Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Intervention
2.3. Blood Samples
2.4. Cell Culture
2.5. ASMC Treatment
2.6. Real Time RTPCR
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Consent for Publication
Abbreviations
| AGEs | Advanced Glycation End Products |
| ANOVA | Analysis of Variance |
| ASMCs | Aortic smooth muscle cells |
| BMI | Body Mass Index |
| CV | Cardiovascular |
| CVD | Cardiovascular Disease |
| CRP | C-Reactive Protein |
| DAMP | Damage-associated molecular pattern |
| EDTA | Ethylenediamine Tetra-acetic Acid |
| ELISA | Enzyme-linked Immunosorbent Assay |
| esRAGEs | Endogenous secreted Receptor for Advanced Glycation End Products |
| HMGB-1 | High Mobility Group Box-1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| PBS | Phosphate buffered saline |
| PON1 | Paraoxonase 1 |
| RAGEs | Receptor for Advanced Glycation End Products |
| sRAGEs | Soluble Receptor for Advanced Glycation End Products |
| TLR4 | Toll-Like Receptor 4 |
| WHO | World Health Organization |
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti- inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto Jr, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabák, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical activity and inflammatory markers over 10 years: Follow-Up in men and women from the Whitehall II Cohort Study. Circulation 2012, 126, 928–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-Reactive Protein and inflammatory markers. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [Green Version]
- Arikawa, A.Y.; Thomas, W.; Schmitz, K.H.; Kurzer, M.S. Sixteen weeks of exercise reduces C- reactive protein levels in young women. Med. Sci. Sports Exerc. 2011, 43, 1002–1009. [Google Scholar] [CrossRef]
- Campbell, K.L.; Campbell, P.T.; Ulrich, C.M.; Wener, M.; Alfano, C.M.; Foster-Schubert, K.; Rudolph, R.E.; Potter, J.D.; McTiernan, A. No reduction in C-reactive protein following a 12-month randomized controlled trial of exercise in men and women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1714–1718. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Neilson, H.K.; Woolcott, C.G.; Wang, Q.; Stanczyk, F.Z.; McTiernan, A.; Jones, C.A.; Irwin, M.L.; Yasui, Y.; Courneya, K.S. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev. Res. 2011, 5, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Lakka, T.A.; Lakka, H.M.; Rankinen, T.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: The HERITAGE Family Study. Eur. Heart J. 2005, 26, 2018–2025. [Google Scholar] [CrossRef]
