Transition of Living Arrangement and Cognitive Impairment Status among Chinese Older Adults: Are They Associated?
Abstract
:1. Introduction
1.1. Background
1.2. Literature Gaps
1.3. Purpose of the Study
2. Methods and Materials
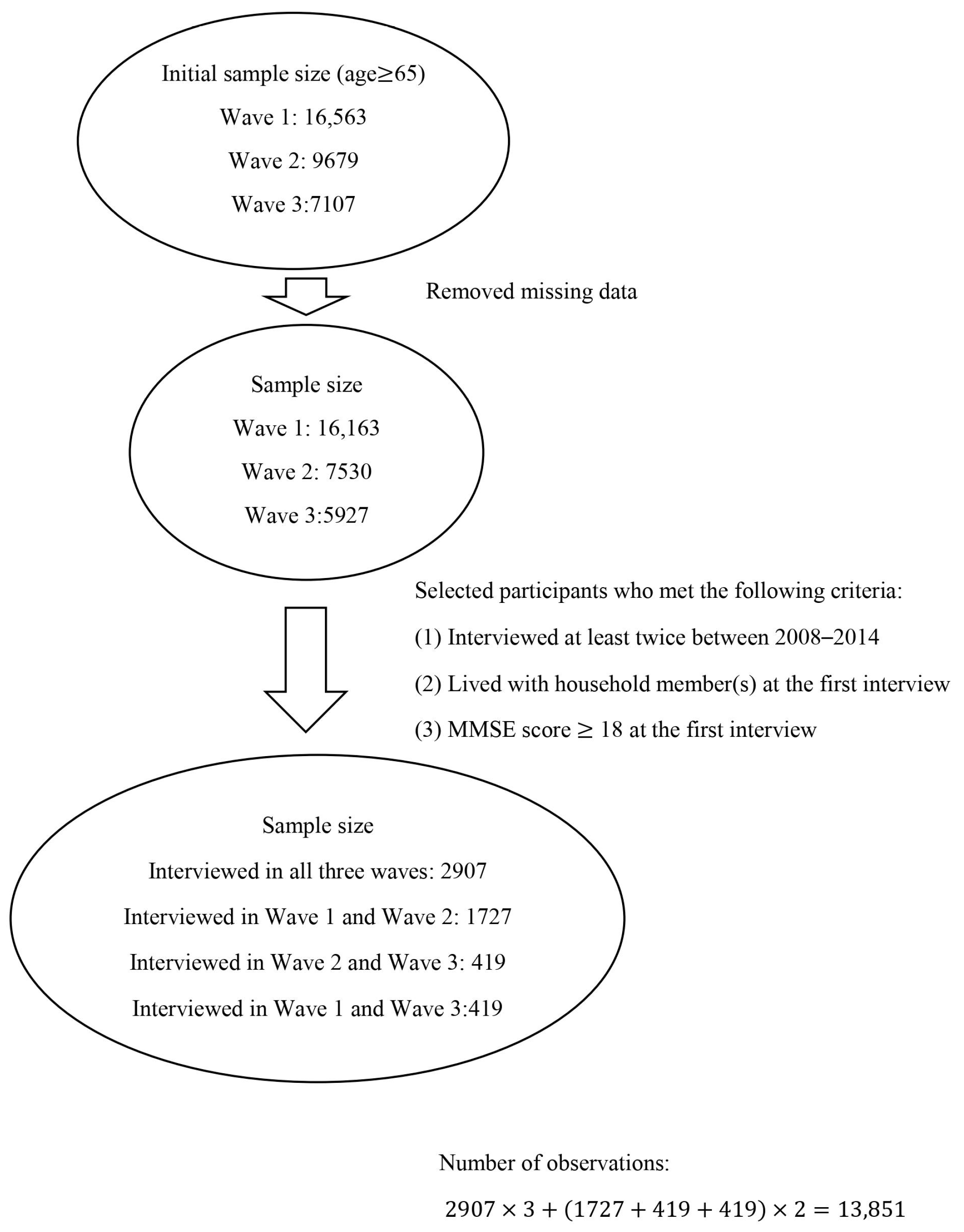
2.1. Study Sample
2.2. Primary Predictor
2.3. Outcome
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Association between Living Arrangement Transition and Changes of Cognitive Function among Chinese Older Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HelpAge International. Global AgeWatch Insights: The Right to Health for Older People, the Right to Be Counted. Available online: http://globalagewatch.org/reports/global-agewatch-insights-2018-report-summary-and-country-profiles/ (accessed on 1 October 2020).
