Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site, Collection and Storage
2.2. Biomass Drying Process
2.3. Aqueous Extraction
2.4. Proximate Composition
2.4.1. Determination of Moisture and Ash Content
2.4.2. Total Protein Content
2.4.3. Total Carbohydrates
2.4.4. Total Lipid Content
2.4.5. Fatty Acid Analysis
2.5. Indexes of Lipid Quality
2.6. Agar Extraction and Quantification
2.7. Antioxidant Capacity
2.7.1. DPPH Radical Scavenging Assay
2.7.2. Ferric Reducing Power Assay (FRAP)
2.7.3. ABTS Radical Scavenging Assay
2.7.4. Total Phenolic Compound Assay (TPC)
2.8. Statistical Analysis
3. Results
3.1. Proximate Analysis
3.2. Biomass Quality
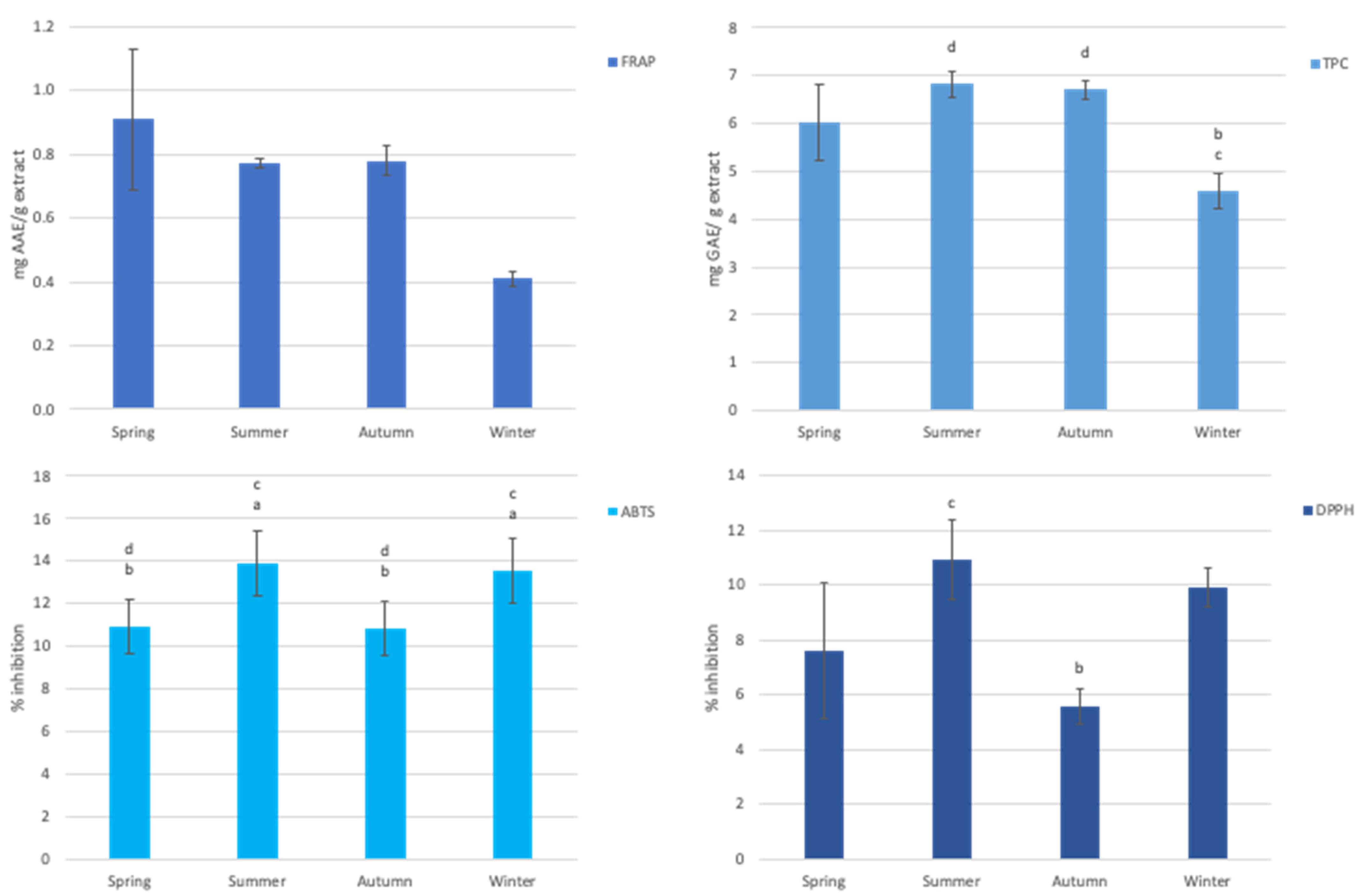
3.3. Antioxidant Capacity of Aqueous Extract of Gelidium Corneum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocha, J.; Peixe, L.; Gomes, N.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bio-prospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Fulton, C.J.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.; Wernberg, T.; Wilson, S.K. Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol. Oceanogr. 2014, 59, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Piñeiro-Corbeira, C.; Barreiro, R.; Cremades, J.; Arenas, F. Seaweed assemblages under a climate change scenario: Functional responses to temperature of eight intertidal seaweeds match recent abundance shifts. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.S.; Periyasamy, C.; Kumar, K.S.; Rao, A.S.; Anantharaman, P. Seaweeds: Distribution, Production and Uses. In Bioprospecting of Algae; Society for Plant Research India: Meerut, India, 2019; pp. 59–78. Available online: https://www.researchgate.net/publication/329504187 (accessed on 15 July 2021).
- Gamal, A.A.E. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Hiqashi-Okaj, K.; Otani, S.; Okai, Y. Potent suppressive effect of a Japanese edible seaweed, Enteromorpha prolifera (Sujiao-nori) on initiation and promotion phases of chemically induced mouse skin tumorigenesis. Cancer Lett. 1999, 140, 21–25. [Google Scholar] [CrossRef]