- Papini, C.B.; Nakamura, P.M.; Zorzetto, L.P.; Thompson, J.L.; Phillips, A.C.; Kokubun, E. The effect of a community-based, primary health care exercise program on inflammatory biomarkers and hormone levels. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Ihalainen, J.K.; Schumann, M.; Eklund, D.; Hämäläinen, M.; Moilanen, E.; Paulsen, G.; Häkkinen, K.; Mero, A.A. Combined aerobic and resistance training decreases inflammation markers in healthy men. Scand. J. Med. Sci. Sports 2018, 28, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Della, G.P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vascul. Pharmacol. 2015, 72, 1–8. [Google Scholar] [PubMed] [Green Version]
- Gopal, P.; Reynaert, N.L.; Scheijen, J.L.; Schalkwijk, C.G.; Franssen, F.M.; Wouters, E.F.; Rutten, E.P. Association of plasma sRAGE but not esRAGE with lung function impairment in COPD. Respir. Res. 2014, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauen Larsen, H.; Marinkovic, G.; Nilsson, P.M.; Nilsson, J.; Engström, G.; Melander, O.; Orho-Melander, M.; Schiopu, A. High Plasma sRAGE (Soluble Receptor for Advanced Glycation End Products) Is Associated With Slower Carotid Intima-Media Thickness Progression and Lower Risk for First-Time Coronary Events and Mortality. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Weinstein, S.J.; Albanes, D.; Taylor, P.R.; Graubard, B.I.; Virtamo, J.; Stolzenberg-Solomon, R.Z. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: A prospective study. Cancer Res. 2011, 71, 3582–3589. [Google Scholar] [CrossRef] [Green Version]
- Creagh-Brown, B.C.; Quinlan, G.J.; Hector, L.R.; Evans, T.W.; Burke-Gaffney, A. Association between preoperative plasma sRAGE levels and recovery from cardiac surgery. Mediat. Inflamm. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Ambrogi, F.; De Cal, M.; Vianello, E.; Ronco, C.; Corsi Romanelli, M.M. Role of the Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Prognostic Factor for Mortality in Hemodialysis and Peritoneal Dialysis Patients. Mediat. Inflamm. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jabaudon, M.; Blondonnet, R.; Pereira, B.; Cartin-Ceba, R.; Lichtenstern, C.; Mauri, T.; Determann, R.M.; Drabek, T.; Hubmayr, R.D.; Gajic, O.; et al. Plasma sRAGE is independently associated with increased mortality in ARDS: A meta-analysis of individual patient data. Intensive Care Med. 2018, 44, 1388–1399. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.J.; Lindberg, S.; Hoffmann, S.; Iversen, A.Z.; Pedersen, S.H.; Møgelvang, R.; Galatius, S.; Flyvbjerg, A.; Jensen, J.S.; Bjerre, M. Dynamic changes in sRAGE levels and relationship with cardiac function in STEMI patients. Clin. Biochem. 