Tetrahexyldecyl Ascorbate (THDC) Degrades Rapidly under Oxidative Stress but Can Be Stabilized by Acetyl Zingerone to Enhance Collagen Production and Antioxidant Effects
Abstract
1. Introduction
2. Results
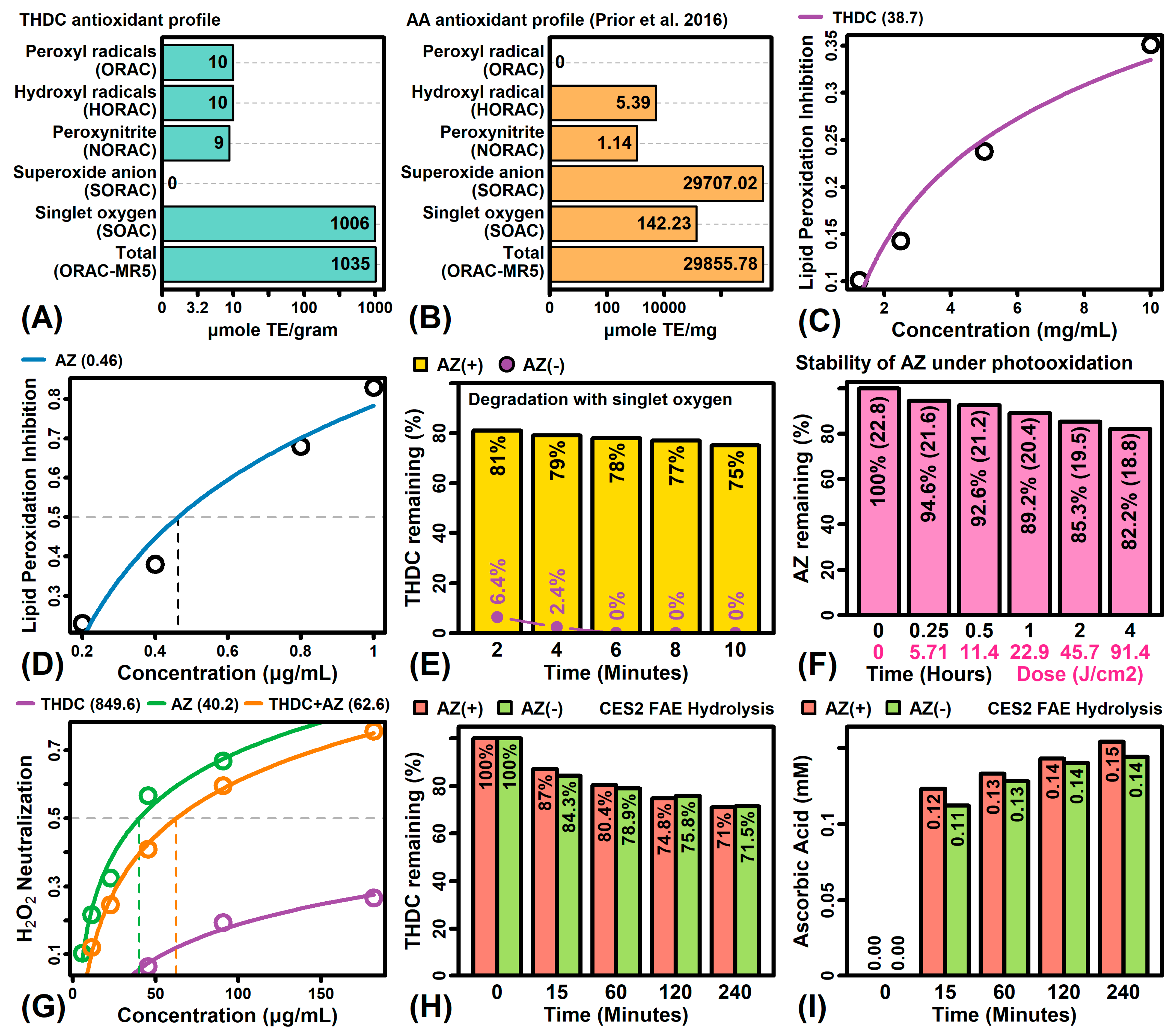