- Cassolato, J.E.; Noseda, M.D.; Pujol, C.A.; Pellizzari, F.M.; Damonte, E.B.; Duarte, M.E. Chemical structure and antiviral activity of the sulfated heterorhamnan isolated from the green seaweed Gayralia oxysperma. Carbohydr. Res. 2008, 343, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, N.C.; Catarino, M.D.; Silva, A.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L. Edible Seaweeds of the World, 1st ed.; Pereira, L., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 15–47. Available online: https://www.researchgate.net/publication/235767788_A_review_of_the_nutrient_composition_of_selected_edible_seaweeds (accessed on 15 July 2021).
- de Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Kolanjinathan, K.; Ganesh, P.; Saranraj, P. Pharmacological importance of seaweeds: A review. World J. Fish Mar. Sci. 2014, 6, 1–15. [Google Scholar] [CrossRef]
- Mendes, A.; Lopes da Silva, T.; Reis, A. DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol. Biotechnol. 2007, 45, 38–44. Available online: https://hrcak.srce.hr/30434 (accessed on 15 July 2021).
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innovative Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the Pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Covington, M. Omega-3 fatty acids. Am. Fam. Physician 2004, 70, 133–140. Available online: https://www.aafp.org/afp/2004/0701/p133.html (accessed on 15 July 2021). [PubMed]
- Hodge, L.; Salome, C.M.; Hughes, J.M.; Liu-Brennan, D.; Rimmer, J.; Allman, M.; Pang, D.; Armour, C.; Woolcock, A.J. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur. Respir. J. 1998, 11, 361–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Mancini Filho, J.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Jayasankar, R.; Kulandaivelu, G. Fatty acid profiles of marine red alga Gracilaria spp. (Rhodophyta, Gigar-tinales). Indian J. Mar. Sci. 1999, 28, 74–76. Available online: https://nopr.niscair.res.in/handle/123456789/25622 (accessed on 15 July 2021).
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Cardoso, M.; Carvalho, G.; Silva, J.; Rodrigues, S.; Pereira, R.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2021; Available online: http://www.algaebase.org (accessed on 15 July 2021).
- Almeida, A.R.G.S.D. Estudo da Flora Algológica do Intertidal da Praia da Vigia. Ph.D. Thesis, University of Algarve, Faro, Portugal, 2007. [Google Scholar]
- Lamouroux, J.V.F. Gelidium Corneum (Hudson). 2020. Available online: https://www.algaebase.org/search/species/detail/?species_id=377 (accessed on 15 July 2021).
