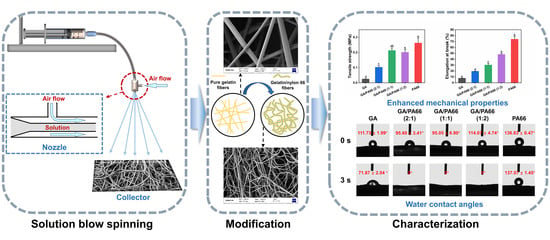
Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Spinning Solution
2.3. Solution Blow Spinning (SBS) Process
2.4. Fiber Morphology
2.5. Fourier Transform Infrared (FTIR) Analysis
2.6. X-ray Diffraction (XRD) Analysis
2.7. Thermal Analysis
2.8. Mechanical Strength
2.9. Water Contact Angle (WCA)
2.10. Water Vapor Permeability (WVP)
2.11. Solvent Resistance
2.12. Statistical Analysis
3. Results and Discussion
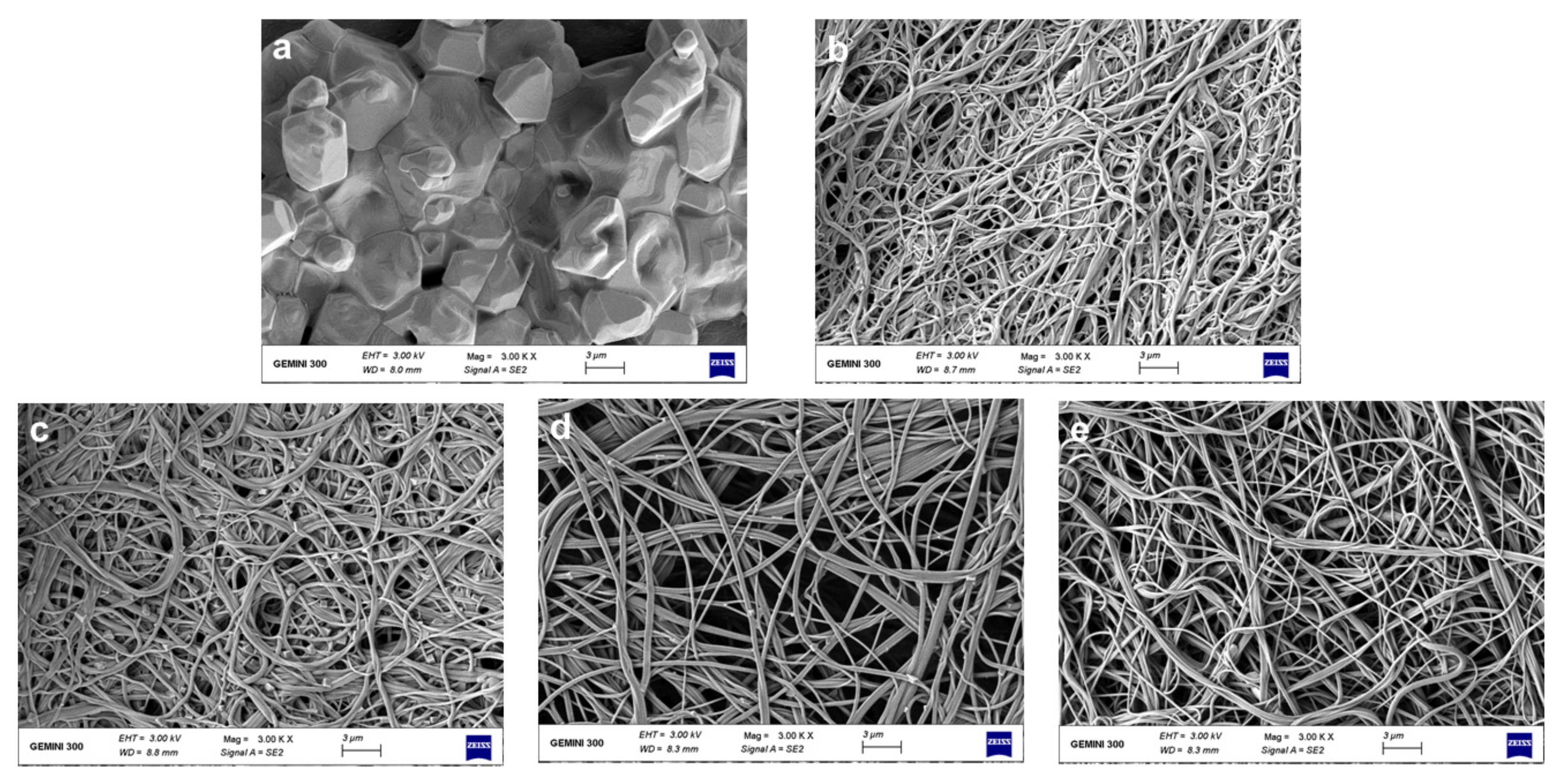
3.1. Morphologies of Nanofibrous Films
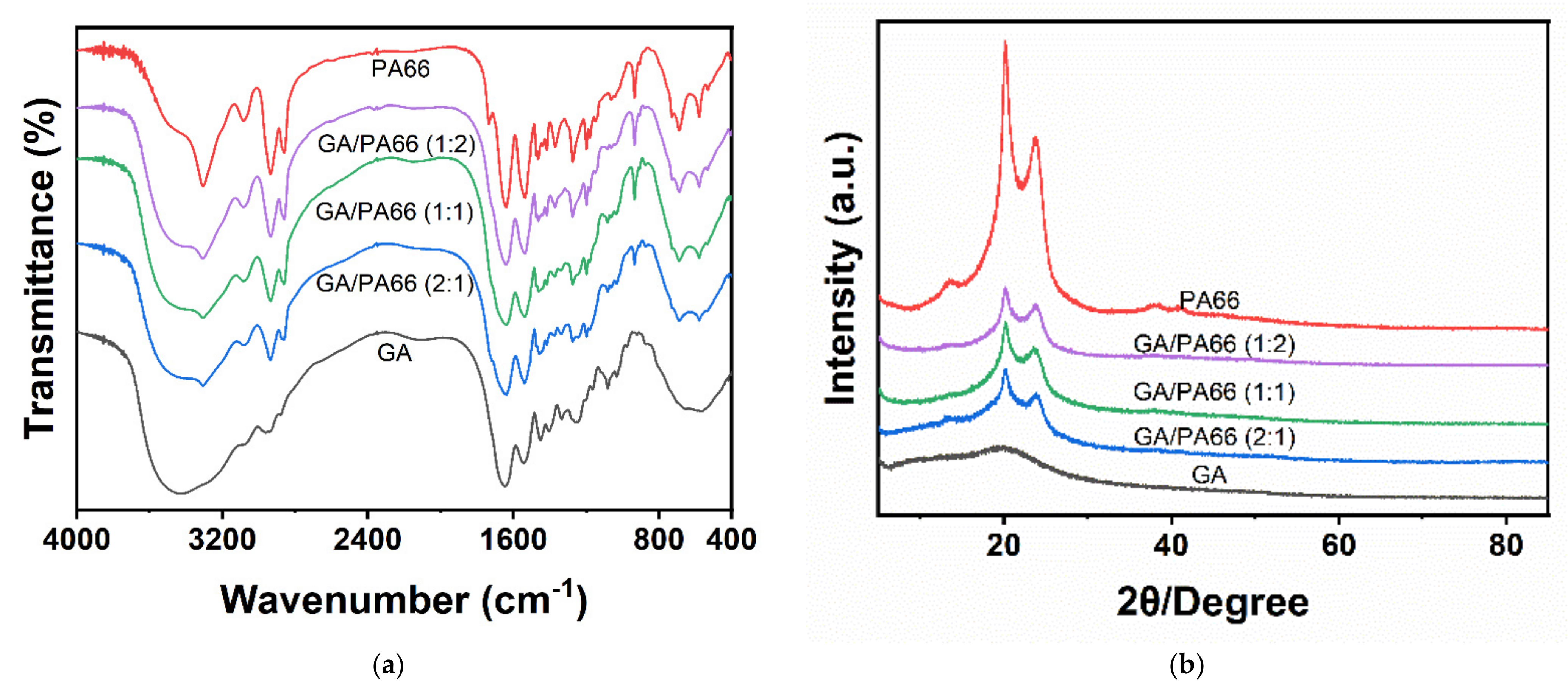
3.2. FTIR Spectra Analysis
3.3. XRD Analysis
3.4. Thermal Analysis
3.5. Mechanical Properties Analysis
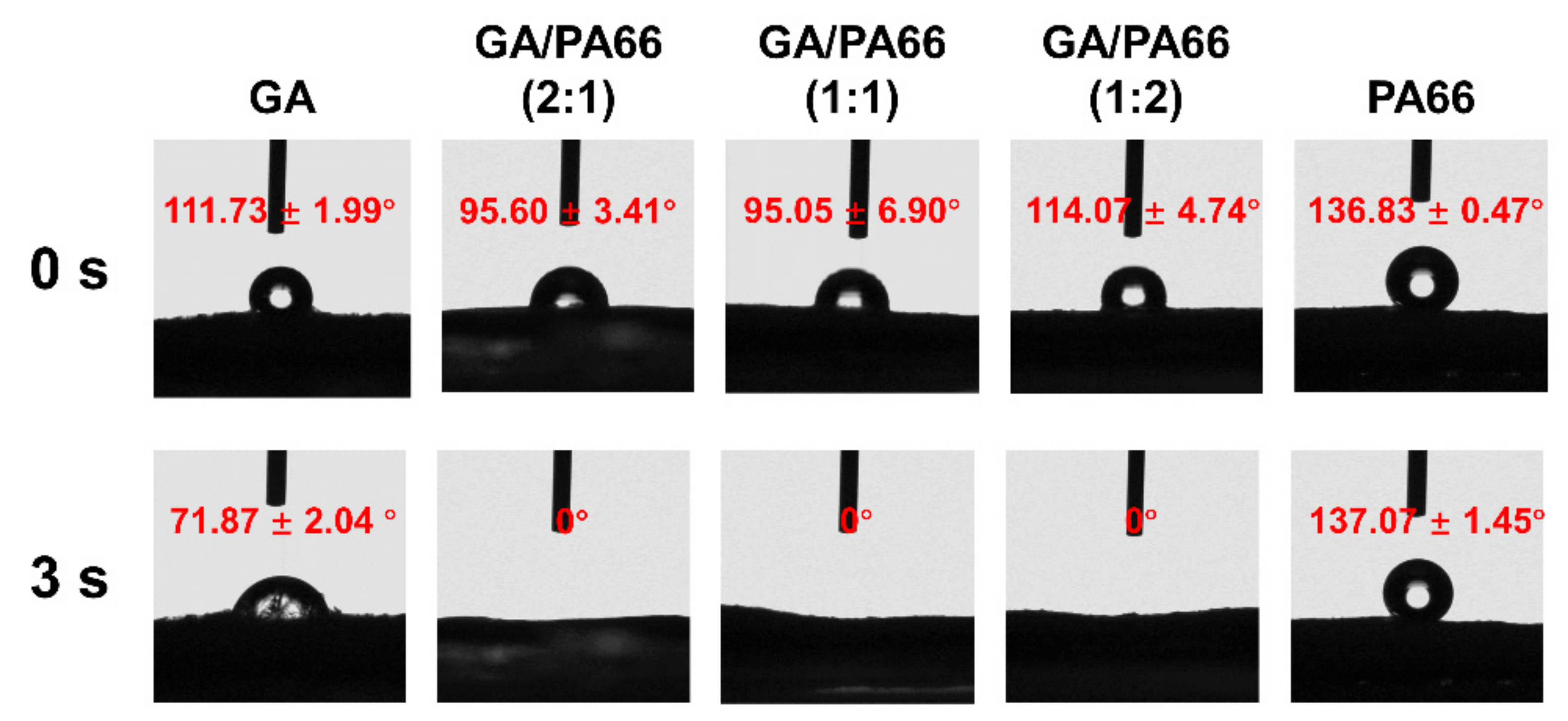
3.6. Water Contact Angles Analysis
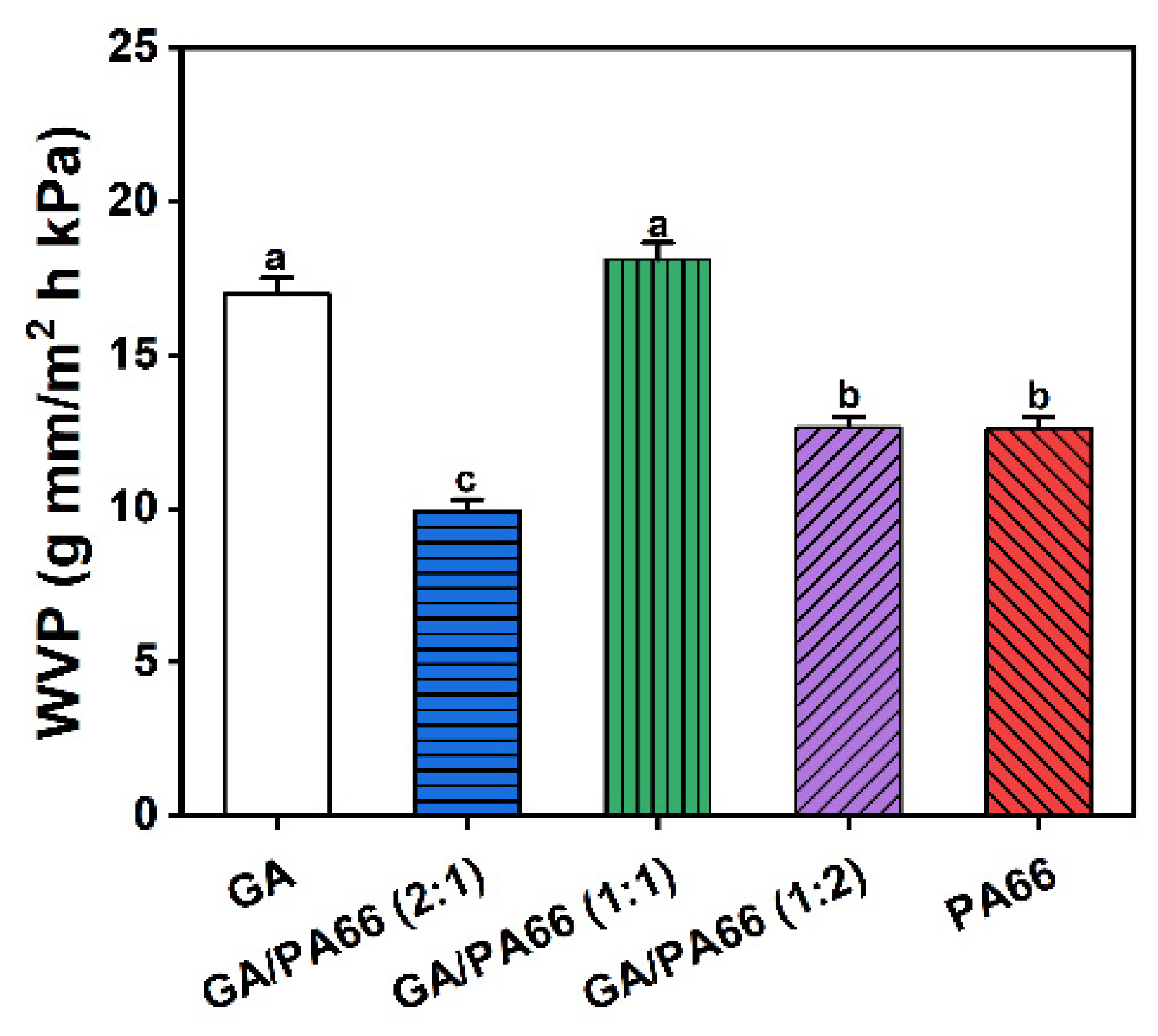
3.7. Water Vapor Permeability Analysis
3.8. Solvent Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, T.; Yu, J.; Li, Q.; Wang, C.F.; Chen, S.; Li, W.; Wang, G. Large-scale fabrication of robust artificial skins from a biodegradable sealant-loaded nanofiber scaffold to skin tissue via microfluidic blow-spinning. Adv. Mater. 2020, 32, 2000982. [Google Scholar] [CrossRef] [PubMed]
- Benavides, R.E.; Jana, S.C.; Reneker, D.H. Nanofibers from scalable gas jet process. ACS Macro Lett. 2012, 1, 1032–1036. [Google Scholar] [CrossRef]
- Dadol, G.C.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B. Solution blow spinning-polyacrylonitrile-assisted cellulose acetate nanofiber membrane. Nanotechnology 2020, 31, 345602. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.T.G.; Rempel, S.P.; Agnol, L.D.; Bianchi, O. The main blow spun polymer systems: Processing conditions and applications. J. Polym. Res. 2020, 27, 205. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Vargas Romero, E.; Lim, L.-T.; Suárez Mahecha, H.; Bohrer, B.M. The effect of electrospun polycaprolactone nonwovens containing chitosan and propolis extracts on fresh pork packaged in linear low-density polyethylene films. Foods 2021, 10, 1110. [Google Scholar] [CrossRef]
- Wang, D.D.; Liu, Y.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D.D. Fabrication and characterization of gelatin/zein nanofiber films loading perillaldehyde for the preservation of chilled chicken. Foods 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Tandon, B.; Kamble, P.; Olsson, R.T.; Blaker, J.J.; Cartmell, S.H. Fabrication and Characterisation of Stimuli Responsive Piezoelectric PVDF and Hydroxyapatite-Filled PVDF Fibrous Membranes. Molecules 2019, 24, 1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sett, S.; Stephansen, K.; Yarin, A.L. Solution-blown nanofiber mats from fish sarcoplasmic protein. Polymer 2016, 93, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Cao, Y.; Rao, J.; Zou, Y.; Zhang, H.; Wu, D.; Chen, K. Application of solution blow spinning to rapidly fabricate natamycin-loaded gelatin/zein/polyurethane antimicrobial nanofibers for food packaging. Food Packag. Shelf Life 2021, 29, 100721. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y. Effects of concentration on nanostructural images and physical properties of gelatin from channel catfish skins. Food Hydrocoll. 