Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
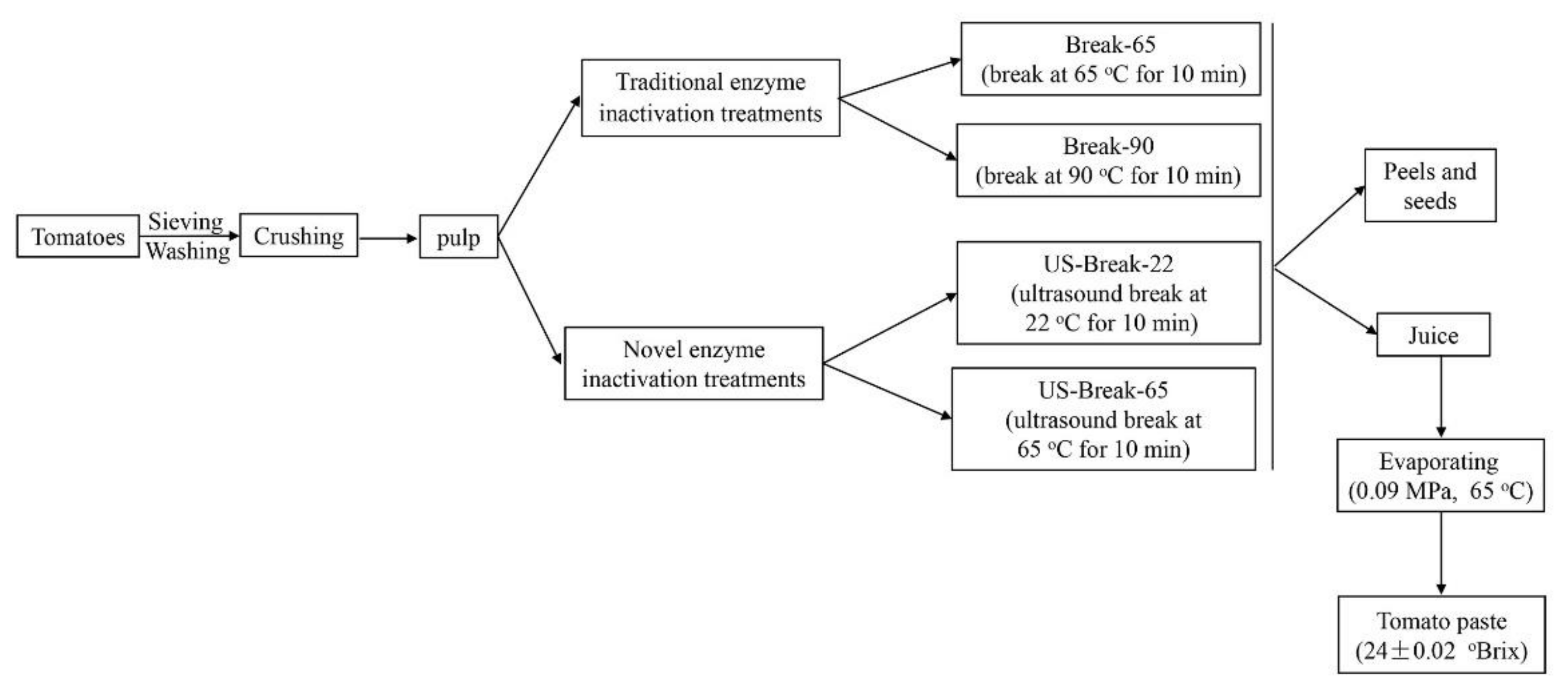
2.2. Tomato Paste Preparation
2.3. Physical Characterization of the Tomato Pastes
2.3.1. Particle Size Distribution of the Tomato Pastes
2.3.2. Rheological Properties of the Tomato Pastes
2.4. Chemical Characterization of the Tomato Pastes
2.4.1. Enzyme Extraction and Analysis
2.4.2. Serum Pectin of the Tomato Pastes
2.4.3. Ascorbic Acid in the Tomato Pastes
2.4.4. Phenolic Compounds in the Tomato Pastes
2.4.5. Carotenoids in the Tomato Pastes
2.5. Hydrophilic and Lipophilic Antioxidant Activities of the Tomato Pastes
2.6. Statistical Analyses
3. Results and Discussion
3.1. Effects of Different Breaking Treatments on PME and PG
3.2. Effects of Different Breaking Treatments on the Content and Physico-Chemical Properties of Serum Pectin
3.2.1. Pectin Content
3.2.2. Degree of Methyl-Esterification (DM) of Serum Pectin
3.2.3. Average Molecular Weight (Mw) of Serum Pectin
3.2.4. Chemical Properties of Serum Pectin
3.3. Effects of Different Breaking Treatments on Particle Size Distribution of Tomato Pastes
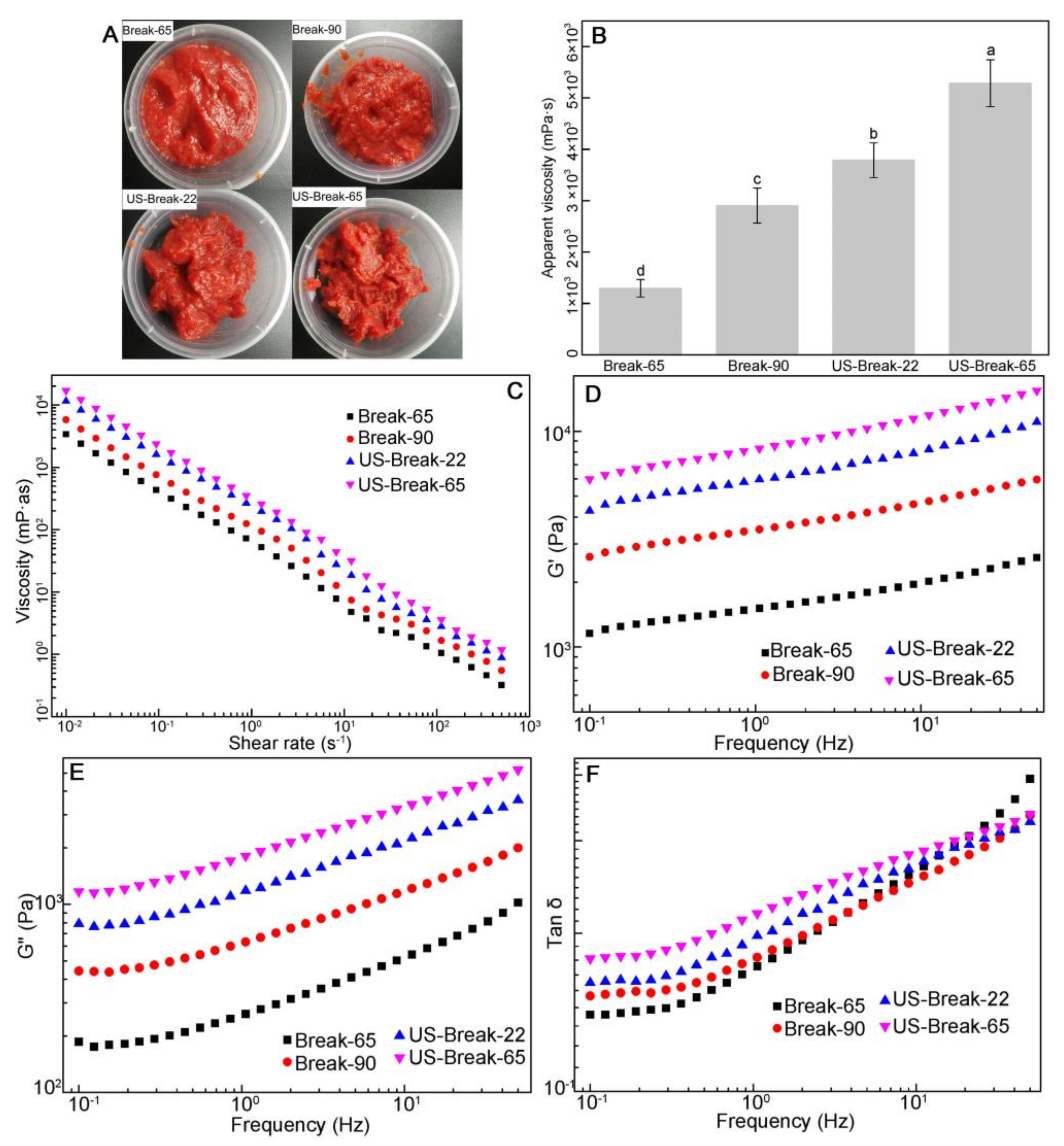
3.4. Effects of Different Breaking Treatments on the Viscosity and Rheological Properties of Tomato Pastes
3.4.1. The Viscosity of Tomato Pastes
3.4.2. The Rheological Properties of the Tomato Pastes
Flow Behavior of the Tomato Pastes
Dynamic Rheological Properties of the Tomato Pastes
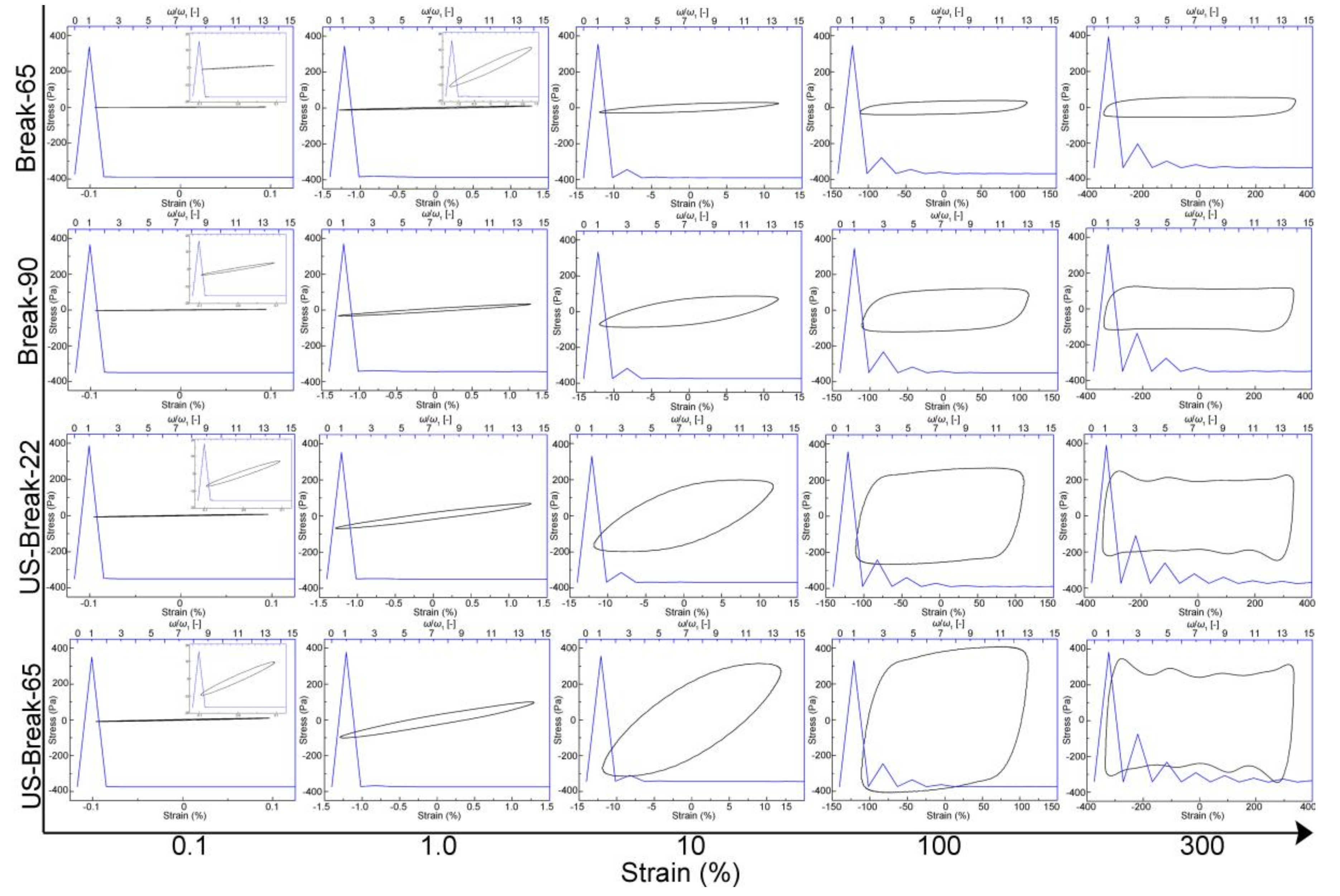
Large-Amplitude Oscillation Shear (LAOS) Behavior of the Tomato Pastes
3.4.3. The Mechanism Underling the Effects of Different Breaking Treatments on the Viscosity and Rheological Properties of Tomato Pastes
3.5. Effects of Different Breaking Treatments on the Nutritional Qualities of Tomato Pastes
3.5.1. Levels of Ascorbic Acid
3.5.2. Levels of Phenolics Compounds
3.5.3. Levels of Carotenoids
3.5.4. Antioxidant Activity of the Tomato Pastes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tulipani, S.; Huelamo, M.M.; Ribalta, M.R.; Estruch, R.; Ferrer, E.E.; Andres-Lacueva, C.; Illan, M.; Lamuela-Raventós, R.M. Oil matrix effects on plasma exposure and urinary excretion of phenolic compounds from tomato sauces: Evidence from a human pilot study. Food Chem. 2012, 130, 581–590. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Farjadian, F.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; de Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Andreou, V.; Dimopoulos, G.; Katsaros, G.; Taoukis, P. Comparison of the application of high pressure and pulsed electric fields technologies on the selective inactivation of endogenous enzymes in tomato products. Innov. Food Sci. Emerg. Technol. 2016, 38, 349–355. [Google Scholar] [CrossRef]