2.1. THDC Is a Poor Antioxidant That Degrades Rapidly in the Presence of Singlet Oxygen
2.2. THDC Activates Type I Interferon Signaling in the Absence of AZ
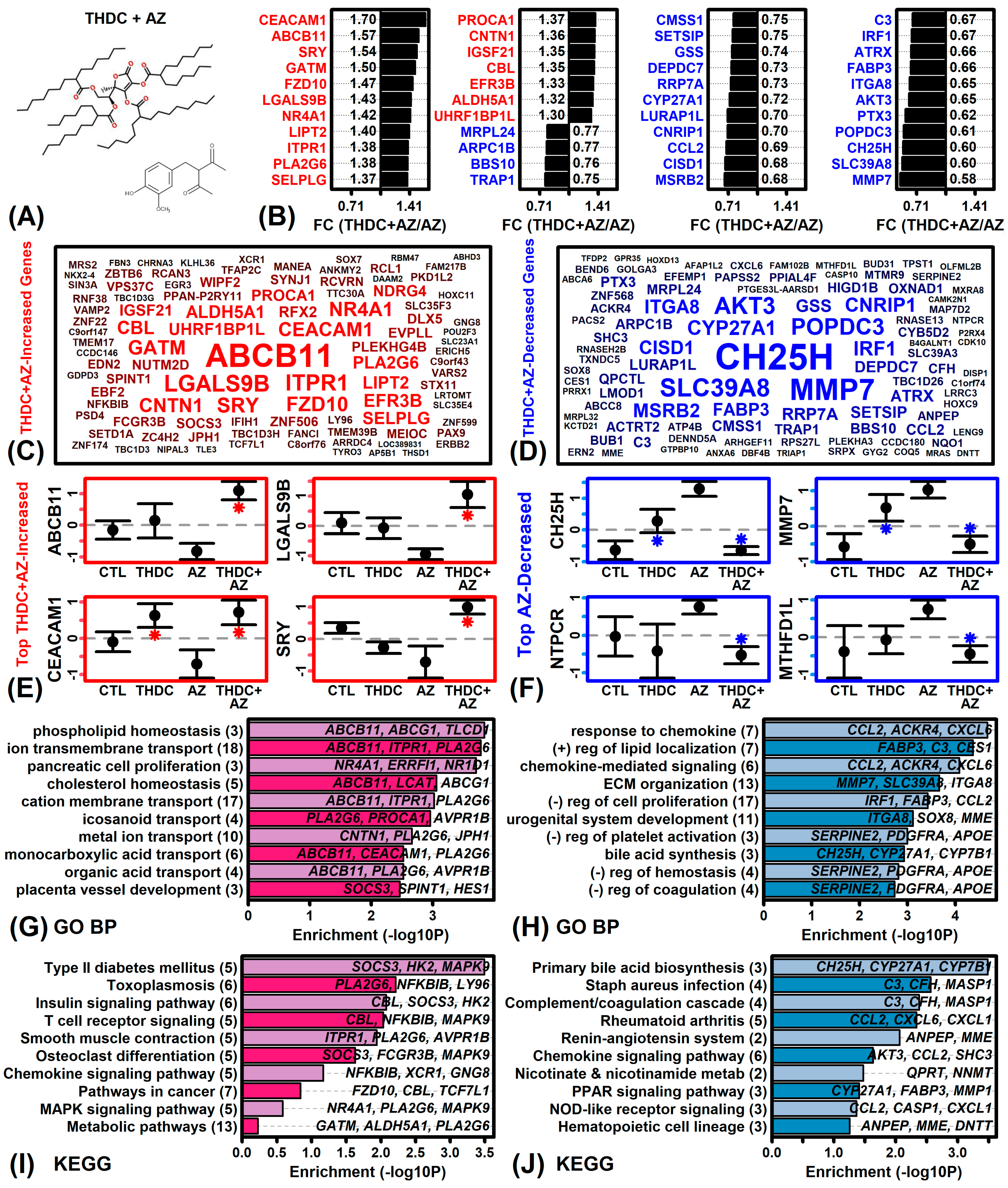
2.3. THDC in the Presence of AZ Up-Regulates Phospholipid Homeostasis Genes