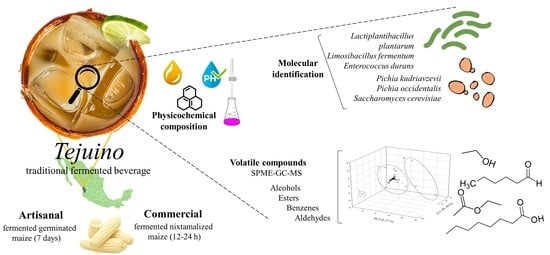
Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Physicochemical Analysis
2.3. Microbiological Analysis
2.4. Isolation of Lactic Acid Bacteria and Yeast
2.5. Identification of LAB and Yeasts Strains
2.6. Analysis of Volatile Compounds by Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (SPME-GC-MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Microbial Quality
3.3. Identification of LAB and Yeast Strains
3.4. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siyuan, S.; Tong, L.; Liu, R. Corn phytochemicals and their health benefits. Food Sci. Human Well. 2018, 73, 185–195. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashau, M.E.; Maliwichi, L.L.; Jideani, A.I.O. Non-alcoholic fermentation of maize (Zea mays) in Sub-Saharan Africa. Fermentation 2021, 7, 158. [Google Scholar] [CrossRef]
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; de la Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araaujo, R.; González, R.L.; Londoño, L.; Ventura, J.; et al. Traditional fermented beverages in Mexico. In Fermented Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 605–635. [Google Scholar]
- Rubio-Castillo, Á.E.; Santiago-López, L.; Vallejo-Cordoba, B.; Hernández-Mendoza, A.; Sayago-Ayerdi, S.G.; González-Córdova, A.F. Traditional non-distilled fermented beverages from Mexico to based on maize: An approach to Tejuino beverage. Int. J. Gastron. Foods Sci. 2021, 23, 100283. [Google Scholar] [CrossRef]
- Wacher-Rodarte, C. Alimentos y bebidas fermentados tradicionales. Biotecnologıa Alimentaria; Grupo Noriega, Editorial LIMUSA: Mexico City, Mexico, 1995; pp. 313–349. [Google Scholar]
- Sangwan, S.; Kumar, S.; Goyal, S. Maize utilisation in food bioprocessing: An overview. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D.P., Kumar, S.S., Eds.; Springer: New Delhi, India, 2014; pp. 119–134. [Google Scholar]
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional fermented foods and beverages from a microbiological and nutritional perspective: The Colombian heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research opportunities: Traditional fermented beverages in Mexico. Cultural, microbiological, chemical, and functional aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef]
- Coelho, E.; Lemos, M.; Genisheva, Z.; Domingues, L.; Vilanova, M.; Oliveira, J.M. Validation of a LLME/GC-MS methodology for quantification of volatile compounds in fermented beverages. Molecules 2020, 25, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- AOAC. International Official Methods of Analysis, 17th ed.; AOAC Int.: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Wisker, E.; Feldheim, W. Metabolizable energy of diets low or high in dietary fiber from fruits and vegetables when consumed by humans. J. Nutr. 1990, 120, 1331–1337. [Google Scholar] [CrossRef]
- Torres-Llanez, M.J.; Vallejo-Cordoba, B.; Díaz-Cinco, M.E.; Mazorra-Manzano, M.A.; González-Córdova, A.F. Characterization of the natural microflora of artisanal Mexican Fresco cheese. Food Control. 2006, 17, 683–690. [Google Scholar] [CrossRef]
- Secretaria de Salud. NOM-111-1994. Bienes y Servicios. Método para la Cuenta de Mohos y Levaduras en Alimentos. Diario Oficial de la Federación. Available online: http://www.economia-noms.gob.mx/normas/noms/1995/111-ssa1.pdf (accessed on 1 August 2021).
- Secretaria de Salud. NOM-092-1994. Bienes y Servicios. Método para la Cuenta de Bacterias Aerobias en Placa. Diario Oficial de la Federación. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=4728921&fecha=15/08/1994&print=true (accessed on 1 August 2021).
- Secretaria de Salud. NOM-112-1994. Bienes y Servicios. Determinación de Bacterias Coliformes. Técnica del Número más Probable. Diario Oficial de la Federación. Available online: http://dof.gob.mx/nota_detalle.php?codigo=4728925&fecha=15/08/1994 (accessed on 1 August 2021).