- Fachin, D.; van Loey, A.M.; Nguyen, B.L.; Verlent, I.; Indrawati, E.; Hendrickx, M. Inactivation kinetics of polygalacturonase in tomato juice. Innov. Food Sci. Emerg. Technol. 2003, 4, 135–142. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.; Trigueros-Andrés, E.; Beltrán, S.; Melgosa, R. Effect of high pressure carbon dioxide on tomato juice: Inactivation kinetics of pectin methylesterase and polygalacturonase and determination of other quality parameters. J. Food Eng. 2018, 239, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.; Fawcett, S.; Barringer, S. Flavor, viscosity, and color analyses of hot and cold break tomato juices. J. Food Sci. 2002, 67, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Santiago, J.S.J.; Kermani, Z.J.; Xu, F.; van Loey, A.; Hendrickx, M.E. The effect of high pressure homogenization and endogenous pectin-related enzymes on tomato purée consistency and serum pectin structure. Innov. Food Sci. Emerg. Technol. 2017, 43, 35–44. [Google Scholar] [CrossRef]
- Wu, J.; Gamage, T.; Vilkhu, K.; Simons, L.; Mawson, R. Effect of thermosonication on quality improvement of tomato juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 186–195. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Illera, A.E.; Sanz, M.; Benito-Román, O.; Varona, S.; Beltrán, S.; Melgosa, R.; Solaesa, A. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov. Food Sci. Emerg. Technol. 2018, 47, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Terefe, N.S.; Gamage, M.; Vilkhu, K.; Simons, L.; Mawson, R.; Versteeg, C. The kinetics of inactivation of pectin methylesterase and polygalacturonase in tomato juice by thermosonication. Food Chem. 2009, 117, 20–27. [Google Scholar] [CrossRef]
- Gao, R.; Ye, F.; Wang, Y.; Lu, Z.; Yuan, M.; Zhao, G. The spatial-temporal working pattern of cold ultrasound treatment in improving the sensory, nutritional and safe quality of unpasteurized raw tomato juice. Ultrason. Sonochem. 2019, 56, 240–253. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 58, 104609. [Google Scholar] [CrossRef]
- Campoli, S.S.; Rojas, M.L.; do Amaral, J.E.P.G.; Canniatti-Brazaca, S.G.; Augusto, P.E.D. Ultrasound processing of guava juice: Effect on structure, physical properties and lycopene in vitro accessibility. Food Chem. 2018, 268, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of straw-berry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Margulis, M.; Margulis, I. Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrason. Sonochem. 2003, 10, 343–345. [Google Scholar] [CrossRef]