2015, 48, 297–301. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Komura, K.; Iwata, Y.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: Association with disease severity. J. Clin. Immunol. 2009, 29, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, X.F.; Zhao, Y.; Yu, J.Y.; Liu, J.S.; Gu, W.J.; Gao, F. Plasma sRAGE is independently associated with high sensitivity C-reactive protein in type 2 diabetes without coronary artery disease. Diabetes Res. Clin. Pract. 2010, 87, 19–22. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.I.; Adachi, H.; Kurita-Nakamura, Y.; Matsui, T.; Yoshida, T.; Sato, A.; Imaizumi, T. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab. Res. Rev. 2007, 23, 368–371. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- D’Amico, F.; Granata, M.; Skarmoutsou, E.; Trovato, C.; Lovero, G.; Gangemi, P.; Longo, V.; Pettinato, M.; Mazzarino, M.C. Biological therapy downregulates the heterodimer S100A8/A9 (calprotectin) expression in psoriatic patients. Inflamm. Res. 2018, 67, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Kishida, K.; Funahashi, T.; Shimomura, I. Three-month treatment with pioglitazone reduces circulating levels of S100A8/A9 (MRP8/14) complex, a biomarker of inflammation, without changes in body mass index, in type 2 diabetics with abdominal obesity. Diabetes Res. Clin. Pract. 2012, 95, 58–60. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Flores-Guerrero, J.L.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Seidu, S.; Dullaart, R.P.; Bakker, S.J. Plasma calprotectin and risk of cardiovascular disease: Findings from the PREVEND prospective cohort study. Atherosclerosis 2018, 275, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.P.; Haugen, E.; Ikemoto, M.; Fujita, M.; Terasaki, F.; Fu, M. S100A8/A9 complex as a new biomarker in prediction of mortality in elderly patients with severe heart failure. Int. J. Cardiol. 2012, 155, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Katashima, T.; Naruko, T.; Terasaki, F.; Fujita, M.; Otsuka, K.; Murakami, S.; Sato, A.; Hiroe, M.; Ikura, Y.; Ueda, M.; et al. Enhanced expression of the S100A8/A9 complex in acute myocardial infarction patients. Circ. J. 2010, 74, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Ueda, M.; Ikemoto, M.; Naruko, T.; Itoh, A.; Tamaki, S.I.; Nohara, R.; Terasaki, F.; Sasayama, S.; Fujita, M. Increased serum levels and expression of S100A8/A9 complexin infiltrated neutrophils in atherosclerotic plaque of unstable angina. Heart 2008, 94, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Tsoporis, J.N.; Overgaard, C.B.; Izhar, S.; Parker, T.G. S100B modulates the hemodynamic response to norepinephrine stimulation. Am. J. Hypertens. 