± 0.5 ab | 21.1 ± 0.04 ab | 20.5 ± 0.8 ab | 26.0 ± 0.3 a |
| 9t12t-18:2 | nd | nd | nd | nd | 0.3 ± 0.1 ab | 0.5 ± 0.1 a | 0.3 ± 0.11 b | 0.003 ± 0.001 c | 0.3 ± 0.1 ab | nd |
| 9c12c-18:2 | 5.2 ± 0.5 d | 7.0 ± 0.1 c | 11.6 ± 0.1 a | 8.6 ± 0.1 b | 2.3 ± 0.3 f | 1.6 ± 0.1 f | 2.0 ± 0.3 f | 3.7 ± 0.01 e | 3.3 ± 0.1 e | 3.3 ± 0.1 e |
| 6c9c12c-18:3 | 1.5 ± 0.22 b | 1.5 ± 0.03 b | 0.9 ± 0.06 c | 1.8 ± 0.05 a | 0.4 ± 0.03 d | 0.2 ± 0.01 d | 0.4 ± 0.03 d | 0.4 ± 0.01 d | 0.4 ± 0.02 d | 0.2 ± 0.01 d |
| 9c12c15c-18:3 | 13.5 ± 1.6 a | 11.0 ± 0.04 b | 11.4 ± 0.1 b | 13.4 ± 0.2 a | 2.3 ± 0.2 d | 0.5 ± 0.1 d | 2.3 ± 0.1 d | 5.1 ± 0.02 c | 2.2 ± 0.1 d | 1.7 ± 0.04 d |
| 6c9c12c15c-18:4 | 7.4 ± 1.1 ab | 6.4 ± 0.02 bc | 5.6 ± 0.04 c | 7.9 ± 0.1 a | 0.1 ± 0.02 e | 0.3 ± 0.03 e | 0.1 ± 0.02 e | 2.0 ± 0.004 d | 2.2 ± 0.1 d | 0.6 ± 0.01 e |
| 8c11c14c-20:3 | 0.2 ± 0.1 de | 0.3 ± 0.1 bcd | 0.4 ± 0.01 bc | 0.2 ± 0.06 de | 0.1 ± 0.01 e | 0.2 ± 0.01 de | 0.1 ± 0.01 e | 0.4 ± 0.01 bc | 0.5 ± 0.02 b | 1.4 ± 0.05 a |
| 11c14c17c-20:3 | 0.1 ± 0.04 b | nd | nd | 0.1 ± 0.02 b | 0.3 ± 0.03 a | nd | 0.3 ± 0.02 a | 0.1 ± 0.004 b | 0.1 ± 0.01 b | 0.1 ± 0.003 b |
| 5c8c11c14c-20:4 | 0.7 ± 0.03 cde | 0.7 ± 0.01 cde | 0.7 ± 0.02 cde | 0.4 ± 0.1 e | 1.1 ± 0.1 cd | 1.3 ± 0.06 cd | 1.1 ± 0.08 cd | 4.0 ± 0.02 a | 2.0 ± 0.1 b | 4.3 ± 0.1 a |
| 13c16c-22:2 | 0.6 ± 0.1 ab | nd | nd | 0.4 ± 0.01 bc | 0.7 ± 0.1 a | 0.2 ± 0.01 de | 0.6 ± 0.1 a | 0.3 ± 0.01 cd | 0.1 ± 0.01 e | 0.3 ± 0.02 cd |
| 5c8c11c14c17c-20:5 | 1.4 ± 0.0 ab | 1.2 ± 0.01 bc | 1.5 ± 0.04 a | 0.8 ± 0.1 d | 0.4 ± 0.1 e | 0.8 ± 0.1 d | 0.3 ± 0.1 e | 1.5 ± 0.02 a | 0.3 ± 0.02 e | 0.9 ± 0.1 cd |
| 4c7c10c13c16c19c-22:6 | 1.9 ± 0.2 b | 1.1 ± 0.1 c | 2.3 ± 0.1 a | 1.8 ± 0.1 b | nd | nd | 0.2 ± 0.11 d | 0.1 ± 0.003 d | nd | nd |
| ΣPUFAs | 32.4 ± 34.0 ab | 29.2 ± 0.1 b | 34.4 ± 0.2 a | 35.4 ± 0.5 a | 7.9 ± 0.5 ef | 5.6 ± 0.1 f | 7.4 ± 0.4 f | 17.5 ± 0.1 c | 11.4 ± 0.5 de | 12.9 ± 0.3 d |
| ΣC18 PUFAs | 27.6 ± 3.3 a | 25.8 ± 0.1 a | 29.4 ± 0.2 a | 31.7 ± 0.4 a | 5.4 ± 0.3 cd | 3.2 ± 0.2 e | 5.0 ± 0.3 cd | 11.2 ± 0.04 b | 8.4 ± 0.3 bc | 5.8 ± 0.1 cd |