- Lipnicki, D.M.; Crawford, J.; Kochan, N.A.; Trollor, J.N.; Draper, B.; Reppermund, S.; Maston, K.; Mather, K.A.; Brodaty, H.; Sachdev, P.S. Sydney Memory and Ageing Study Team. Risk Factors for Mild Cognitive Impairment, Dementia and Mortality: The Sydney Memory and Ageing Study. J. Am. Med. Dir. Assoc. 2017, 18, 388–395. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Mukadam, N. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Jack, C.R., Jr. Mild Cognitive Impairment: Ten Years Later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The World Health Organization (WHO). Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Available online: https://www.who.int/mental_health/neurology/dementia/guidelines_risk_reduction/en/ (accessed on 21 September 2020).
- Kuang, W.; Gao, M.; Tian, L.; Wan, Y.; Qiu, P. Trends in the prevalence of cognitive impairment in Chinese older adults: Based on the Chinese Longitudinal Healthy Longevity Survey cohorts from 1998 to 2014. Int. Health 2020, 12, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, J.; Wimo, A.; Fratiglioni, L.; Qiu, C. The economic burden of dementia in China, 1990–2030: Implications for health policy. Bull. World Health Organ. 2017, 95, 18–26. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Liu, G.G. Cognitive impairment and mortality among the oldest-old Chinese. Int. J. Geriatr. Psychiatry 2016, 31, 1345–1353. [Google Scholar] [CrossRef]
- Feng, Z.; Falkingham, J.; Liu, X.; Vlachantoni, A. Changes in living arrangements and mortality among older people in China. SSM-Popul. Health 2017, 3, 9–19. [Google Scholar] [CrossRef]
- Evans, I.; Llewellyn, D.J.; Matthews, F.E.; Woods, R.T.; Brayne, C.; Clare, L. CFAS-Wales research team. Living alone and cognitive function in later life. Arch. Gerontol. Geriatr. 2019, 81, 222–233. [Google Scholar] [CrossRef]
- Imamura, H.; Uchiyama, E.; Akiyama, M.; Kaneko, I.; Takebayashi, T.; Nishiwaki, Y. Relationship of living arrangement with the decline in functional capacity in elderly people by gender: A longitudinal observational study. Environ. Health Prev. Med. 2020, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Mazzuco, S.; Meggiolaro, S.; Ongaro, F.; Toffolutti, V. Living arrangement and cognitive decline among older people in Europe. Ageing Soc. 2016, 37, 1111–1133. [Google Scholar] [CrossRef]
- Roystonn, K.; Abdin, E.; Shahwan, S.; Zhang, Y.; Sambasivam, R.; Vaingankar, J.A.; Mahendran, R.; Chua, H.C.; Chong, S.A.; Subramaniam, M. Living arrangements and cognitive abilities of community-dwelling older adults in Singapore. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2020, 20, 625–635. [Google Scholar] [CrossRef]
- Wick, J.Y. Aging in place: Our house Is a very, very, very fine house. Consult. Pharm.® 2017, 32, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, Z. Health and Living Arrangement Transitions among China’s Oldest-old. In Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Yi, Z., Poston, D.L., Vlosky, D.A., Gu, D., Eds.; Springer Science & Business Media: Berlin, Germany, 2008; pp. 215–234. [Google Scholar]
- Feng, Z.; Jones, K.; Wang, W.W. An exploratory discrete-time multilevel analysis of the effect of social support on the survival of elderly people in China. Soc. Sci. Med. 2015, 130, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, T.; Han, B. Does co-residence with adult children associate with better psychological well-being among the oldest old in China? Aging Ment. Health 2014, 18, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, J.; Qi, J. Intergenerational coresidence and subjective well-being of older adults in China: The moderating effect of living arrangement preference and intergenerational contacts. Demogr. Res. 2019, 41, 1347–1372. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Poston, D.L.; Vlosky, D.A.; Gu, D. Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Springer Science & Business Media: Berlin, Germany, 2008; Volume 20. [Google Scholar]