- Chen, Y.S.; Yanagida, F.; Shinohara, T. Isolation and identification of lactic acid bacteria from soil using an enrichment procedure. Lett. Appl. Microbiol. 2005, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Maifreni, M.; Rondinini, G. Microbiological characterization of artisanal Montasio cheese: Analysis of its indigenous lactic acid bacteria. FEMS Microbiol. Lett. 2003, 229, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Díaz, R.; González-Córdova, A.F.; Estrada-Montoya, M.D.C.; Méndez-Romero, J.I.; Mazorra-Manzano, M.A.; Soto-Valdez, H.; Vallejo-Cordoba, B. Volatile and sensory evaluation of Mexican Fresco cheese as affected by specific wild Lactococcus lactis strains. J. Dairy Sci. 2020, 103, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiran, P.; Kerdchoechuen, O.; Laohakunjit, N. Combined effects of fermentation and germination on nutritional compositions, functional properties and volatiles of maize seeds. J. Cereal Sci. 2016, 71, 207–216. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Lara-Núñez, A.; García-Ayala, B.B.; Garza-Aguilar, S.M.; Flores-Sánchez, J.; Sánchez-Camargo, V.A.; Bravo-Alberto, C.E.; Vázquez-Santana, S.; Vázquez-Ramos, J.M. Glucose and sucrose differentially modify cell proliferation in maize during germination. Plant Physiol. Biochem. 2017, 113, 20–31. [Google Scholar] [CrossRef]
- Gernah, D.I.; Ariahu, C.C.; Ingbian, E.K. Effects of malting and lactic fermentation on some chemical and functional properties of maize (Zea mays). Am. J. Food Technol. 2011, 6, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Ben Omar, N.; Ampe, F. Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 2000, 66, 3664–3673. [Google Scholar] [CrossRef] [Green Version]
- Cocolin, L.; Manzano, M.; Cantoni, C.; Comi, G. Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl. Environ. Microbiol. 2001, 67, 5113–5121. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Ruiz, G.; Guyot, J.P.; Ruiz-Teran, F.; Morlon-Guyot, J.; Wacher, C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: A functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003, 69, 4367–4374. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cataluña, A.; Elizaquível, P.; Carrasco, P.; Espinosa, J.; Reyes, D.; Wacher, C.; Aznar, R. Diversity and dynamics of lactic acid bacteria in Atole agrio, a traditional maize-based fermented beverage from South-Eastern Mexico, analysed by high throughput sequencing and culturing. Antonie Leeuwenhoek 2018, 111, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Espinoza, A.S. Identificación de la Capacidad Biotransformadora, de Bacterias Acido Lácticas Nativas de Tres Productos Artesanales (Queso Adobera, Tejuino y Pulque) para Convertir Acido Linoleico en Acido Linoleico Conjugado [Centro de Investigación y Asistencia en Tecnología del Estado de Jalisco]. 2017. Available online: https://ciatej.repositorioinstitucional.mx/jspui/bitstream/1023/426/1/Sandra Jimenez Espinoza.pdf (accessed on 8 August 2021).
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Chaves-López, C.; Rossi, C.; Maggio, F.; Paparella, A.; Serio, A. Changes occurring in spontaneous maize fermentation: An overview. Fermentation 2020, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Grijalva-Vallejos, N.; Aranda, A.; Matallana, E. Evaluation of yeasts from Ecuadorian chicha by their performance as starters for alcoholic fermentations in the food industry. Int. J. Food Microbiol. 2020, 317, 108462. [Google Scholar] [CrossRef]