2009, 23, 577–584. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Lee, B.-T. Fabrication and characterization of cross-linked gelatin electro-spun nano-fibers. J. Biomed. Sci. Eng. 2010, 3, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, R.; Sun, L.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. 3D-printed biopolymers for tissue engineering application. Int. J. Polym. Sci. 2014, 2014, 829145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ouyang, H.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Rose, J.B.; Sidney, L.E.; Patient, J.; White, L.J.; Dua, H.S.; El Haj, A.J.; Hopkinson, A.; Rose, F.R.A.J. In vitro evaluation of electrospun blends of gelatin and PCL for application as a partial thickness corneal graft. J. Biomed. Mater. Res.-Part A 2019, 107, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Ji, G.; Wu, S.; Shen, J. Preparation and characterization of nylon 66/montmorillonite nanocomposites with co-treated montmorillonites. Eur. Polym. J. 2003, 39, 1641–1646. [Google Scholar] [CrossRef]
- Ahmadloo, E.; Gharehaghaji, A.A.; Latifi, M.; Saghafi, H.; Mohammadi, N. Effect of PA66 nanofiber yarn on tensile fracture toughness of reinforced epoxy nanocomposite. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 233, 2033–2043. [Google Scholar] [CrossRef]
- Lee, J.E.; Han, D.H.; Kim, Y.M.; Choi, K.M. Comparison of mechanical and thermal properties of polyamide-66/rubber/fiber alloy composites. Asian J. Chem. 2013, 25, 5237–5244. [Google Scholar] [CrossRef]