| ΣC20 PUFAs | 2.3 ± 0.3 bc | 2.2 ± 0.1 bc | 2.6 ± 0.03 b | 1.5 ± 0.5 e | 1.8 ± 0.1 cd | 2.3 ± 0.2 bc | 1.7 ± 0.04 cd | 6.0 ± 0.1 a | 2.9 ± 0.2 b | 6.7 ± 0.2 a |
| n6/n3 PUFA | 0.3 ± 0.02 e | 0.5 ± 0.002 e | 0.7 ± 0.002 de | 0.5 ± 0.002 e | 1.7 ± 0.2 b | 2.5 ± 0.3 a | 1.6 ± 0.2 b | 1.0 ± 0.003 cd | 1.4 ± 0.03 bc | 2.9 ± 0.03 a |
| PUFA/SFA | 0.6 ± 0.04 b | 0.6 ± 0.003 b | 0.6 ± 0.003 b | 0.8 ± 0.02 a | 0.1 ± 0.01 e | 0.1 ± 0.002 e | 0.1 ± 0.01 e | 0.3 ± 0.002 c | 0.2 ± 0.01 d | 0.2 ± 0.01 d |
| UI | 123.9 ± 6.3 b | 112.8 ± 0.5 c | 119.2 ± 0.5 bc | 133.3 ± 1.5 a | 42.3 ± 1.3 g | 28.6 ± 0.8 h | 42.2 ± 0.8 g | 78.9 ± 0.4 d | 55.8 ± 0.8 f | 67.7 ± 1.3 e |
| AI | 1.0 ± 0.2 de | 1.1 ± 0.01 de | 1.2 ± 0.03 d | 0.8 ± 0.01 e | 2.5 ± 0.1 b | 6.7 ± 0.7 a | 2.4 ± 0.06 b | 2.1 ± 0.01 bc | 2.5 ± 0.04 b | 1.6 ± 0.04 c |
| TI | 0.6 ± 0.04 e | 0.7 ± 0.003 e | 0.6 ± 0.002 e | 0.5 ± 0.01 e | 5.0 ± 0.2 b | 11.1 ± 0.8 a | 5.2 ± 0.2 b | 2.0 ± 0.01 d | 3.2 ± 0.1 c | 4.0 ± 0.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Adilah, H.; Al-Sharrah, T.K.; Al-Bader, D.; Ebel, R.; Küpper, F.C.; Kumari, P. Assessment of Arabian Gulf Seaweeds from Kuwait as Sources of Nutritionally Important Polyunsaturated Fatty Acids (PUFAs). Foods 2021, 10, 2442. https://doi.org/10.3390/foods10102442
Al-Adilah H, Al-Sharrah TK, Al-Bader D, Ebel R, Küpper FC, Kumari P. Assessment of Arabian Gulf Seaweeds from Kuwait as Sources of Nutritionally Important Polyunsaturated Fatty Acids (PUFAs). Foods. 2021; 10(10):2442. https://doi.org/10.3390/foods10102442
Chicago/Turabian StyleAl-Adilah, Hanan, Tahani Khalaf Al-Sharrah, Dhia Al-Bader, Rainer Ebel, Frithjof Christian Küpper, and Puja Kumari. 2021. "Assessment of Arabian Gulf Seaweeds from Kuwait as Sources of Nutritionally Important Polyunsaturated Fatty Acids (PUFAs)" Foods 10, no. 10: 2442. https://doi.org/10.3390/foods10102442
APA StyleAl-Adilah, H., Al-Sharrah, T. K., Al-Bader, D., Ebel, R., Küpper, F. C., & Kumari, P. (2021). Assessment of Arabian Gulf Seaweeds from Kuwait as Sources of Nutritionally Important Polyunsaturated Fatty Acids (PUFAs). Foods, 10(10), 2442. https://doi.org/10.3390/foods10102442