- Tan, J.; Kerr, W.L. Rheological properties and microstructure of tomato puree subject to continuous high pressure homogeni-zation. J. Food Eng. 2015, 166, 45–54. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, K.; Zhang, Z.; Liu, F.; Hu, H.; Pan, S. Changes on the rheological properties of pectin-enriched mango nectar by high intensity ultrasound. LWT 2018, 91, 414–422. [Google Scholar] [CrossRef]
- Duvarci, O.C.; Yazar, G.; Kokini, J.L. The SAOS, MAOS and LAOS behavior of a concentrated suspension of tomato paste and its prediction using the Bird-Carreau (SAOS) and Giesekus models (MAOS-LAOS). J. Food Eng. 2017, 208, 77–88. [Google Scholar] [CrossRef]
- Augusto, P.E.; Ibarz, A.; Cristianini, M. Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: Viscoelastic properties and the Cox–Merz rule. J. Food Eng. 2013, 114, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wen, C.; Wang, M.; Wang, S.; Dong, N.; Lei, Z.; Lin, S.; Zhu, B. Enhancing the hardness of potato slices after boiling by combined treatment with lactic acid and calcium chloride: Mechanism and optimization. Food Chem. 2019, 308, 124832. [Google Scholar] [CrossRef]
- Gao, R.; Ye, F.; Lu, Z.; Wang, J.; Shen, X.L.; Zhao, G. A novel two-step ultrasound post-assisted lye peeling regime for tomatoes: Reducing pollution while improving product yield and quality. Ultrason. Sonochem. 2018, 45, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.D.; Kokini, J. Rheological properties and conformation of tomato paste pectins, citrus and apple pectins. J. Food Sci. 1987, 52, 1658–1664. [Google Scholar] [CrossRef]
- Adekunte, A.; Tiwari, B.; Cullen, P.; Scannell, A.; O’Donnell, C. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Andres-Lacueva, C.; Waterhouse, A.; Lamuela-Raventos, R.M. Effect of tomato industrial processing on phenolic profile and hydrophilic antioxidant capacity. LWT 2012, 47, 154–160. [Google Scholar] [CrossRef]
- Rigano, M.M.; Raiola, A.; Tenore, G.C.; Monti, D.M.; del Giudice, R.; Frusciante, L.; Barone, A. Quantitative trait loci pyra-miding can improve the nutritional potential of tomato (Solanum lycopersicum) fruits. J. Agric. Food Chem. 2014, 62, 11519–11527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, K.-C. Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. LWT 2008, 41, 450–459. [Google Scholar] [CrossRef]
- Raviyan, P.; Zhang, Z.; Feng, H. Ultrasonication for tomato pectinmethylesterase inactivation: Effect of cavitation intensity and temperature on inactivation. J. Food Eng. 2005, 70, 189–196. [Google Scholar] [CrossRef]
- Crelier, S.; Robert, M.-C.; Claude, J.; Juillerat, M.-A. Tomato (Lycopersicon esculentum) pectin methylesterase and polygalac-turonase behaviors regarding heat- and pressure-induced inactivation. J. Agric. Food Chem. 2001, 49, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Vercet, A.; Lopez, P.; Burgos, J. Free radical production by manothermosonication. Ultrasonics 1998, 36, 615–618. [Google Scholar] [CrossRef]