2009, 22, 1048–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, G.K.; Loeb, A.; David Gordon, D.; Thompson, M.M. Expression of Smooth Muscle-specific a-Isoactin in Cultured Vascular Smooth Muscle Cells: Relationship between Growth and Cytodifferentiation. J. Cell Biol. 1986, 102, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Tsoporis, J.N.; Fazio, A.; Rizos, I.K.; Izhar, S.; Proteau, G.; Salpeas, V.; Rigopoulos, A.; Sakadakis, E.; Toumpoulis, I.K.; Parker, T.G. Increased right atrial appendage apoptosis is associated with differential regulation of candidate MicroRNAs 1 and 133A in patients who developed atrial fibrillation after cardiac surgery. J. Mol. Cell. Cardiol. 2018, 121, 25–32. [Google Scholar] [CrossRef]
- Fagerhol, M.K.; Nielsen, H.G.; Vetlesen, A.; Sandvik, K.; Lyberg, T. Increase in plasma calprotectin during long-distance running. Scand. J. Clin. Lab. Investig. 2005, 65, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Lechtermann, A.; Fobker, M.; Brandt, B.; Sorg, C.; Völker, K.; Nacken, W. The response of the novel pro-inflammatory molecules S100A8/A9 to exercise. Int. J. Sports Med. 2006, 27, 751–758. [Google Scholar] [CrossRef]
- Rochette, E.; Merlin, E.; Hourdé, C.; Evrard, B.; Peraira, B.; Echaubard, S.; Duché, P. Inflammatory response 24h post-exercise in youth with juvenile idiopathic arthritis. Int. J. Sports Med. 2018, 39, 867–874. [Google Scholar]
- Levitova, A.; Hulejova, H.; Spiritovic, M.; Pavelka, K.; Senolt, L.; Husakova, M. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res. Ther. 2016, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Acar, A.; Guzel, S.; Sarifakioglu, B.; Guzel, E.C.; Guzelant, A.Y.; Karadag, C.; Kiziler, L. Calprotectin levels in patients with rheumatoid arthritis to assess and association with exercise treatment. Clin. Rheumatol. 2016, 35, 2685–2692. [Google Scholar] [CrossRef]
- Evans, R.K.; Antczak, A.J.; Lester, M.; Yanovich, R.; Israeli, E.; Moran, D.S. Effects of a 4-Month Recruit Training Program on Markers of Bone Metabolism. Med. Sci. Sports Exerc. 2008, 40, 660–670. [Google Scholar] [CrossRef]