- Ercolini, D. PCR-DGGE fingerprinting: Novel strategies for detection of microbes in food. J. Microbiol. Methods 2004, 56, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.; Fell, J.; Boekhout, T. The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C., Fell, J.W., Boekhout, T., Eds.; Elsevier Science Ltd: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Chan, G.F.; Gan, H.M.; Ling, H.L.; Rashid, N.A.A. Genome sequence of Pichia kudriavzevii M12, a potential producer of bioethanol and phytase. Eukaryot. Cell. 2012, 11, 1300–1301. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, L.; Abdelaliem, Y.F.; Mahfoz, S.A. Biodiesel production by Pichia occidentalis MH879824. Int. J. Curr. Microbiol. 2019, 8, 2975–2987. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Ciani, M.; Mannazzu, I.; Domizio, P. Microbiota of fermented beverages. Fermentation 2018, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- Dirzo, G.S.; Ferrer, C.E.L.; Valadez, M.F.; Garfias, A.L.J.; Rodríguez, J.A.A.; Cruz, E.J.M.; Chilpa, R.R. Estudio preliminar del Axokot, bebida tradicional fermentada, bajo una perspectiva transdisciplinaria. Investig. Univ. Multidiscip. Rev. Investig. La Univ. Simón Bolívar 2010, 9, 12. [Google Scholar]
- de la Fuente-Salcido, N.M.; Castañeda-Ramírez, J.C.; García-Almendárez, B.E.; Bideshi, D.K.; Salcedo-Hernández, R.; Barboza-Corona, J.E. Isolation and characterization of bacteriocinogenic lactic bacteria from M-Tuba and Tepache, two traditional fermented beverages in México. Food Sci. Nutr. 2015, 3, 434–442. [Google Scholar] [CrossRef]
- Ramos, C.L.; Schwan, R.F. Technological and nutritional aspects of indigenous Latin America fermented foods. Curr. Opin. Food Sci. 2017, 13, 97–102. [Google Scholar] [CrossRef]
- Ogodo, A.C.; Ugbogu, O.C.; Onyeagba, R.A.; Okereke, H.C. Effect of lactic acid bacteria consortium fermentation on the proximate composition and in-Vitro starch/protein digestibility of maize. Am. J. Microbiol. Biotechnol. 2017, 4, 35–43. [Google Scholar]
- Chaves, R.D.; Pradella, F.; Turatti, M.A.; Amaro, E.C.; da Silva, A.R.; dos Santos Farias, A.; Pereira, J.L.; Khaneghah, A.M. Evaluation of Staphylococcus spp. in food and kitchen premises of Campinas, Brazil. Food Control 2018, 84, 463–470. [Google Scholar] [CrossRef]
- Cheng, F. Volatile flavor compounds in yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Omemu, A.M.; Oyewole, O.B.; Bankole, M.O. Significance of yeasts in the fermentation of maize for ogi production. Food Microbiol. 2007, 24, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on Riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.K.; Kim, Y.S. Distinctive formation of volatile compounds in fermented rice inoculated by different molds, yeasts, and lactic acid bacteria. Molecules 2019, 24, 2123. [Google Scholar] [CrossRef] [Green Version]