- Zhang, L. Living Arrangements and Subjective Well-Being among the Chinese Elderly. Open J. Soc. Sci. 2015, 3, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Ren, Q.; Treiman, D.J. Living Arrangements of the Elderly in China and Consequences for Their Emotional Well-being. Chin. Sociol. Rev. 2015, 47, 255–286. [Google Scholar] [CrossRef]
- Sereny, M. Living Arrangements of Older Adults in China: The Interplay Among Preferences, Realities, and Health. Res. Aging 2011, 33, 172–204. [Google Scholar] [CrossRef]
- Li, L.W.; Zhang, J.; Liang, J. Health among the oldest-old in China: Which living arrangements make a difference? Soc. Sci. Med. 2009, 68, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Sereny, M.D.; Gu, D. Living arrangement concordance and its association with self-rated health among institutionalized and community-residing older adults in China. J. Cross-Cult. Gerontol. 2011, 26, 239–259. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Z. Social support and self-reported quality of life China’s oldest old. In Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Springer Science & Business Media: Berlin, Germany, 2008; pp. 357–376. [Google Scholar]
- Lund, R.; Due, P.; Modvig, J.; Holstein, B.E.; Damsgaard, M.T.; Andersen, P.K. Cohabitation and marital status as predictors of mortality—an eight year follow-up study. Soc. Sci. Med. 2002, 55, 673–679. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, Y.-C.; Chiang, T.; Liu, C.-T.; Shelley, M. Living Arrangements and Sleep-Related Outcomes Among Older Adults in China: A Panel Analytic Approach. Int. J. Aging Hum. Dev. 2020, 91, 111–126. [Google Scholar] [CrossRef]
- Ren, L.; Zheng, Y.; Wu, L.; Gu, Y.; He, Y.; Jiang, B.; Zhang, J.; Zhang, L.; Li, J. Investigation of the prevalence of Cognitive Impairment and its risk factors within the elderly population in Shanghai, China. Sci. Rep. 2018, 8, 3575. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.D.; Pezzin, L.E.; Rice, J.B. Stability and changes in living arrangements: Relationship to nursing home admission and timing of placement. The Journals of Gerontology. Ser. B Psychol. Sci. Soc. Sci. 2010, 65, 783–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y. Towards deeper research and better policy for healthy aging—Using the unique data of Chinese Longitudinal Healthy Longevity Survey. China Econ. J. 2012, 5, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Gu, D. General data quality assessment of the CLHLS. In Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Springer Science & Business Media: Berlin, Germany, 2008; pp. 39–60. [Google Scholar]
- Pezzotti, P.; Scalmana, S.; Mastromattei, A.; Di Lallo, D. Progetto Alzheimer Working Group. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: A prospective observational study. BMC Fam. Pract. 2008, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Jockusch, J.; Hopfenmuller, W.; Nitschke, I. Chewing function and related parameters as a function of the degree of dementia: Is there a link between the brain and the mouth? J. Oral Rehabil. 2021. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.G.; Peterson, R.A.; Therneau, T.M.; Sanford, B.L.; Stone, R.M.; Mandrekar, S.J. Application of multi-state models in cancer clinical trials. Clin. Trials 2018, 15, 489–498. [Google Scholar] [CrossRef]
- Meira-Machado, L.; de Uña-Alvarez, J.; Cadarso-Suárez, C.; Andersen, P.K. Multi-state models for the analysis of time-to-event data. Stat. Methods Med Res. 2009, 18, 195–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therneau, T.M. Package “Survival”. Available online: https://cran.r-project.org/web/packages/survival/survival.pdf (accessed on 3 December 2020).