- Kotani, K.; Caccavello, R.; Sakane, N.; Yamada, T.; Taniguchi, N.; Gugliucci, A. Influence of Physical Activity Intervention on Circulating Soluble Receptor for Advanced Glycation end Products in Elderly Subjects. J. Clin. Med. Res. 2011, 3, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Farinha, J.B.; Krause, M.; Rodrigues-Krause, J.; Reischak-Oliveira, A. Exercise for type 1 diabetes mellitus management: General considerations and new directions. Med. Hypotheses 2017, 104, 147–153. [Google Scholar] [CrossRef]
- Choi, K.M.; Han, K.A.; Ahn, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Baik, S.H.; Choi, D.S.; et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2012, 97, 3751–3758. [Google Scholar] [CrossRef] [Green Version]
- Sponder, M.; Campean, I.A.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Graf, S.; Dalos, D.; Strametz-Juranek, J. Long-term physical activity leads to a significant increase in serum sRAGE levels: A sign of decreased AGE-mediated inflammation due to physical activity? Heart Vessels 2018, 33, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Santilli, F.; Vazzana, N.; Iodice, P.; Lattanzio, S.; Liani, R.; Bellomo, R.G.; Lessiani, G.; Perego, F.; Saggini, R.; Davì, G. Effects of high-amount-high-intensity exercise on in vivo platelet activation: Modulation by lipid peroxidation and AGE/RAGE axis. Thromb. Haemost. 2013, 110, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Effects of resistance training at different loads on inflammatory markers in young adults. Eur. J. Appl. Physiol. 2017, 117, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Markovitch, D.; Betts, J.A.; Mazzatti, D.; Turner, J.; Tyrrell, R.M. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: A randomized-controlled trial. J. Appl. Physiol. 2010, 108, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachi, A.L.; Barros, M.P.; Vieira, R.P.; Rocha, G.A.; de Andrade, P.; Victorino, A.B.; Ramos, L.R.; Gravina, C.F.; Lopes, J.D.; Vaisberg, M.; et al. Combined Exercise Training Performed by Elderly Women Reduces Redox Indexes and Proinflammatory Cytokines Related to Atherogenesis. Oxid. Med. Cell Longev. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tartibian, B.; FitzGerald, L.Z.; Azadpour, N.; Maleki, B.H. A randomized controlled study examining the effect of exercise on inflammatory cytokine levels in post-menopausal women. Post Reprod. Health 2015, 21, 9–15. [Google Scholar] [CrossRef]