- Vercet, A.; Sánchez, C.; Burgos, J.; Montañés, L.; Lopez Buesa, P. The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. J. Food Eng. 2002, 53, 273–278. [Google Scholar] [CrossRef]
- Lin, H.; Aizawa, K.; Inakuma, T.; Yamauchi, R.; Kato, K. Physical properties of water-soluble pectins in hot- and cold-break tomato pastes. Food Chem. 2005, 93, 403–408. [Google Scholar] [CrossRef]
- Luh, B.; Daoud, H. Effect of break temperature yd holding time on pectin and pectic enzyme in tomato pulp. J. Food Sci. 1971, 36, 1039–1043. [Google Scholar] [CrossRef]
- Ciruelos, A.; Gonzalez, C.; Latorre, A.; Ruiz, R.; Rodriguez, A. Effect of heat treatment on the pectins of tomatoes during tomato paste manufacturing. Acta Hortic. 2001, 542, 181–186. [Google Scholar] [CrossRef]
- Santiago, J.S.J.; Christiaens, S.; van Loey, A.M.; Hendrickx, M.E. Deliberate processing of carrot purées entails tailored serum pectin structures. Innov. Food Sci. Emerg. Technol. 2016, 33, 515–523. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, S.; van Buggenhout, S.; Houben, K.; Chaula, D.; van Loey, A.; Hendrickx, M.E. Unravelling process-induced pectin changes in the tomato cell wall: An integrated approach. Food Chem. 2012, 132, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, M.C.; Greve, L.C.; Labavitch, J.M. Changes in cell wall pectins accompanying tomato (Lycopersicon esculentum Mill.) paste manufacture. J. Agric. Food Chem. 2002, 50, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Houben, K.; Jolie, R.P.; Fraeye, I.; van Loey, A.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Lin, H.; Qin, X.; Aizawa, K.; Inakuma, T.; Yamauchi, R.; Kato, K. Chemical properties of water-soluble pectins in hot- and cold-break tomato pastes. Food Chem. 2005, 93, 409–415. [Google Scholar] [CrossRef]
- Hwang, J.; Pyun, Y.; Kokini, J. Sidechains of pectins: Some thoughts on their role in plant cell walls and foods. Food Hydrocoll. 1993, 7, 39–53. [Google Scholar] [CrossRef]
- Wu, B.; Patel, B.; Fei, X.; Jones, O.; Campanella, O.; Reuhs, B. Variations in physical-chemical properties of tomato suspensions from industrial processing. LWT 2018, 93, 281–286. [Google Scholar] [CrossRef]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Hemar, Y.; Balaban, M.O.; Liao, X. The effect of ultrasound on particle size, color, viscosity and polyphenol oxidase activity of diluted avocado puree. Ultrason. Sonochem. 2015, 27, 567–575. [Google Scholar] [CrossRef]