- Conroy, R.M.; Golden, J.; Jeffares, I.; O’Neill, D.; McGee, H. Boredom-proneness, loneliness, social engagement and depression and their association with cognitive function in older people: A population study. Psyhology Health Med. 2010, 15, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, P.; Dong, B. Associations between social networks, social contacts, and cognitive function among Chinese nonagenarians/centenarians. Arch. Gerontol. Geriatr. 2015, 60, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-C.J.; Liu, Y.-Y. Influence of social support on cognitive function in the elderly. BMC Health Serv. Res. 2003, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, Z. Health and living arrangement transitions among China’s oldest-old. Res. Aging 2005, 27, 526–555. [Google Scholar] [CrossRef]
- Gu, D.; Sautter, J.; Pipkin, R.; Zeng, Y. Sociodemographic and health correlates of sleep quality and duration among very old Chinese. Sleep 2010, 33, 601–610. [Google Scholar] [CrossRef] [Green Version]
| Overall | Normal (25 ≤ MMSE ≤ 30) | Mild (18 ≤ MMSE ≤ 24) | Moderate (10 ≤ MMSE ≤ 17) | Severe (0 ≤ MMSE ≤ 9) | |
|---|---|---|---|---|---|
| Primary Predictor | n (%) | n (%) | n (%) | n (%) | n (%) |
| Living arrangement | |||||
| Stayed with household member(s) | 12,876 (92.96) | 9747 (75.7) | 2270 (17.63) | 461 (3.58) | 398 (3.09) |
| Became alone | 917 (6.62) | 675 (73.61) | 166 (18.1) | 44 (4.8) | 32 (3.49) |
| Moved to an institution | 58 (0.42) | 32 (55.17) | 16 (27.59) | 3 (5.17) | 7 (12.07) |
| Covariates | |||||
| Age | |||||
| 65–80 | 7007 (50.59) | 6214 (88.68) | 694 (9.9) | 68 (0.97) | 31 (0.44) |
| 81–95 | 5522 (39.87) | 3691 (66.84) | 1337 (24.21) | 277 (5.02) | 217 (3.93) |
| Above 95 | 1322 (9.54) | 549 (41.53) | 421 (31.85) | 163 (12.33) | 189 (14.3) |
| Gender | |||||
| Male | 7107 (51.31) | 5902 (83.04) | 899 (12.65) | 168 (2.36) | 138 (1.94) |
| Female | 6744 (48.69) | 4552 (67.5) | 1553 (23.03) | 340 (5.04) | 299 (4.43) |
| Marital status | |||||
| Married | 7703 (55.61) | 6589 (85.54) | 900 (11.68) | 126 (1.64) | 88 (1.14) |
| Others | 6148 (44.39) | 3865 (62.87) | 1552 (25.24) | 382 (6.21) | 349 (5.68) |
| Received formal education | |||||
| No | 6790 (49.02) | 4335 (63.84) | 1732 (25.51) | 398 (5.86) | 325 (4.79) |
| Yes | 7061 (50.98) | 6119 (86.66) | 720 (10.2) | 110 (1.56) | 112 (1.59) |
| Residential areas | |||||
| Urban | 6547 (47.27) | 5032 (76.86) | 1028 (15.7) | 267 (4.08) | 220 (3.36) |
| Rural | 7304 (52.73) | 5422 (74.23) | 1424 (19.5) | 241 (3.3) | 217 (2.97) |
| Smoking status | |||||
| No | 10,810 (78.04) | 7944 (73.49) | 2036 (18.83) | 435 (4.02) | 395 (3.65) |
| Yes | 3041 (21.96) | 2510 (82.54) | 416 (13.68) | 73 (2.4) | 42 (1.38) |
| Alcohol use status | |||||
| No | 11,125 (80.32) | 8217 (73.86) | 2068 (18.59) | 446 (4.01) | 394 (3.54) |
| Yes | 2726 (19.68) | 2237 (82.06) | 384 (14.09) | 62 (2.27) | 43 (1.58) |
| Number of times suffering from chronic conditions that required inpatient treatments in the past two years | |||||
| None | 10,943 (79.01) | 8358 (76.38) | 1881 (17.19) | 380 (3.47) | 324 (2.96) |
| 1–2 | 2530 (18.27) | 1830 (72.33) | 506 (20) | 102 (4.03) | 92 (3.64) |
| Above 2 | 378 (2.73) | 266 (70.37) | 65 (17.2) | 26 (6.88) | 21 (5.56) |
| Life satisfaction | |||||
| Good | 8727 (63.01) | 6800 (77.92) | 1473 (16.88) | 309 (3.54) | 145 (1.66) |
| Neutral | 4350 (31.41) | 3279 (75.38) | 821 (18.87) | 163 (3.75) | 87 (2) |
| Bad | 573 (4.14) | 369 (64.4) | 151 (26.35) | 30 (5.24) | 23 (4.01) |
| Not able to answer | 201 (1.45) | 6 (2.99) | 7 (3.48) | 6 (2.99) | 182 (90.55) |