- Spina, R.; Cavalcante, B. Evaluation of grinding of unfilled and glass fiber reinforced polyamide 6,6. Polymers 2020, 12, 2288. [Google Scholar] [CrossRef] [PubMed]
- Cakal Sarac, E.; Haghighi Poudeh, L.; Berktas, I.; Saner Okan, B. Scalable fabrication of high-performance graphene/polyamide 66 nanocomposites with controllable surface chemistry by melt compounding. J. Appl. Polym. Sci. 2021, 138, 49972. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Zhang, X.; Liu, H.; Zhang, H.; Zhang, X. Polyamide 66 and amino-functionalized multi-walled carbon nanotube composites and their melt-spun fibers. J. Mater. Sci. 2019, 54, 11056–11068. [Google Scholar] [CrossRef]
- Xiong, Y.; Ren, C.; Zhang, B.; Yang, H.; Lang, Y.; Min, L.; Zhang, W.; Pei, F.; Yan, Y.; Li, H.; et al. Analyzing the behavior of a porous nano-hydroxyapatite/polyamide 66 (n-HA/PA66) composite for healing of bone defects. Int. J. Nanomed. 2014, 9, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, J.M.; Schvezov, C.E.; Rosenberger, M.R. Influence of Experimental Variables on the Measure of Contact Angle in Metals Using the Sessile Drop Method. Procedia Mater. Sci. 2015, 8, 742–751. [Google Scholar] [CrossRef]
- Hasanpour Ardekani-Zadeh, A.; Hosseini, S.F. Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydr. Polym. 2019, 223, 115108. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Zhang, Y.; Yarin, A.L.; Davis, S.C.; Pourdeyhimi, B. Solution Blowing of Soy Protein Fibers. Biomacromolecules 2011, 12, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Sengor, M.; Ozgun, A.; Corapcioglu, G.; Ipekoglu, M.; Garipcan, B.; Ersoy, N.; Altintas, S. Core–shell PVA/gelatin nanofibrous scaffolds using co-solvent, aqueous electrospinning: Toward a green approach. J. Appl. Polym. Sci. 2018, 135, 46582. [Google Scholar] [CrossRef]