- Fito, P.; Clemente, G.; Sanz, F. Rheological behaviour of tomato concentrate (hot break and cold break). J. Food Eng. 1983, 2, 51–62. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Valencia, C.; Gallegos, C.; Ciruelos, A.; Latorre, A. Influence of processing on the rheological properties of tomato paste. J. Sci. Food Agric. 2002, 82, 990–997. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of non-linear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Szopinski, D.; Luinstra, G.A. Viscoelastic properties of aqueous guar gum derivative solutions under large amplitude oscillatory shear (LAOS). Carbohydr. Polym. 2016, 153, 312–319. [Google Scholar] [CrossRef]
- Anvari, M.; Tabarsa, M.; Joyner, H.S. Large amplitude oscillatory shear behavior and tribological properties of gum extracted from Alyssum homolocarpum seed. Food Hydrocoll. 2018, 77, 669–676. [Google Scholar] [CrossRef]
- Giner, J.; Gimeno, V.; Espachs, A.; Elez, P.; Barbosa-Cánovas, G.V.; Martín, O. Inhibition of tomato (Licopersicon esculentum Mill.) pectin methylesterase by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2000, 1, 57–67. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the micro-structure, pectin, carbohydrates and rheological properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef]
- Rojas, M.; Leite, T.S.; Cristianini, M.; Alvim, I.D.; Augusto, P.E. Peach juice processed by the ultrasound technology: Changes in its microstructure improve its physical properties and stability. Food Res. Int. 2016, 82, 22–33. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality pa-rameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef]
- Esparza, L.M.A.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Do Amaral Souza, F.d.C.; Gomes Sanders Moura, L.; de Oliveira Bezerra, K.; Paiva Lopes Aguiar, J.; Moreira Mar, J.; Sanches, E.A.; dos Santos, F.F.; Bakry, A.M.; Nicolau Paulino, B.; Campelo, P.H. Thermosonication applied on camu–camu nectars processing: Effect on bioactive compounds and quality parameters. Food Bioprod. Process. 2019, 116, 212–218. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remón, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Changes in phenolic profile and antioxidant activity during production of diced tomatoes. Food Chem. 2011, 126, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Moreno, E.R.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality en-hancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of thermosonication on bio-active compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, R.; Salucci, M.; Leonardi, C.M.; Ferracane, R.; Catasta, G.; Azzini, E.; Maiani, G. Effect of domestic cooking on human bioavailability of naringenin, chlorogenic acid, lycopene and β-arotene in cherry tomatoes. Eur. J. Nutr. 2004, 43, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Ultra-performance liquid chromatographic separation of geometric isomers of carotenoids and antioxidant activities of 20 tomato cultivars and breeding lines. Food Chem. 2012, 132, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dai, Y.; Kakuda, Y.; Mittal, G.; Xue, S.J. Effect of heating and exposure to light on the stability of lycopene in tomato purée. Food Control. 