- Cavaco, M.; Duarte, A.; Bernardino, S.; Afonso, C. Sustainable Use of Seaweeds from S. Martinho do Porto, Portugal–Past, Present, and Future Perspective; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The seaweed resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Rahikainen, M.; Yang, B. Macroalgae as food and feed ingredients in the Baltic Sea region–regulation by the European Union. Available online: https://www.submariner-network.eu/images/grass/GRASS_O3.4a_EU_regulation_of_seaweed_food_and_feed.pdf (accessed on 16 July 2021).
- Hegde, M.V.; Zanwar, A.A.; Adekar, S.P. (Eds.) Omega-3 Fatty Acids: Keys to Nutritional; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- FDA, Food and Drug Administration. National Institutes of Health, Health and Hunan Services. 2021. Available online: https://fdasis.nlm.nih.gov/srs/auto/gelidium%20corneum (accessed on 21 June 2021).
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- de Quirós, A.R.B.; Frecha-Ferreiro, S.; Vidal-Pérez, A.M.; López-Hernández, J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010, 231, 495–498. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Sánchez-Meraz, M.A.; Gamboa-Gómez, C.I. Hypoglycemic and antioxidant effects of five commercial turmeric (Curcuma longa) supplements. J. Food Biochem. 2020, 44, e13389. [Google Scholar] [CrossRef]
- Fatima, M.R.; Dinesh, S.; Mekata, T.; Itami, T.; Sudhakaran, R. Therapeutic efficiency of Portieria hornemannii (Rhodophyta) against Vibrio parahaemolyticus in experimentally infected Oreochromis mossambicus. Aquaculture 2016, 450, 369–374. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. Appendix D, Guidelines for collaborative study procedures to validate characteristics of a method of analysis. In Official Methods of Analysis of AOAC International, 20th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Soares, C.; Sousa, S.; Machado, S.; Vieira, E.; Carvalho, A.P.; Ramalhosa, M.J.; Morais, S.; Correia, M.; Oliva-Teles, T.; Domingues, V.; et al. Bioactive Lipids of Seaweeds from the Portuguese North Coast: Health Benefits versus Potential Contamination. Foods 2021, 10, 1366. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Adão, P.; Reboleira, J.; Teles, M.; Santos, B.; Ribeiro, N.; Teixeira, C.M.; Guedes, M.; Pessoa, J.C. Bernardino, S. Enhancement of the Antioxidant and Antimicrobial Activities of Porphyran through Chemical Modification with Tyrosine Derivatives. Molecules 2021, 26, 2916. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomo-lybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Figueroa, F.L.; Korbee, N. Interactive effects of UV radiation and nutrients on ecophysiology: Vulnerability and adaptation to climate change. In Seaweeds and Their Role in Globally Changing Environments; Springer: Dordrecht, The Netherlands, 2010; pp. 157–182. [Google Scholar] [CrossRef]
- Quintano, E.; Díez, I.; Muguerza, N.; Figueroa, F.L.; Gorostiaga, J.M. Depth influence on biochemical performance and thallus size of the red alga Gelidium corneum. Mar. Ecol. 2018, 39, e12478. [Google Scholar] [CrossRef]
- Quintano, E.; Ganzedo, U.; Díez, I.; Figueroa, F.L.; Gorostiaga, J.M. Solar radiation (PAR and UVA) and water temperature in relation to biochemical performance of Gelidium corneum (Gelidiales, Rhodophyta) in subtidal bottoms off the Basque coast. J. Sea Res. 2013, 83, 47–55. [Google Scholar] [CrossRef]
- Rasyid, A.; Ardiansyah, A.; Pangestuti, R. Nutrient composition of dried seaweed Gracilaria gracilis. Indones. J. Mar. Sci. 2019, 24, 1–6. [Google Scholar] [CrossRef]