- Miles, E.A.; Rees, D.; Banerjee, T.; Cazzola, R.; Lewis, S.; Wood, R.; Oates, R.; Tallant, A.; Cestaro, B.; Yaqoob, P.; et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis 2008, 196, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA Intern. Med. 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorand, B.; Baumert, J.; Döring, A.; Herder, C.; Kolb, H.; Rathmann, W.; Giani, G.; Koenig, W.; KORA Group. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis 2006, 184, 216–224. [Google Scholar] [CrossRef] [PubMed]

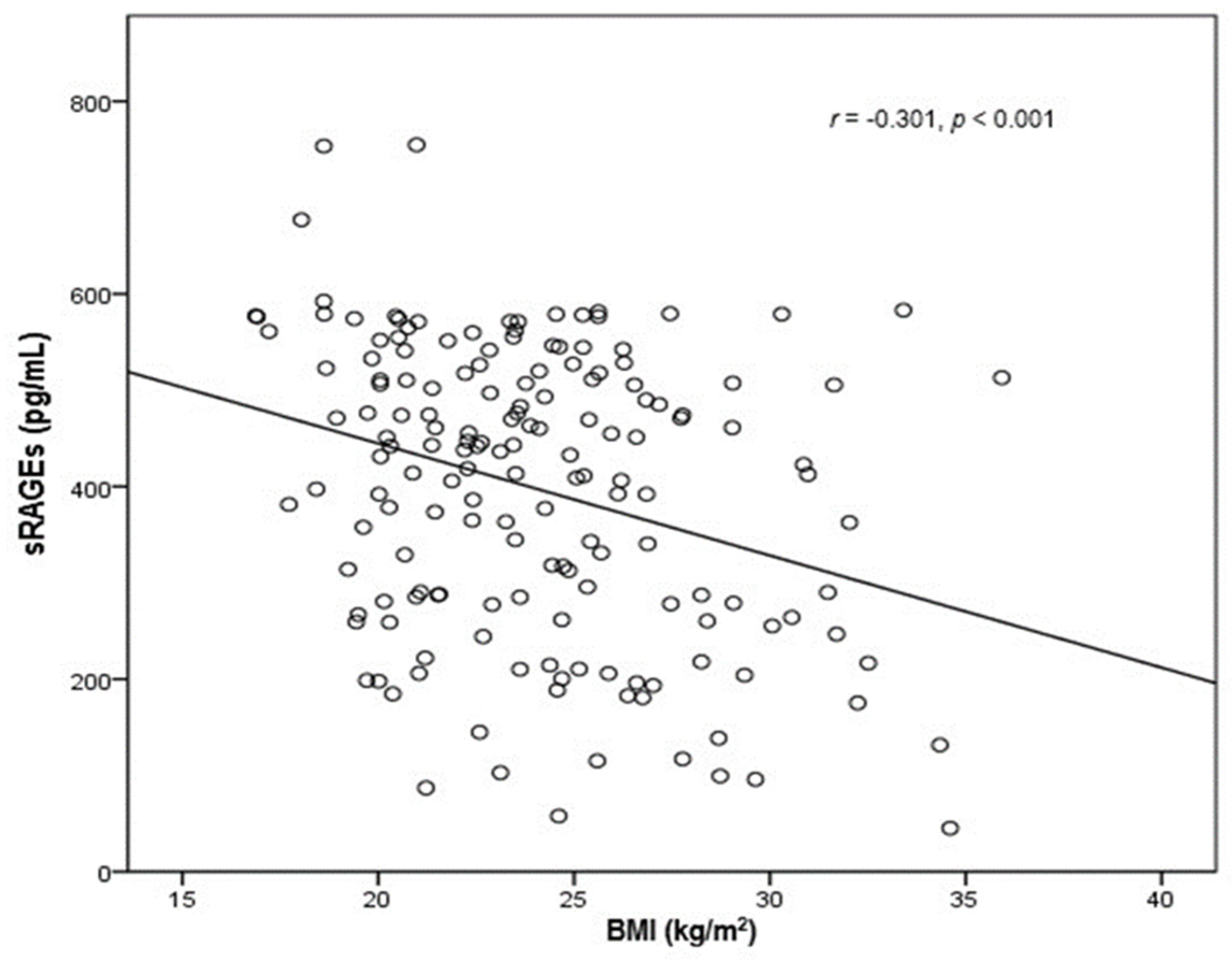


| Age (years) | 21.07 ± 1.63 |
| Weight (kg) | 78.12 ± 13.47 |
| Height (cm) | 180.13 ± 6.3 |
| BMI (kg/m²) | 24.06 ± 3.87 |
| Systolic BP (mm Hg) | 125.88 ± 13.88 |
| Diastolic BP (mm Hg) | 77.29 ± 9.19 |
| BMI (Median Value = 24.035) | |||
|---|---|---|---|
| Below Median | Above Median | ||
| sRAGEs (pg/mL) | |||
| Day 0 | 431.8 ± 138.5 | 363.67 ± 153.48 | p = 0.003 |
| Day 28 | 240.93 ± 179.66 | 199.36 ± 181.77 | p = 0.14 |
| p < 0.001 | p < 0.001 | ||
| S100A8/A9 (ng/mL) | |||
| Day 0 | 606.7 ± 232.5 | 645.9 ± 240.9 | p = 0.287 |
| Day 28 | 506.9 ± 228.8 | 434.4 ± 176.7 | p = 0.023 |
| p = 0.002 | p < 0.001 | ||
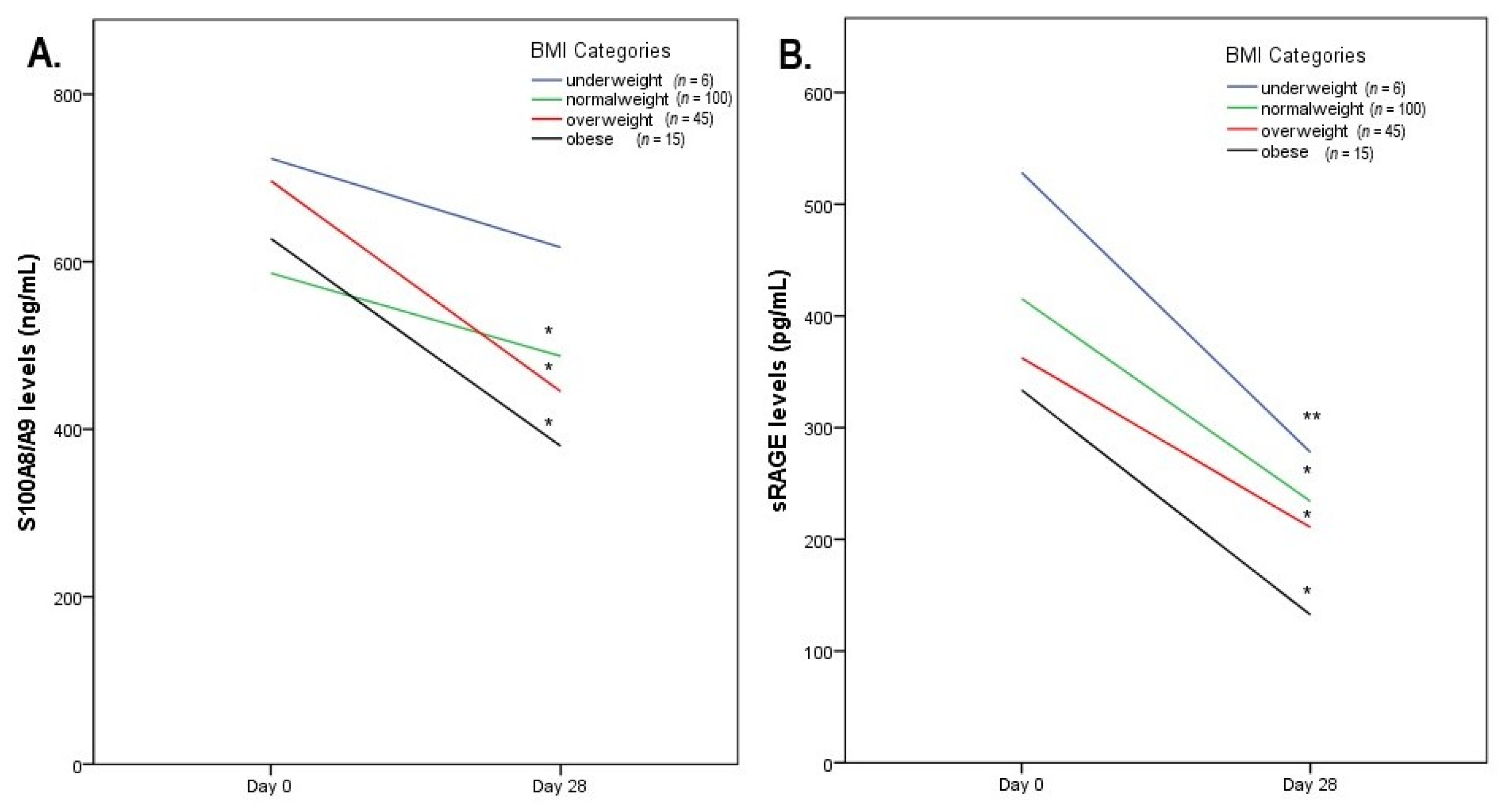
| BMI | ||||
|---|---|---|---|---|
| Underweight (n = 6) | Normal Weight (n = 100) | Overweight (n = 45) | Obese (n = 15) | |
| sRAGEs (pg/mL) | ||||
| Day 0 | 528.3 ± 115.4 | 415.4 ± 140.8 a | 362.47 ± 153.2 a,b | 333.5 ± 165.3 a,b |
| Day 28 | 278 ± 188.4 | 234.1 ± 175.8 | 210.7 ± 206.9 | 132.4 ± 103.1 a,b |
| p = 0.016 | p < 0.001 | p < 0.001 | p < 0.001 | |
| S100A8/A9 (ng/mL) | ||||
| Day 0 | 723.2 ± 165.8 | 586.3 ± 226.2 a | 700.7 ± 256.8 b | 627.5 ± 218.9 |
| Day 28 | 617.1 ± 283.1 | 487.2 ± 218.6 | 444.3 ± 181.5 a | 379.9 ± 112.9 b |
| p = 0.346 | p < 0.001 | p < 0.001 | p < 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drosatos, I.-A.; Tsoporis, J.N.; Izhar, S.; Gupta, S.; Tsirebolos, G.; Sakadakis, E.; Triantafyllis, A.S.; Rigopoulos, A.; Rigopoulos, D.; Rallidis, L.S.; et al. Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits. Biomolecules 2021, 11, 1354. https://doi.org/10.3390/biom11091354
Drosatos I-A, Tsoporis JN, Izhar S, Gupta S, Tsirebolos G, Sakadakis E, Triantafyllis AS, Rigopoulos A, Rigopoulos D, Rallidis LS, et al. Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits. Biomolecules. 2021; 11(9):1354. https://doi.org/10.3390/biom11091354
Chicago/Turabian StyleDrosatos, Ioannis-Alexandros, James N. Tsoporis, Shehla Izhar, Sahil Gupta, George Tsirebolos, Eleftherios Sakadakis, Andreas S. Triantafyllis, Angelos Rigopoulos, Dimitrios Rigopoulos, Loukianos S. Rallidis, and et al. 2021. "Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits" Biomolecules 11, no. 9: 1354. https://doi.org/10.3390/biom11091354