- Lu, L.; Yang, B.; Liu, J. Flexible multifunctional graphite nanosheet/electrospun-polyamide 66 nanocomposite sensor for ECG, strain, temperature and gas measurements. Chem. Eng. J. 2020, 400, 125928. [Google Scholar] [CrossRef]
- Gu, A.; Wu, J.; Shen, L.; Zhang, X.; Bao, N. High-strength GO/PA66 nanocomposite fibers via in situ precipitation and polymerization. Polymers 2021, 13, 1688. [Google Scholar] [CrossRef]
- Noguchi, T.; Niihara, K.; Kawamoto, K.; Fukushi, M.; Jinnai, H.; Nakajima, K.; Endo, M. Preparation of high-performance carbon nanotube/polyamide composite materials by elastic high-shear kneading and improvement of properties by induction heating treatment. J. Appl. Polym. Sci. 2021, 138, 50512. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, C.; Wang, P.; Zhang, Y.; Zhang, H. Electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite nanotube for active food packaging. Carbohydr. Polym. 2020, 247, 116711. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Jiang, W.; Zhou, H.; He, J. Preparation and properties of composite phase-change nanofiber membrane by improved bubble electrospinning. Mater. Res. Express 2021, 8, 55011. [Google Scholar] [CrossRef]