- Rasyid, A.; Handayani, T. Evaluation of the biochemical composition of tropical red seaweeds Galaxaura rugosa and Gelidiella acerosa from Ujung Genteng waters, Indonesia. Aquac. Aquar. Conserv. Legis. 2019, 12, 601–609. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thor-kelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Barral-Martínez, M.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Tailoring hybrid carrageenans from Mastocarpus stellatus red seaweed using microwave hydrodiffusion and gravity. Carbohydr. Polym. 2020, 248, 116830. [Google Scholar] [CrossRef] [PubMed]
- Gamero-Vega, G.; Palacios-Palacios, M.; Quitral, V. Nutritional Composition and Bioactive Compounds of Red Seaweed: A Mini-Review. J. Food Nutr. Res. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Jang, K.H.; Lee, D.; Kang, H.R.; Kim, T.Y.; Lee, B.H.; Choi, B.W.; Kim, S.; Shin, J. Monoglycerides from the brown alga Sargassum sagamianum: Isolation, synthesis, and biological activity. Bioorganic Med. Chem. Lett. 2008, 18, 3589–3592. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative evaluation and selection of a method for lipid and fatty acid ex-traction from macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia turuturu. J. Appl. Phycol. 2013, 25, 425–432. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Nichols, P.D. Seasonal lipid composition in macroalgae of the northeastern Pacific Ocean. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- Liang, Y.; Wen, Z. Bio-based nutraceuticals from biorefining. In Advances in Biorefineries; Woodhead Publishing: Cambridge, UK, 2014; pp. 596–623. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Lopez-Hernandez, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ganesan, K.; Rao, P.S. Seasonal variation in nutritional composition of Kappaphycus alvarezii (Doty) Doty—An edible seaweed. J. Food Sci. Technol. 2015, 52, 2751–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngan, Y.; Price, I.R. Seasonal growth and reproduction of intertidal algae in the Townsville region (Queensland, Australia). Aquat. Bot. 1980, 9, 117–134. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.R.K.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional value of selected macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and functional bioactivity value of selected Azorean macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Cérantola, S.; Breton, F.; Gall, E.A.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Montero, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Application of Hansen solubility approach for the subcritical and supercritical selective extraction of phlorotannins from Cystoseira abies-marina. RSC Adv. 2016, 6, 94884–94895. [Google Scholar] [CrossRef]
- Patarra, R.F.; Iha, C.; Pereira, L.; Neto, A.I. Concise review of the species Pterocladiella capillacea (SG Gmelin) Santelices Hommersand. J. Appl. Phycol. 2019, 1–22. [Google Scholar] [CrossRef]
- Silva, P.; Pereira, L. Concise review of Osmundea pinnatifida (Hudson) Stackhouse. J. Appl. Phycol. 2020, 32, 2761–2771. [Google Scholar] [CrossRef]
- Martínez, B.; Rico, J.M. Seasonal variation of p content and major n pools in Palmaria palmata (Rhodophyta) 1. J. Phycol. 2002, 38, 1082–1089. [Google Scholar] [CrossRef] [Green Version]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.P.; Villaume, C.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Lemus, A.; Bird, K.; Kapraun, D.F.; Koehn, F. Agar yield, quality and standing crop biomass of Gelidium serrulatum, Gelidium floridanum and Pterocladia capillacea in Venezuela. Food Hydrocoll. 1991, 5, 469–479. [Google Scholar] [CrossRef]