2008, 19, 514–520. [Google Scholar] [CrossRef]
- Murakami, K.; Honda, M.; Takemura, R.; Fukaya, T.; Diono, W.; Kanda, H.; Goto, M. Effect of thermal treatment and light irradiation on the stability of lycopene with high Z-isomers content. Food Chem. 2018, 250, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effects of industrial tomato paste processing on ascorbic acid, flavonoids and ca-rotenoids and their stability over one-year storage. J. Sci. Food Agric. 2012, 92, 23–28. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Stacewicz-Sapuntzakis, M.; Bowen, P.E. Effects of heat treatment on the carotenoid and tocopherol composition of tomato. J. Food Sci. 2012, 77, C1109–C1114. [Google Scholar] [CrossRef]
- Richelle, M.; Lambelet, P.; Rytz, A.; Tavazzi, I.; Mermoud, A.-F.; Juhel, C.; Borel, P.; Bortlik, K. The proportion of lycopene isomers in human plasma is modulated by lycopene isomer profile in the meal but not by lycopene preparation. Br. J. Nutr. 2011, 107, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive com-pounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Tomas, M.; Beekwilder, J.; Hall, R.; Sagdic, O.; Boyacioglu, D.; Capanoglu, E. Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017, 220, 51–58. [Google Scholar] [CrossRef] [PubMed]



| Properties | Control | Break-65 | Break-90 | US-Break-22 | US-Break-65 |
|---|---|---|---|---|---|
| pectin content (mg GalA/g serum) | 10.91 ± 0.85 e | 14.65 ± 1.11 d | 22.85 ± 2.17 c | 26.37 ± 1.33 b | 31.50 ± 3.81 a |
| GalA(mol%) | 81.42 ± 0.85 a | 49.22 ± 0.59 d | 69.74 ± 2.20 b | 49.59 ± 1.01 d | 59.66 ± 0.56 c |
| Fuc(mol%) | 0.37 ± 0.02 c | 0.65 ± 0.03 a | 0.40 ± 0.04 c | 0.66 ± 0.05 a | 0.52 ± 0.03 b |
| Rha(mol%) | 3.00 ± 0.29 c | 5.47 ± 0.31 a | 2.46 ± 0.22 c | 5.72 ± 0.24 a | 4.09 ± 0.22 b |
| Gal(mol%) | 8.36 ± 0.76 d | 26.83 ± 1.02 a | 15.44 ± 0.20 b | 26.73 ± 1.44 c | 21.15 ± 0.20 b |
| Xyl(mol%) | 3.25 ± 0.20 c | 8.44 ± 0.31 a | 6.11 ± 0.39 b | 8.17 ± 0.40 a | 6.67 ± 0.30 b |
| Ara(mol%) | 2.96 ± 0.22 d | 8.93 ± 0.24 a | 5.27 ± 0.28 c | 8.67 ± 0.56 a | 7.45 ± 0.30 b |
| Man(mol%) | 0.64 ± 0.03 a | 0.47 ± 0.01 c | 0.57 ± 0.01 b | 0.47 ± 0.02 c | 0.47 ± 0.01 c |
| Linearity | 4.55 ± 0.26 a | 0.98 ± 0.02 d | 2.36 ± 0.26 b | 0.99 ± 0.44 d | 1.50 ± 0.04 c |
| Side chain length | 3.78 ± 0.31 d | 6.56 ± 0.50 bc | 8.42 ± 0.10 a | 6.18 ± 0.23 c | 7.00 ± 0.12 b |