- Vilela, J.D.A.S.; de Figueiredo Vilela, L.; Ramos, C.L.; Schwan, R.F. Physiological and genetic characterization of indigenous Saccharomyces cerevisiae for potential use in productions of fermented maize-based-beverages. Braz. J. Microbiol. 2020, 51, 1297–1307. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic higher alcohols in wine: Implication on aroma and palate attributes during Chardonnay aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Piao, Y.; Zhang, X.; Zhao, C.; Hou, Y.; Shi, Z. Analysis of volatile compounds from a malting process using headspace solid-phase micro-extraction and GC–MS. Food Res. Int. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Romero-Medina, A.; Estarrón-Espinosa, M.; Verde-Calvo, J.R.; Lelièvre-Desmas, M.; Escalona-Buendía, H.B. Renewing traditions: A sensory and chemical characterisation of mexican pigmented corn beers. Foods 2020, 9, 886. [Google Scholar] [CrossRef]
- Annan, N.T.; Poll, L.; Sefa-Dedeh, S.; Plahar, W.A.; Jakobsen, M. Volatile compounds produced by Lactobacillus fermentum, Saccharomyces cerevisiae and Candida krusei in single starter culture fermentations of Ghanaian maize dough. J. Appl. Microbiol. 2003, 94, 462–474. [Google Scholar] [CrossRef]
- Fadida, T.; Selilat-Weiss, A.; Poverenov, E. N-hexylimine-chitosan, a biodegradable and covalently stabilized source of volatile, antimicrobial hexanal. Next generation controlled-release system. Food Hydrocoll. 2015, 48, 213–219. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Zhang, P.; Ajlouni, S.; Fang, Z. Changes in phenolic content, antioxidant activity, and volatile compounds during processing of fermented sorghum grain tea. Cereal Chem. 2020, 97, 612–625. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Artisanal Tejuino | Commercial Tejuino | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | AR | AW | AY | AZ | TC | TL | TM | TT |
| 1 Moisture | 89.8 ± 0.22 bc | 92.66 ± 0.34 d | 89.73 ± 0.07 bc | 91.66 ± 1.20 cd | 87.70 ± 1.01 b | 84.65 ± 0.32 a | 88.29 ± 0.02 b | 83.35 ± 3.77 a |
| 1 TS | 10.2 ± 0.22 bc | 7.34 ± 0.34 a | 10.28 ± 0.07 bc | 8.34 ± 1.20 ab | 12.30 ± 1.01 c | 15.36 ± 0.33 d | 11.72 ± 0.02 c | 16.65 ± 3.77 d |
| 1 Protein | 18.60 ± 0.92 f | 27.05 ± 0.32 g | 16.50 ± 0.12 e | 6.17 ± 0.08 d | 2.84 ± 0.14 bc | 2.32 ± 0.25 b | 3.00 ± 0.25 c | 1.23 ± 0.01 a |
| 1 Fat | 7.67 ± 0.00 d | 9.01 ± 0.58 d | 5.67 ± 0.00 c | 4.85 ± 0.38 c | 4.72 ± 0.04 b | 4.23 ± 0.00 b | 4.02 ± 0.48 b | 2.04 ± 0.00 a |
| 1 CHOS * | 59.42 ± 1.25 d | 49.35 ± 0.10 e | 63.62 ± 0.14 c | 77.53 ± 1.23 ab | 79.27 ± 1.24 ab | 76.70 ± 0.97 b | 79.32 ± 0.69 ab | 79.52 ± 3.77 a |
| 1 Ash | 3.78 ± 0.57 d | 7.24 ±0.02 e | 4.01 ± 0.13 d | 2.73 ± 0.08 c | 0.87 ± 0.06 a | 2.39 ± 0.04 c | 1.93 ± 0.01 b | 0.56 ± 0.01 a |
| pH | 2.91 ± 0.01 a | 3.52 ± 0.01 c | 2.91 ± 0.01 a | 3.48 ± 0.01 b | 3.63 ± 0.01 d | 3.96 ± 0.01 e | 3.52 ± 0.01 c | 3.36 ± 0.01 b |
| Acidity ‡ | 0.4 ± 0.01 b | 0.76 ± 0.01 f | 0.68 ± 0.01 e | 1.22 ± 0.01 g | 0.27 ± 0.02 a | 0.57 ± 0.03 d | 0.55 ± 0.02 d | 0.47 ± 0.01 c |
| Artisanal Tejuino | Commercial Tejuino | |||||||
|---|---|---|---|---|---|---|---|---|
| Microorganisms | AR | AW | AY | AZ | TC | TL | TM | TT |
| MY | 8.33 ± 0.01 g | 8.23 ± 0.05 f | 8.47 ± 0.01 h | 7.32 ± 0.02 d | 7.26 ± 0.04 c | 6.76 ± 0.02 b | 7.93 ± 0.03 e | 4.74 ± 0.04 a |
| 44AMB | 8.66 ± 0.01 e | 8.23 ± 0.01 c | 8.40 ± 0.01 d | 7.27 ± 0.02 a | 7.15 ± 0.01 a | 7.11 ± 0.38 a | 7.59 ± 0.05 b | 7.17 ± 0.06 a |