- Rabiei, R.; Phang, S.M.; Lim, P.E.; Salleh, A.; Sohrabipour, J.; Ajdari, D.; Zarshenas, G.A. Productivity, biochemical composition and biofiltering performance of agarophytic seaweed, Gelidium elegans (Red algae) grown in shrimp hatchery effluents in Malaysia, Iran. J. Fish. Sci. 2016, 15, 53–74. [Google Scholar]
- Li, Y.; Zhao, M.; Gomez, L.P.; Senthamaraikannan, R.; Padamati, R.B.; O’Donnell, C.P.; Tiwari, B.K. Investigation of enzyme-assisted methods combined with ultra-sonication under a controlled alkali pretreatment for agar extraction from Gelidium sesquipedale. Food Hydrocoll. 2021, 120, 106905. [Google Scholar] [CrossRef]
- Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Effect of alkali treatment time and ex-traction time on agar from Gracilaria vermiculophylla. In Nineteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2007; pp. 65–69. [Google Scholar] [CrossRef]
- Kumar, V.; Fotedar, R. Agar extraction process for Gracilaria cliftonii (Withell, Millar, Kraft, 1994). Carbohydr. Polym. 2009, 78, 813–819. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatan, Mexico. J. Appl. Phycol. 1997, 9, 533–539. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [Green Version]
- Rødde, R.S.H.; Vårum, K.M.; Larsen, B.A.; Myklestad, S.M. Seasonal and geographical variation in the chemical composition of the red alga Palmaria palmata (L.) Kuntze. Bot. Mar. 2004, 47, 125–133. [Google Scholar] [CrossRef]
- Robledo, D.; Freile-Pelegrin, Y. Chemical and mineral composition of six potentially edible seaweed species of Yucatan. Bot. Mar. 1997, 40, 301–306. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Santelices, B. Production ecology of Gelidium. In International Workshop on Gelidium; Springer: Dordrecht, The Netherlands, 1991; pp. 31–44. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res.-Granthaalayah 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Banach, J.L.; Hoek-van den Hil, E.F.; van der Fels-Klerx, H.J. Food safety hazards in the European seaweed chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- Yeh, T.S.; Hung, N.H.; Lin, T.C. Analysis of iodine content in seaweed by GC-ECD and estimation of iodine intake. J. Food Drug Anal. 2014, 22, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Gomes, A.; Cardoso, C.; Afonso, C.; Campos, A.M.; Gomes, R.; Falé, P.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Commercial Red Seaweed in Portugal (Gelidium sesquipedale and Pterocladiella capillacea, Florideophyceae): Going beyond a single-purpose product approach by valorizing bioactivity. Thalass. Int. J. Mar. Sci. 2020, 36, 213–224. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of red algae from Jeju Island. Algae 2006, 21, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Murugan, K.; Iyer, V.V. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine algae. Vitro Cell. Dev. Biol.-Anim. 2013, 49, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient ex-traction and antioxidant capacity of mycosporine-like amino acids from red alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Incharoensakdi, A.; Häder, D.P.; Sinha, R.P. Ultra-violet radiation-induced generation of reactive oxygen species, DNA damage and in-duction of UV-absorbing compounds in the cyanobacterium Rivularia sp. HKAR-4. S. Afr. J. Bot. 2014, 90, 163–169. [Google Scholar] [CrossRef] [Green Version]

| Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|
| Moisture (% fw) | 52.53 ± 1.99 bc | 62.69 ± 1.21 a | 63.19 ± 0.43 a | 45.61 ± 8.22 |
| Ash (% fw) | 12.68 ± 0.73 | 11.30 ± 0.84 d | 14.12 ± 0.85 | 13.21 ± 0.45 b |
| Protein (%) | 10.44 ± 0.19 bcd | 14.61 ± 0.71 acd | 16.25 ± 0.33 ab | 15.47 ± 0.21 ab |
| Lipids (%) | 2.75 ± 0.28 abd | 2.16 ± 0.10 ad | 2.57 ± 0.04 d | 0.93 ± 0.04 abc |
| Agar (%) | 5.99 ± 0.88 | 6.33 ± 2.43 | 6.01 ± 1.90 | 8.70 ± 0.97 |
| Carbohydrates (%) | 39.50 ± 3.29 c | 29.78 ± 1.60 d | 24.84 ± 5.06 ad | 39.08 ± 2.71 bc |
| Spring | Summer | Autumn | Winter | ||
|---|---|---|---|---|---|
| Tridecyclic acid | C13:0 | 0.55 ± 0.18 | 0.60 ± 0.16 | 0.87 ± 0.28 | 0.67 ± 0.09 |
| Myristic acid | C14:0 | 5.86 ± 0.24 bd | 4.13 ± 0.13 acd | 6.33 ± 0.14 b | 8.49 ± 0.17 abc |
| Palmitic acid | C16:0 | 45.97 ± 0.53 bcd | 42.79 ± 0.33 acd | 56.07 ± 0.93 abd | 63.33 ± 0.99 abc |
| Stearic acid | C18.0 | 2.26 ± 1.26 | 1.47 ± 1.00 | 1.46 ± 0.03 | 2.03 ± 0.18 |
| Sum SFA% | 55.05 ± 1.14 bcd | 49.36 ± 0.70 acd | 64.46 ± 0.90 abd | 75.12 ± 0.67 abc | |
| Myristoleic acid | C14:1 | 0.37 ± 0.13 | 0.44 ± 0.07 | 0.85 ± 0.00 | 0.52 ± 0.03 |
| Palmitoleic acid | C16:1 n-7 | 1.46 ± 0.01 b | 0.899 ± 0.08 acd | 1.08 ± 0.23 b | 1.42 ± 0.12 b |
| Oleic acid | C18:1 n-9 | 7.23 ± 0.55 b | 5.47 ± 0.06 acd | 7.56 ± 0.28 b | 7.48 ± 0.14 b |
| Sum MUFA% | 11.03 ± 0.27 b | 8.08 ± 0.34 acd | 11.06 ± 1.17 b | 11.05 ± 0.67 b | |
| Linoleic acid | C18:2 n-6 | 0.94 ± 0.38 | 0.75 ± 0.23 | 0.71 ± 0.07 | 0.52 ± 0.04 |
| Arachidonic acid | C20:4 n-6 | 14.68 ± 1.48 bcd | 18.87 ± 0.81 acd | 10.98 ± 0.78 abd | 5.95 ± 0.18 abc |
| Eicosapentaenoic acid | C20:5 n-3 | 17.64 ± 0.14 bcd | 22.68 ± 0.51 acd | 9.49 ± 0.28 abd | 6.87 ± 0.29 abc |
| Sum PUFA% | 33.92 ± 1.18 bcd | 42.56 ± 1.04 acd | 21.18 ± 1.07 abd | 13.83 ± 0.36 abc | |
| Nutritional Indexes | |||||
| Σ n-3 | 17.91 ± 0.13 bcd | 22.68 ± 0.51 acd | 9.49 ± 0.28 abd | 7.08 ± 0.23 abc | |
| Σ n-6 | 16.01 ± 1.07 bcd | 19.88 ± 0.61 acd | 11.9 ± 0.79 abd | 6.74 ± 0.16 abc | |
| AI index | 1.15 ± 0.02 bcd | 0.93 ± 0.01 acd | 1.94 ± 0.10 abd | 2.89 ± 0.09 abc | |
| TI Index | 0.82 ± 0.02 | 0.60 ± 0.01 | 1.64 ± 0.10 | 2.54 ± 0.05 | |
| n-6/n-3 ratio | 0.89 ± 0.05 c | 0.88 ± 0.02 c | 1.23 ± 0.05 abd | 0.95 ± 0.02 c | |
| h/H ratio | 0.87 ± 0.01 bcd | 1.08 ± 0.01 acd | 0.52± 0.02 abd | 0.35± 0.01 abc | |
| Iodine and Contaminants (mg kg−1 dw) | Spring (June) | Autumn (November) |
|---|---|---|
| Lead | 0.24 ± 0.12 | 0.52 ± 0.16 |
| Arsenic | 1.50 ± 0.1 | 1.60 ± 0.28 |
| Cadmium | 0.18 ± 0.02 | 0.12 ± 0.04 |
| Mercury | <0.007 | <0.007 |
| Tin | <0.5 | <0.5 |
| Iodine | 236.67 ± 0.12 c | 150.00 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaco, M.; Duarte, A.; Freitas, M.V.; Afonso, C.; Bernardino, S.; Pereira, L.; Martins, M.; Mouga, T. Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods 2021, 10, 2394. https://doi.org/10.3390/foods10102394
Cavaco M, Duarte A, Freitas MV, Afonso C, Bernardino S, Pereira L, Martins M, Mouga T. Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods. 2021; 10(10):2394. https://doi.org/10.3390/foods10102394
Chicago/Turabian StyleCavaco, Mário, Adriana Duarte, Marta V. Freitas, Clélia Afonso, Susana Bernardino, Leonel Pereira, Mendelson Martins, and Teresa Mouga. 2021. "Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal" Foods 10, no. 10: 2394. https://doi.org/10.3390/foods10102394