| DM | 34.39 ± 1.41 a | 20.89 ± 1.71 c | 36.63 ± 2.30 a | 19.53 ± 1.43 c | 27.75 ± 2.91 b |
| Mw | 196.10 ± 7.57 a | 67.27 ± 9.77 d | 161.23 ± 10.88 b | 74.09 ± 1.89 d | 131.38 ± 4.19 c |
| Compound | Control | Break-65 | Break-90 | US-Break-22 | US-Break-65 |
|---|---|---|---|---|---|
| rutin | 185.22 ± 15.1 c | 196.35 ± 10.86 bc | 204.01 ± 10.18 b | 221.38 ± 14.86 a | 209.41 ± 10.77 ab |
| quercetin | 18.99 ± 0.80 b | 20.75 ± 0.90 b | 25.63 ± 0.45 a | 27.64 ± 1.78 a | 26.37 ± 2.23b a |
| naringenin | 79.43 ± 3.03 c | 95.77 ± 9.01 b | 111.93 ± 7.57 a | 114.41 ± 4.15 a | 99.85 ± 7.01 b |
| naringenin-7-O-glucoside | 22.08 ± 0.69 a | 21.17 ± 1.22 a | 22.73 ± 0.88 a | 24.05 ± 2.77 a | 23.87 ± 0.80 a |
| total flavonols/flavanone | 305.72 ± 13.72 d | 331.39 ± 10.17 c | 366.29 ± 12.52 b | 393.54 ± 20.43 a | 361.67 ± 16.47 b |
| protocatechuic acid | 2.33 ± 0.13 d | 3.04 ± 0.08 c | 2.57 ± 0.14 d | 4.22 ± 0.12 a | 3.89 ± 0.27 b |
| ferulic acid | 11.03 ± 0.06 d | 13.87 ± 1.08 b | 12.11 ± 0.23 c | 15.74 ± 0.54 a | 14.55 ± 0.36 b |
| caffeic acid | 13.76 ± 0.91 c | 16.9 ± 0.62 b | 14.74 ± 0.71 c | 17.91 ± 0.50 a | 18.53 ± 1.29 a |
| p-coumaric acid | 13.94 ± 1.25 b | 14.88 ± 0.63 b | 13.41 ± 0.22 b | 16.87 ± 0.25 a | 16.71 ± 1.03 a |
| gentistic acid | 16.92 ± 0.24 b | 20.26 ± 0.52 a | 17.61 ± 1.30 b | 20.41 ± 1.85 a | 20.82 ± 2.10 a |
| chlorogenic acid | 17.22 ± 1.88 c | 19.41 ± 1.18 bc | 17.58 ± 0.92 c | 25.52 ± 2.13 a | 20.75 ± 1.46 b |
| total phenolic acid | 76.48 ± 1.18 d | 88.37 ± 0.89 c | 78.01 ± 1.20 d | 100.69 ± 0.52 a | 94.88 ± 0.47 b |
| total phenolic | 382.20 ± 13.25 d | 419.76 ± 10.80 c | 444.30 ± 13.61 c | 494.23 ± 20.27 a | 456.55 ± 16.58 b |
| Compound | Control | Break-65 | Break-90 | US-Break-22 | US-Break-65 |
|---|---|---|---|---|---|
| cis-lutein/ cis-lutein-5,8-epoxides | 0.21 ± 0.01 ab | 0.16 ± 0.01 bc | 0.15 ± 0.01 c | 0.22 ± 0.02 a | 0.18 ± 0.02 b |
| all-trans-lutein | 4.07 ± 0.15 a | 3.36 ± 0.13 b | 3.30 ± 0.44 b | 3.51 ± 0.31 b | 4.15 ± 0.15 a |
| 13-cis-lutein | 0.22 ± 0.02 b | 0.34 ± 0.04 a | 0.32 ± 0.04 a | 0.38 ± 0.04 a | 0.33 ± 0.02 a |
| total lutein | 4.50 ± 0.16 ab | 3.86 ± 0.12 c | 3.77 ± 0.45 c | 4.11 ± 0.29 bc | 4.67 ± 0.18 a |
| 15-cis-β-carotene | 1.41 ± 0.14 c | 1.87 ± 0.08 b | 1.84 ± 0.14 b | 2.12 ± 0.03 a | 1.87 ± 0.16 b |
| di-cis-β-carotene | 1.55 ± 0.09 c | 1.84 ± 0.09 b | 1.89 ± 0.14 b | 2.02 ± 0.16 ab | 2.13 ± 0.09 a |
| all-trans-β-carotene | 18.08 ± 1.71 b | 18.06 ± 0.92 b | 18.00 ± 1.65 b | 20.76 ± 0.86 a | 20.35 ± 0.89 ab |
| 13-cis-β-carotene | 0.85 ± 0.07 b | 1.10 ± 0.03 b | 1.45 ± 0.21 a | 1.43 ± 0.20 a | 1.38 ± 0.14 a |
| total β-carotene | 21.90 ± 1.57 b | 22.87 ± 0.99 b | 23.18 ± 1.45 b | 26.33 ± 0.94 a | 25.73 ± 0.84 a |