| TCB * | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 |
| Lactic Acid Bacteria | ||||||||
| Lactobacillus spp. | 8.28 ± 0.02 c | 8.13 ± 0.03 c | 8.33 ± 0.01 c | 6.98 ± 0.01 a | 7.18 ± 0.03 a | 7.14 ± 0.33 a | 7.64 ± 0.01 b | 7.13 ± 0.01a |
| Lactococcus spp. | 8.46 ± 0.10 b | 8.22 ± 0.01 b | 8.47 ± 0.01 b | 7.19 ± 0.05 a | 7.28 ± 0.03 a | 6.86 ± 0.68 a | 7.42 ± 0.02 a | 7.15 ± 0.07 a |
| Streptococcus spp. | 8.11 ± 0.01 d | 8.18 ± 0.05 d | 8.46 ± 0.10 e | 7.18 ± 0.01 bc | 7.26 ± 0.01 bc | 6.87 ± 0.80 ab | 6.89 ± 0.11 b | 7.15 ± 0.07 bc |
| Commercial Tejuino | Artisanal Tejuino | ||||
|---|---|---|---|---|---|
| Strain | Identification | Accession | Strain | Identification | Accession |
| 1 | Pichia occidentalis | MN904761.1 | 4 | Saccharomyces cerevisiae | MT649488.1 |
| 2 | Pichia kudriavzevii | JF715184.1 | 5 | Saccharomyces cesevisiae | |
| 3 | Pichia kudriavzevii | 6 | Saccharomyces cerevisiae | ||
| 7 | Acetobacter orientalis | MT416429.1 | 11 | Lactococcus lactis | MT645510.1 |
| 9 | Enterococcus faecium | MK332450.1 | 17 | Lactiplantibacillus plantarum | CP050805.1 |
| 12 | Limosilactobacillus fermentum | 18 | Lactiplantibacillus plantarum | ||
| 13 | Limosilactobacillus fermentum | MT613608.1 | 20 | Lactiplantibacillus plantarum | |
| 14 | Limosilactobacillus fermentum | 10 | Enterococcus durans | MT604840.1 | |
| 15 | Limosilactobacillus fermentum | 27 | Enterococcus durans | ||
| 16 | Limosilactobacillus fermentum | 28 | Enterococcus hirae | KX752853.1 | |
| 21 | Staphylococcus warneri | MT642942.1 | |||
| 22 | Staphylococcus warneri | ||||
| 8 23 | Enterococcus durans Enterococcus durans | MT604840.1 | |||
| 24 | Enterococcus durans | ||||
| 25 | Enterococcus durans | ||||
| 26 | Enterococcus durans | ||||
| Artisanal Tejuino | Commercial Tejuino | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Volatile Compound | RT | AR | AW | AY | TC | TL | TM | TT |
| Esters | |||||||||
| 1 | Ethyl acetate | 6.01 | 0.104 ± 0.06 a | 0.081 ± 0.05 a | 1.041 ± 1.29 a | ND | 0.008 ± 0.01 a | 0.049 ± 0.02 a | 0.008 ± 0.01 a |
| 2 | Butanoic acid, ethyl ester | 10.2 | ND | ND | ND | ND | ND | ND | 0.010 ± 0.00 a |
| 3 | Pentanoic acid, 3-methyl-, ethyl ester | 21.36 | 0.008 ± 0.01 a | ND | 0.021 ± 0.00 a | ND | ND | ND | ND |
| 4 | Propanoic acid, 2-hydroxy-, ethyl ester, (S) | 26.4 | ND | ND | 0.005 ± 0.00 a | ND | ND | ND | ND |
| 5 | Octanoic acid, ethyl ester | 30.76 | 0.42 ± 0.18 a | 0.139 ± 0.07 a | ND | 0.907 ± 0.77 a | 0.381 ± 0.4 1 a | 0.019 ± 0.02 a | 0.005 ± 0.00 a |
| 6 | Nonanoic acid, ethyl ester | 34.86 | 0.026 ± 0.01 a | 0.0372 ± 0.05a | ND | 0.015 ± 0.01a | ND | ND | DN |
| 7 | Decanoic acid, methyl ester | 37.47 | ND | ND | ND | ND | ND | ND | DN |
| 8 | Decanoic acid, ethyl ester * | 39.19 | 0.998 ± 0.4 d | 0.046 ± 0.02 abc | 0.875 ± 0.06 cd | 0.252 ± 0.14 a | 0.753 ± 0.61 bcd | 0.035 ± 0.02 ab | 0.008 ± 0.00 bcd |
| 9 | Octanoic acid, 3-methylbutyl ester | 39.82 | 0.029 ± 0.01 | ND | ND | ND | ND | ND | ND |
| 10 | Butanedioic acid, diethyl ester | 40.58 | 0.005 ± 0.00 a | ND | ND | ND | 0.015 ± 0.00 a | ND | ND |
| 11 | Ethyl 9-decenoate | 40.68 | 0.027 ± 0.01 a | ND | ND | 0.044 ± 0.02 a | ND | 0.004 ± 0.00 a | ND |