- Baseer, A.; Koenneke, A.; Zapp, J.; Khan, S.A.; Schneider, M. Design and characterization of surface-crosslinked gelatin nanoparticles for the delivery of hydrophilic macromolecular drugs. Macromol. Chem. Phys. 2019, 220, 1900260. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Cheng, Z.; Kang, L.; Zhang, Y.; Zhao, X.; Zhao, S.; Gao, B. Novel anti-fouling polyethersulfone/polyamide 66 membrane preparation for air filtration by electrospinning. Mater. Lett. 2017, 192, 12–16. [Google Scholar] [CrossRef]
- Chi, E.; Tang, Y.; Wang, Z. In situ SAXS and WAXD investigations of polyamide 66/reduced graphene oxide nanocomposites during uniaxial deformation. ACS Omega 2021, 6, 11762–11771. [Google Scholar] [CrossRef] [PubMed]
- Karam, L.; Jama, C.; Mamede, A.S.; Boukla, S.; Dhulster, P.; Chihib, N.E. Nisin-activated hydrophobic and hydrophilic surfaces: Assessment of peptide adsorption and antibacterial activity against some food pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 10321–10328. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Nahvi, Z.; Zandi, M. Antioxidant peptide-loaded electrospun chitosan/poly(vinyl alcohol) nanofibrous mat intended for food biopackaging purposes. Food Hydrocoll. 2019, 89, 637–648. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Water vapor and oxygen barrier properties of extrusion-blown poly(lactic acid)/cellulose nanocrystals nanocomposite films. Compos. Part A Appl. Sci. Manuf. 2018, 114, 204–211. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Kariminejad, M.; Mortazavian, A.M.; Sadeghi, E.; Hasanvand, S. Physico-mechanical and structural properties of eggshell membrane gelatin- chitosan blend edible films. Int. J. Biol. Macromol. 2018, 107, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Ahammed, S.; Liu, F.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Improvement of the water resistance and ductility of gelatin film by zein. Food Hydrocoll. 2020, 105, 105804. [Google Scholar] [CrossRef]