| 15-cis-lycopene | 1.71 ± 0.08 b | 1.93 ± 0.11 b | 1.99 ± 0.17 b | 2.36 ± 0.19 ab | 2.47 ± 0.18 a |
| 13-cis-lycopene | 4.25 ± 0.18 d | 4.70 ± 0.17 cd | 5.16 ± 0.20 bc | 5.41 ± 0.23 ab | 5.96 ± 0.58 a |
| 9,13-di-cis-lycopene | 1.03 ± 0.11 d | 1.32 ± 0.08 c | 1.61 ± 0.22 b | 1.68 ± 0.06 ab | 1.87 ± 0.14 a |
| 9-cis-lycopene | 1.88 ± 0.01 b | 2.23 ± 0.13 b | 2.73 ± 0.25 a | 2.79 ± 0.13 a | 2.65 ± 0.20 a |
| 9′-cis-lycopene | 0.32 ± 0.03 a | 0.29 ± 0.00 a | 0.29 ± 0.00 a | 0.29 ± 0.01 a | 0.30 ± 0.03 a |
| 5,9-cis-lycopene | 1.20 ± 0.18 c | 1.34 ± 0.05 bc | 1.54 ± 0.05 b | 1.92 ± 0.06 a | 1.94 ± 0.11 a |
| 5-cis-lycopene | 3.13 ± 0.16 c | 3.64 ± 0.24 b | 4.29 ± 0.26 a | 4.22 ± 0.31 a | 4.19 ± 0.11 a |
| 5′-cis-lycopene | 0.34 ± 0.02 b | 0.39 ± 0.02 b | 0.38 ± 0.03 b | 0.47 ± 0.04 a | 0.46 ± 0.03 a |
| all-trans-lycopene | 109.89 ± 2.91 a | 95.52 ± 5.91 bc | 87.34 ± 4.63 c | 103.56 ± 6.85 ab | 103.14 ± 11.62 ab |
| total lycopene | 123.74 ± 2.78 a | 111.36 ± 6.08 b | 105.33 ± 5.12 b | 122.69 ± 6.93 a | 122.98 ± 11.89 a |
| total carotenoids | 150.13 ± 4.19 a | 138.08 ± 5.28 b | 132.28 ± 6.89 b | 153.13 ± 5.92 a | 153.38 ± 11.4 a |
| Control | Break-65 | Break-90 | US-Break-22 | US-Break-65 | ||
|---|---|---|---|---|---|---|
| ABTS (µmol TE/100g DW) | H d | 2279.7 ± 188.5 ab | 1938.3 ± 170.5 bc | 1812.7 ± 134.2 c | 2464.8 ± 276.2 a | 1998.1 ± 169.5 bc |
| L e | 1010.1 ± 158.2 c | 1325.9 ± 276.1 b | 1500.2 ± 138.6 ab | 1742.9 ± 86.4 a | 1798.8 ± 113.3 a | |
| T f | 3289.8 ± 312.4 c | 3264.2 ± 233.4 c | 3313.0 ± 20.5 c | 4207.6 ± 279.5 b | 3796.9 ± 273.5 a | |
| DPPH (µmol TE/100g DW) | H | 932.7 ± 81.5 b | 787.8 ± 102.3 c | 698.3 ± 79.3 c | 1247.2 ± 47.6 a | 823.8 ± 78.5 b |
| L | 539.7 ± 74.6 c | 705.2 ± 85.0 b | 832.4 ± 73.8 b | 955.2 ± 132.1 a | 1037.2 ± 118.2 a | |
| T | 1472.3 ± 83.4 c | 1493.2 ± 154.3 c | 1530.7 ± 152.5 c | 2202.4 ± 26.0 a | 1861.0 ± 86.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wu, Z.; Ma, Q.; Lu, Z.; Ye, F.; Zhao, G. Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste. Foods 2021, 10, 2395. https://doi.org/10.3390/foods10102395
Gao R, Wu Z, Ma Q, Lu Z, Ye F, Zhao G. Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste. Foods. 2021; 10(10):2395. https://doi.org/10.3390/foods10102395
Chicago/Turabian StyleGao, Ruiping, Zhen Wu, Qian Ma, Zhiqiang Lu, Fayin Ye, and Guohua Zhao. 2021. "Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste" Foods 10, no. 10: 2395. https://doi.org/10.3390/foods10102395
APA StyleGao, R., Wu, Z., Ma, Q., Lu, Z., Ye, F., & Zhao, G. (2021). Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste. Foods, 10(10), 2395. https://doi.org/10.3390/foods10102395