| 12 | Undecanoic acid, methyl ester | 40.82 | ND | ND | ND | ND | ND | ND | ND |
| 13 | Benzeneacetic acid, ethyl ester | 44.12 | 0.008 ± 0.00 a | 0.024 ± 0.01 a | 0.023 ± 0.00 a | ND | ND | ND | ND |
| 14 | Acetic acid, 2-phenylethyl ester * | 45.45 | 0.107 ± 0.11 a | 0.175 ± 0.07 a | 0.491 ± 0.05 a | 0.148 ± 0.18 a | 0.349 ± 0.02 a | 0.998 ± 0.52 b | 0.043 ± 0.03 ab |
| 15 | Dodecanoic acid, ethyl ester | 46.84 | 0.430 ± 0.15 ab | 0.052 ± 0.02 ab | 0.516 ± 0.03 ab | 0.017 ± 0.01 a | 0.056 ± 0.01 b | 0.01 ± 0.01 b | 0.007 ± 0.00 b |
| 16 | Pentanoic acid, 3-methylbutyl ester | 48.25 | 0.017 ± 0.01 a | ND | 0.027 ± 0.02a | ND | ND | ND | ND |
| 17 | Benzenepropanoic acid, ethyl ester | 48.83 | 0.040 ± 0.02 a | ND | 0.218 ± 0.05a | ND | ND | ND | ND |
| 18 | 2(3H)-Furanone, dihydro-5-pentyl | 58.3 | 0.077 ± 0.04 a | 0.382 ± 0.14 a | ND | 0.31 ± 0.17 a | ND | ND | ND |
| 19 | Nonanoic acid, 9-oxo-, ethyl ester | 61.81 | 0.003 ± 0.00 a | ND | 0.002 ± 0.00 a | ND | ND | ND | ND |
| 20 | Diethyl suberate | 62.47 | 0.019 ± 0.01 | ND | ND | ND | ND | ND | ND |
| 21 | Pentadecanoic acid, ethyl ester | 63.97 | 0.004 ± 0.00 a | ND | 0.025 ± 0.00 a | ND | ND | ND | ND |
| 22 | Hexadecanoic acid, methyl ester | 68.38 | 0.008 ± 0.002 a | ND | ND | ND | ND | ND | ND |
| 23 | Hexadecanoic acid, ethyl ester * | 71.83 | 0.28 ± 0.15 a | 0.082 ± 0.02 a | 2.19 ± 0.36 b | 0.016 ± 0.01 a | 0.121 ± 0.02 a | 0.022 ± 0.00 a | 0.059 ± 0.04 a |
| 24 | Decanedioic acid, diethyl ester | 78.89 | 0.043 ± 0.02 | ND | ND | ND | ND | ND | ND |
| 25 | 8-Nonenoic acid, ethyl ester | 81.85 | 0.016 ± 0.01 | ND | ND | ND | ND | ND | ND |
| 26 | (E)-9-Octadecenoic acid ethyl ester | 98.55 | 0.298 ± 0.23 a | ND | 3.93 ± 0.69 b | ND | 0.098 ± 0.02 a | ND | 0.022 ± 0.01 a |
| Alcohols | |||||||||
| 27 | Ethanol* | 7.06 | 4.169 ± 1.56 b | 3.095 ± 1.72 ab | 4.404 ± 0.36 b | 4.21± 3.06 b | 0.9 ± 0.52 a | 1.67 ± 0.75 a | 1.038 ± 0.45 a |
| 28 | 1-Pentanol* | 20.34 | 0.101 ± 0.04 a | 0.135 ± 0.06 ª | 0.15 ± 0.01 a | 0.199 ± 0.18 a | 0.036 ± 0.01 a | 0.033 ± 0.02 a | 0.022 ± 0.01 a |
| 29 | 2,6-Octadien-1-ol,3,7-dimethyl-,acetate, (Z) | 41.62 | ND | ND | ND | ND | 0.029 ± 0.00 a | 0.001 ± 0.00 a | 0.009 ± 0.00 b |
| 30 | Benzyl alcohol | 48.24 | ND | 0.006 ± 0.00 | ND | ND | ND | ND | ND |
| 31 | Phenylethyl Alcohol * | 50.69 | 1.125 ± 0.43 cd | 2.096 ± 0.78 e | 1.597 ± 0.18 de | 0.901 ± 0.7 bc | 0.322 ± 0.06 ab | 0.225 ± 0.12 ab | 0.16 ± 0.01 a |
| Organic acids | |||||||||
| 32 | Alpha-pyrone-6-carboxylic acid | 28.95 | ND | ND | ND | 0.035 ± 0.03a | ND | 0.003 ± 0.00a | ND |
| 33 | Acetic acid | 30.2 | ND | 0.022 ± 0.02 a | 0.557 ± 0.04 a | ND | ND | 0.003 ± 0.00 a | 0.067 ± 0.04 a |
| 34 | Butanoic acid | 38.06 | ND | ND | ND | ND | ND | ND | 0.183 ± 0.04 a |
| 35 | Octanoic acid | 58.85 | 0.161 ± 0.07 a | 0.118 ± 0.12 a | 0.32 ± 0.04 a | 1.264 ± 0.92 b | 0.019 ± 0.02 a | 0.072 ± 0.08 a | 0.02 ± 0.00 a |
| 36 | Sorbic Acid | 63.57 | ND | ND | ND | ND | ND | ND | 0.023 ± 0.00 |
| 37 | n-Decanoic acid | 72.6 | 0.065 ± 0.02 b | 0.063 ± 0.02 ab | ND | 0.257 ± 0.14 a | ND | 0.054 ± 0.02 ab | 0.033 ± 0.03 ab |
| 38 | Benzenepropanoic acid, α-(1-hydroxyethyl) | 73.29 | 0.153 ± 0.06 a | ND | 0.286 ± 0.01 a | ND | ND | ND | ND |