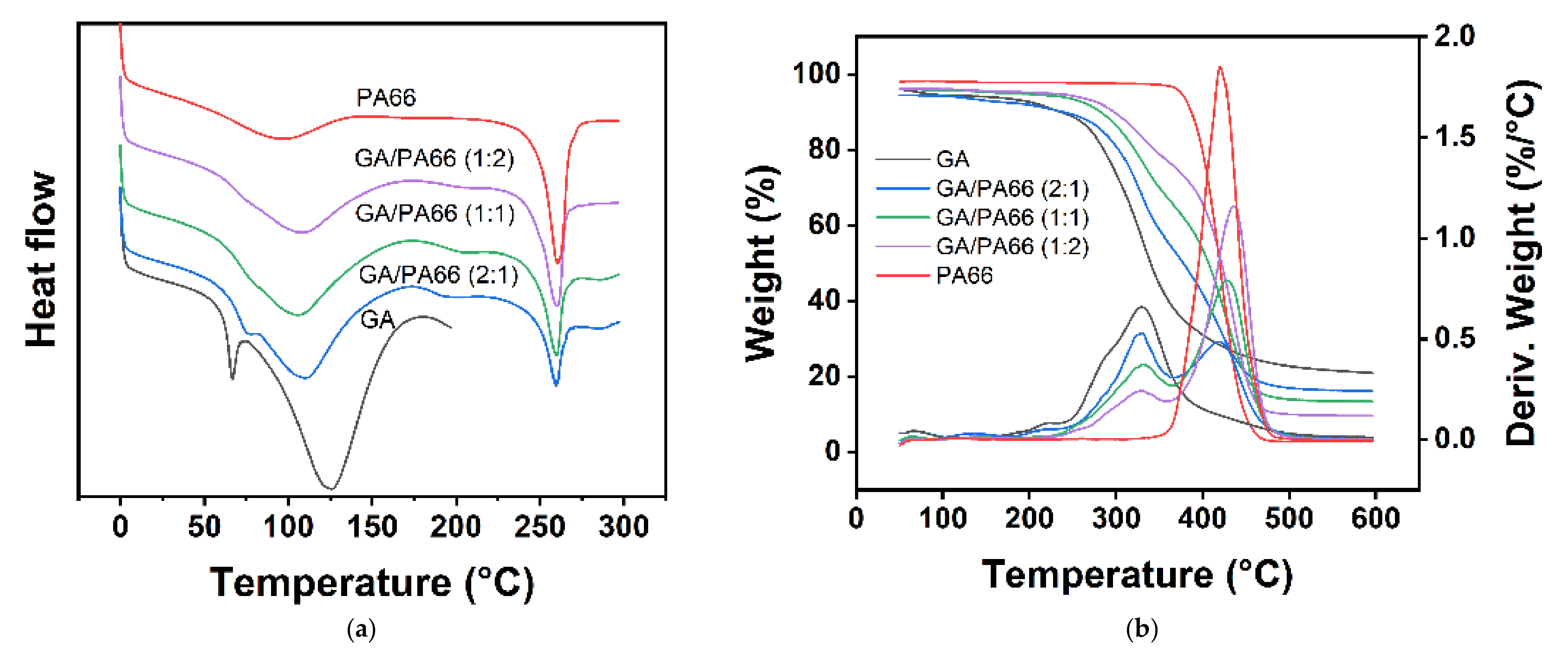

| Sample | DSC Parameters | TGA Parameters | |||||
|---|---|---|---|---|---|---|---|
| Tm, GA (°C) | ΔHm, GA (J/g) | Tm, PA66 (°C) | ΔHm, PA66 (J/g) | T10wt% (°C) 1 | Tmax (°C) 2 | Wred (%) 3 | |
| GA | 126.50 | 145.10 | / | / | 236.70 | 329.59 | 21.04 |
| GA/PA66 (2:1) | 110.20 | 148.7 | 259.71 | 21.87 | 240.17 | 330.22 | 16.20 |
| GA/PA66 (1:1) | 106.22 | 115.3 | 259.81 | 28.74 | 282.64 | 427.51 | 13.50 |
| GA/PA66 (1:2) | 108.28 | 102.9 | 260.39 | 38.52 | 298.13 | 435.59 | 9.70 |
| PA66 | / | / | 260.43 | 65.61 | 387.09 | 420.18 | 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Shen, C.; Zou, Y.; Wu, D.; Zhang, H.; Chen, K. Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film. Foods 2021, 10, 2339. https://doi.org/10.3390/foods10102339
Yang Z, Shen C, Zou Y, Wu D, Zhang H, Chen K. Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film. Foods. 2021; 10(10):2339. https://doi.org/10.3390/foods10102339
Chicago/Turabian StyleYang, Zhichao, Chaoyi Shen, Yucheng Zou, Di Wu, Hui Zhang, and Kunsong Chen. 2021. "Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film" Foods 10, no. 10: 2339. https://doi.org/10.3390/foods10102339
APA StyleYang, Z., Shen, C., Zou, Y., Wu, D., Zhang, H., & Chen, K. (2021). Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film. Foods, 10(10), 2339. https://doi.org/10.3390/foods10102339