| Health status | |||||
| Good | 6762 (48.82) | 5437 (80.41) | 1033 (15.28) | 207 (3.06) | 85 (1.26) |
| Neutral | 4909 (35.44) | 3708 (75.53) | 914 (18.62) | 190 (3.87) | 97 (1.98) |
| bad | 1977 (14.27) | 1302 (65.86) | 499 (25.24) | 105 (5.31) | 71 (3.59) |
| Not able to answer | 203 (1.47) | 7 (3.45) | 6 (2.96) | 6 (2.96) | 184 (90.64) |
| Sleep quality | |||||
| Good | 8889 (64.18) | 6882 (77.42) | 1475 (16.59) | 303 (3.41) | 229 (2.58) |
| Bad | 4962 (35.82) | 3572 (71.99) | 977 (19.69) | 205 (4.13) | 208 (4.19) |
| Wave | |||||
| 2009 | 5053 (36.48) | 4038 (79.91) | 1015 (20.09) | 0 (0) | 0 (0) |
| 2012 | 5053 (36.48) | 3746 (74.13) | 848 (16.78) | 268 (5.3) | 191 (3.78) |
| 2014 | 3745 (27.04) | 2670 (71.3) | 589 (15.73) | 240 (6.41) | 246 (6.57) |
| Normal/Mild to Moderate/Severe | Moderate/Severe to Moderate/Severe | |||
|---|---|---|---|---|
| Primary Predictor | HR | 95% CI | HR | 95% CI |
| Living arrangement | ||||
| Stayed with household member(s) | ||||
| Became alone | 0.66 ** | (0.52, 0.83) | 0.91 | (0.46, 1.77) |
| Moved to an institution | 0.94 | (0.50, 1.77) | 2.14 | (0.88, 5.20) |
| Covariates | ||||
| Age | ||||
| 65–80 | ||||
| 81–95 | 3.82 ** | (3.05, 4.79) | 5.31 * | (1.15, 24.47) |
| Above 95 | 7.68 ** | (5.96, 9.90) | 7.00 * | (1.43, 34.33) |
| Gender | ||||
| Male | ||||
| Female | 1.14 | (0.97, 1.34) | 0.92 | (0.51, 1.63) |
| Marital status | ||||
| Married | ||||
| Others | 1.72 ** | (1.45, 2.05) | 0.79 | (0.42, 1.47) |
| Received formal education | ||||
| No | ||||
| Yes | 0.55 ** | (0.46, 0.65) | 0.50 | (0.23, 1.09) |
| Residential areas | ||||
| Urban | ||||
| Rural | 0.99 | (0.87, 1.12) | 0.89 | (0.64, 1.24) |
| Smoking status | ||||
| No | ||||
| Yes | 0.95 | (0.78, 1.17) | 0.59 | (0.28, 1.23) |
| Alcohol use status | ||||
| No | ||||
| Yes | 0.84 | (0.69, 1.04) | 0.82 | (0.38, 1.76) |
| Number of times suffering from chronic conditions that required inpatient treatments in the past two years | ||||
| None | ||||
| 1–2 | 1.00 | (0.85, 1.18) | 0.97 | (0.64, 1.49) |
| Above 2 | 1.33 | (0.97, 1.82) | 0.93 | (0.40, 2.16) |
| Life satisfaction | ||||
| Good | ||||
| Neutral | 1.20 * | (1.01, 1.43) | 1.56 | (0.85, 2.85) |
| Bad | 1.70 ** | (1.22, 2.37) | 1.44 | (0.50, 4.12) |
| Not able to answer | 3.91 ** | (2.09, 7.33) | 1.48 | (0.28, 7.74) |
| Health status | ||||
| Good | ||||
| Neutral | 1.11 | (0.92, 1.32) | 0.87 | (0.44, 1.75) |
| Bad | 1.63 ** | (1.31, 2.03) | 1.16 | (0.52, 2.58) |
| Not able to answer | 3.25 ** | (1.72, 6.16) | 2.35 | (0.43, 12.76) |
| Sleep quality | ||||
| Good | ||||
| Bad | 1.06 | (0.92, 1.22) | 1.03 | (0.72, 1.46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Lin, C.-H.; Chang, J.-R.; Liu, C.-T.; Shelley, M.; Chang, Y.-C. Transition of Living Arrangement and Cognitive Impairment Status among Chinese Older Adults: Are They Associated? Medicina 2021, 57, 961. https://doi.org/10.3390/medicina57090961
Lee Y-H, Lin C-H, Chang J-R, Liu C-T, Shelley M, Chang Y-C. Transition of Living Arrangement and Cognitive Impairment Status among Chinese Older Adults: Are They Associated? Medicina. 2021; 57(9):961. https://doi.org/10.3390/medicina57090961
Chicago/Turabian StyleLee, Yen-Han, Chia-Hung Lin, Jia-Ren Chang, Ching-Ti Liu, Mack Shelley, and Yen-Chang Chang. 2021. "Transition of Living Arrangement and Cognitive Impairment Status among Chinese Older Adults: Are They Associated?" Medicina 57, no. 9: 961. https://doi.org/10.3390/medicina57090961
APA StyleLee, Y.-H., Lin, C.-H., Chang, J.-R., Liu, C.-T., Shelley, M., & Chang, Y.-C. (2021). Transition of Living Arrangement and Cognitive Impairment Status among Chinese Older Adults: Are They Associated? Medicina, 57(9), 961. https://doi.org/10.3390/medicina57090961