| 39 | 9-Decenoic acid | 79.15 | ND | ND | ND | 0.0222 ± 0.01 | ND | ND | ND |
| 40 | Benzoic acid | 88.42 | ND | ND | ND | ND | 0.004 ± 0.00 a | ND | 0.154 ± 0.00 a |
| Terpenes | |||||||||
| 41 | β-Phellandrene | 12.64 | ND | ND | ND | ND | 0.002 ± 0.00 | ND | ND |
| 42 | β-Pinene | 15.93 | ND | ND | ND | ND | 0.012 ± 0.00 a | ND | 0.0048 ± 0.00 a |
| 43 | β-Myrcene | 16.02 | ND | ND | ND | ND | ND | 0.001 ± 0.00 | ND |
| 44 | D-Limonene | 17.56 | 0.012 ± 0.01 a | 0.003 ± 0.00 a | ND | ND | 0.299 ± 0.11 a | 0.026 ± 0.01 a | 0.096 ± 0.07 a |
| 45 | γ-Terpinene | 19.48 | ND | ND | ND | ND | 0.06 ± 0.01 | ND | ND |
| 46 | o-Cymene | 21.67 | ND | ND | ND | ND | 0.075 ± 0.01 a | ND | 0.007 ± 0.00 a |
| 47 | trans-α-Bergamotene | 36.17 | ND | ND | ND | ND | ND | 0.004 ± 0.00 a | 0.009 ± 0.00 a |
| 48 | Terpinen-4-ol | 37.81 | ND | ND | ND | ND | 0.008 ± 0.00 a | 0.005 ± 0.00 a | ND |
| 49 | L-α-Terpineol | 40.79 | ND | ND | ND | ND | 0.026 ± 0.00 a | 0.003 ± 0.00 a | 0.004a ± 0.00 a |
| 50 | β-Bisabolene | 41.29 | ND | ND | ND | ND | 0.145 ± 0.01 a | 0.023 ± 0.01 a | 0.042 ± 0.01 b |
| Aldehydes | |||||||||
| 51 | Hexanal | 12.62 | ND | 0.011 ± 0.00 a | ND | ND | ND | ND | ND |
| 52 | Nonanal | 29.6 | ND | 0.038 ± 0.01 a | ND | 0.014 ± 0.02 a | ND | ND | ND |
| 53 | 3-Furaldehyde | 32 | 0.006 ± 0.00 b | 0.013 ± 0.01 ab | ND | 0.068 ± 0.063 ab | ND | 0.002 ± 0.00 ab | 0.017 ± 0.01 a |
| 54 | 2,4-Heptadienal, (E,E) | 33.52 | ND | 0.025 ± 0.01 | ND | ND | ND | ND | ND |
| 55 | Benzaldehyde * | 34.11 | 0.046 ± 0.02 c | 0.172 ± 0.06 ab | 0.041 ± 0.00 bc | 0.17 ± 0.14 abc | 0.034 ± 0.00 abc | 0.007 ± 0.00 d | 0.013 ± 0.00 a |
| 56 | 2-Nonenal, (E) | 35.11 | ND | 0.078 ± 0.03a | ND | ND | ND | ND | ND |
| 57 | 2-Furancarboxaldehyde, 5-methyl- | 37.11 | 0.007 ± 0.00 b | 0.023 ± 0.01 ab | 0.006 ± 0.00 ab | 0.049 ± 0.04 ab | 0.004 ± 0.00 b | ND | 0.013 ± 0.00 a |
| 58 | 2-Decenal, (E) | 39.33 | ND | 0.107 ± 0.03 | ND | ND | ND | ND | ND |
| 59 | Benzaldehyde, 4-methyl | 39.58 | ND | 0.099 ± 0.04 a | 0.007 ± 0.00 a | 0.236 ± 0.18 a | ND | 0.014 ± 0.01 a | 0.027 ± 0.01 a |
| 60 | 2,4-Nonadienal, (E,E) | 41.23 | 0.005 ± 0.00 a | 0.111 ± 0.03 a | 0.003 ± 0.00 a | ND | ND | ND | ND |
| 61 | 2-Undecenal | 42.77 | ND | 0.057 ± 0.01 | ND | ND | ND | ND | ND |
| 62 | 3-Acetyl-1H-pyrroline | 55.11 | 0.002 ± 0.00 a | 0.008 ± 0.00 b | 0.003 ± 0.00 a | 0.032 ± 0.02 a | ND | ND | ND |
| Benzenes | |||||||||
| 63 | Toluene | 9.97 | ND | 0.002 ± 0.00 a | ND | 0.008 ± 0.01 a | 0.002 ± 0.00 a | ND | 0.001 ± 0.00 a |
| 64 | Pyridine | 19.4 | ND | ND | ND | ND | ND | ND | ND |
| 65 | Ether, 3-methyl-2-butenyl o-tolyl | 16.07 | ND | ND | ND | 0.0383 ± 0.03 | ND | ND | ND |
| 66 | Benzene, 1,1’-(1,2-cyclobutanediyl)bis-, cis | 21.69 | 0.014 ± 0.00 a | 0.014 ± 0.01 a | ND | ND | ND | ND | ND |
| 67 | Benzofuran, 2-methyl- | 37.19 | ND | ND | ND | ND | ND | 0.007 ± 0.00 | ND |
| 68 | Naphthalene | 42.33 | 0.003 ± 0.00 a | ND | 0.013 ± 0.00 a | 0.006 ± 0.01 a | ND | 0.021 ± 0.01 a | ND |
| 69 | Oxime-, methoxy-phenyl | 43.03 | 0.028 ± 0.01 ab | 0.022 ± 0.01 a | 0.168 ± 0.00 a | ND | 0.019 ± 0.02 ab | 0.008 ± 0.01 ab | 0.025 ± 0.00 a |
| 70 | Phenol, 2-methoxy | 47.29 | ND | 0.019 ± 0.01a | ND | 0.024 ± 0.01 a | ND | ND | ND |
| 71 | Mequinol | 47.57 | ND | ND | ND | ND | ND | ND | 0.002 ± 0.00 |
| 72 | Benzene, 1,4-diethoxy- | 51.56 | ND | 0.054 ± 0.02 | ND | ND | ND | ND | ND |
| 73 | Creosol | 53.49 | 0.069 ± 0.03 bcd | 0.796 ± 0.03 d | 0.022 ± 0.00 a | 0.033 ± 0.02 ab | 0.043 ± 0.01 abc | 0.002 ± 0.00 abc | 0.004 ± 0.00 abcd |
| 74 | Phenol, 3-methyl | 56.22 | ND | ND | ND | ND | ND | 0.005 ± 0.00 | ND |
| 75 | Phenol, 4-ethyl-2-methoxy- * | 57.54 | 0.222 ± 0.09 a | 0.59 ± 0.21 b | 0.283 ± 0.02 ab | 0.22 ± 0.13ab | 0.033 ± 0.00 ab | 0.031 ± 0.01 ab | 0.215 ± 0.01 a |
| 76 | p-Cresol | 59.66 | 0.006 ± 0.00 b | 0.032 ± 0.02 ab | 0.028 ± 0.00 ab | 0.005 ± 0.00 ab | 0.001 ± 0.00 a | 0.003 ± 0.00 ab | 0.003 ± 0.00 ab |
| 77 | Phenol, 3-ethyl | 59.79 | ND | 0.013 ± 0.00 ab | 0.012 ± 0.00 a | ND | ND | 0.004 ± 0.00 ab | ND |
| 78 | Phenol, 4-ethyl | 64.74 | 0.293 ± 0.11 ab | 0.55 ± 0.197 abc | 0.21 ± 0.02 a | 0.741 ± 0.49 c | 0.017 ± 0.00 a | 0.013 ± 0.01 bc | 0.072 ± 0.00 c |
| 79 | 2-Methoxy-4-vinylphenol | 66.73 | 0.08 ± 0.03 a | 0.1 ± 0.04 a | 1.428 ± 0.14 b | 0.446 ± 0.24 a | ND | ND | 0.004 ± 0.00 a |
| Others | |||||||||
| 80 | 2-Vinylfuran | 11.7 | ND | ND | ND | 0.005 ± 0.00 | ND | ND | ND |
| 81 | Methyl vinyl ketone | 14.95 | ND | ND | 0.008 ± 0.00 | ND | ND | ND | ND |
| 82 | Propene | 15.6 | ND | ND | ND | ND | ND | 0.001 ± 0.00 a | ND |
| 83 | Methanesulfonic anhydride | 19.52 | 0.001 ± 0.00 a | 0.013 ± 0.00 a | ND | 0.002 ± 0.00 a | ND | ND | ND |
| 84 | Furan, 2-pentyl | 20.62 | ND | ND | ND | 0.035 ± 0.04 a | ND | ND | ND |
| 85 | Cyclopentane, methyl | 27.6 | 0.005 ± 0.00 a | ND | ND | 0.063 ± 0.06 a | ND | ND | ND |
| 86 | Ethanone, 1-(2-furanyl) | 33.89 | ND | ND | ND | 0.06 ± 0.07 a | ND | 0.005 ± 0.01 a | ND |
| 87 | Cyclopropane, pentyl | 35.27 | 0.007 ± 0.00 a | 0.019 ± 0.01 a | ND | 0.138 ± 0.13 a | ND | ND | ND |
| 88 | Hexadecane | 37.52 | ND | 0.007 ± 0.00 | ND | ND | ND | ND | ND |
| 89 | Bicyclo[6.1.0]nonane, 9-(1-methylethylidene) | 40.31 | ND | 0.019 ± 0.01 | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Castillo, Á.E.; Méndez-Romero, J.I.; Reyes-Díaz, R.; Santiago-López, L.; Vallejo-Cordoba, B.; Hernández-Mendoza, A.; Sáyago-Ayerdi, S.G.; González-Córdova, A.F. Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification. Foods 2021, 10, 2446. https://doi.org/10.3390/foods10102446
Rubio-Castillo ÁE, Méndez-Romero JI, Reyes-Díaz R, Santiago-López L, Vallejo-Cordoba B, Hernández-Mendoza A, Sáyago-Ayerdi SG, González-Córdova AF. Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification. Foods. 2021; 10(10):2446. https://doi.org/10.3390/foods10102446
Chicago/Turabian StyleRubio-Castillo, Ángel Eduardo, José I. Méndez-Romero, Ricardo Reyes-Díaz, Lourdes Santiago-López, Belinda Vallejo-Cordoba, Adrián Hernández-Mendoza, Sonia G. Sáyago-Ayerdi, and Aarón F. González-Córdova. 2021. "Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification" Foods 10, no. 10: 2446. https://doi.org/10.3390/foods10102446
APA StyleRubio-Castillo, Á. E., Méndez-Romero, J. I., Reyes-Díaz, R., Santiago-López, L., Vallejo-Cordoba, B., Hernández-Mendoza, A., Sáyago-Ayerdi, S. G., & González-Córdova, A. F. (2021). Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification. Foods, 10(10), 2446. https://doi.org/10.3390/foods10102446