While Repressing Chemokine Signaling Genes
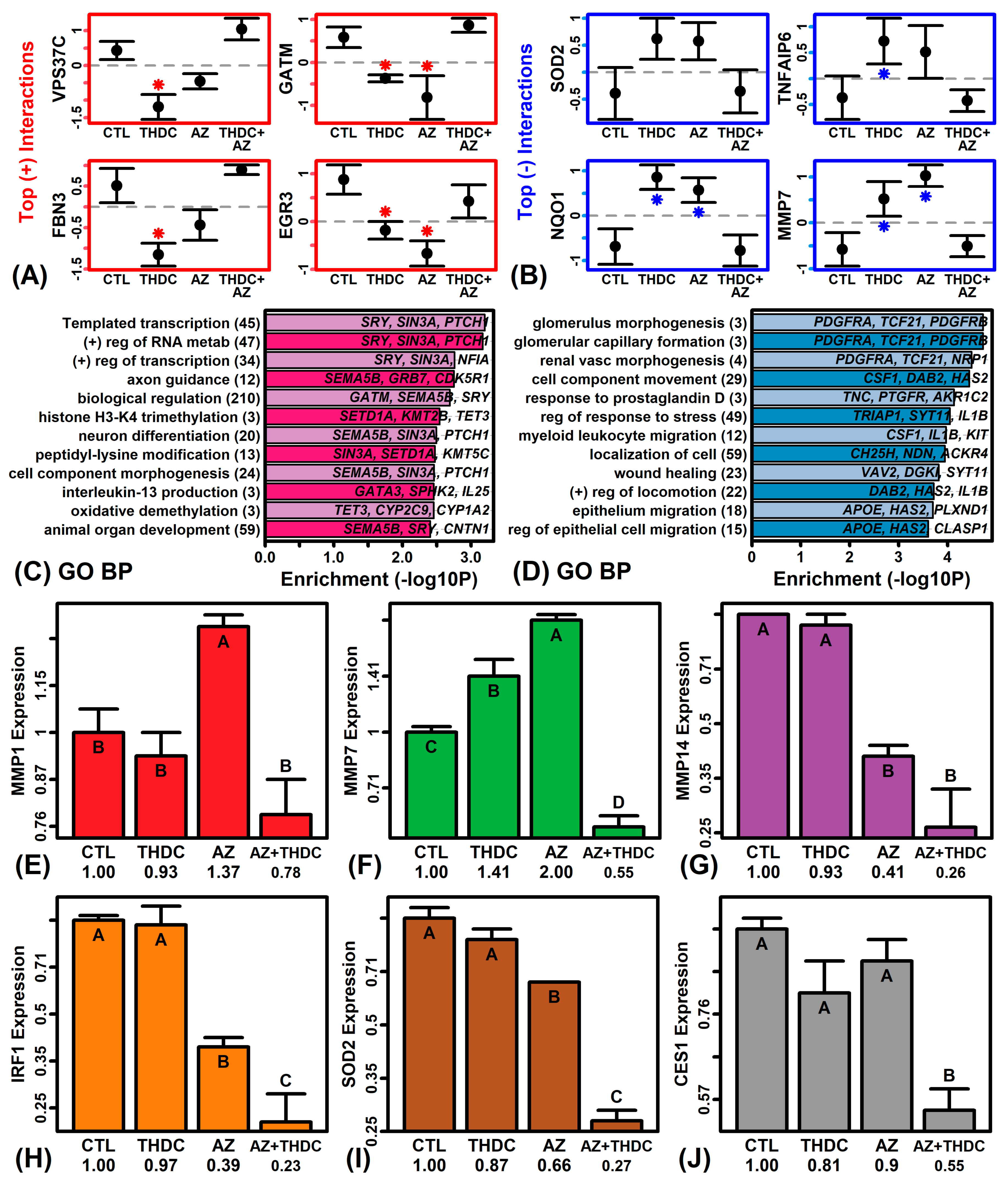
2.4. MMP7 and NQO1 Have Differential Responses to THDC in AZ(−) and AZ(+) Conditions
2.5. AZ Moderates Pro-Inflammatory Gene Expression Changes Observed with THDC Treatment
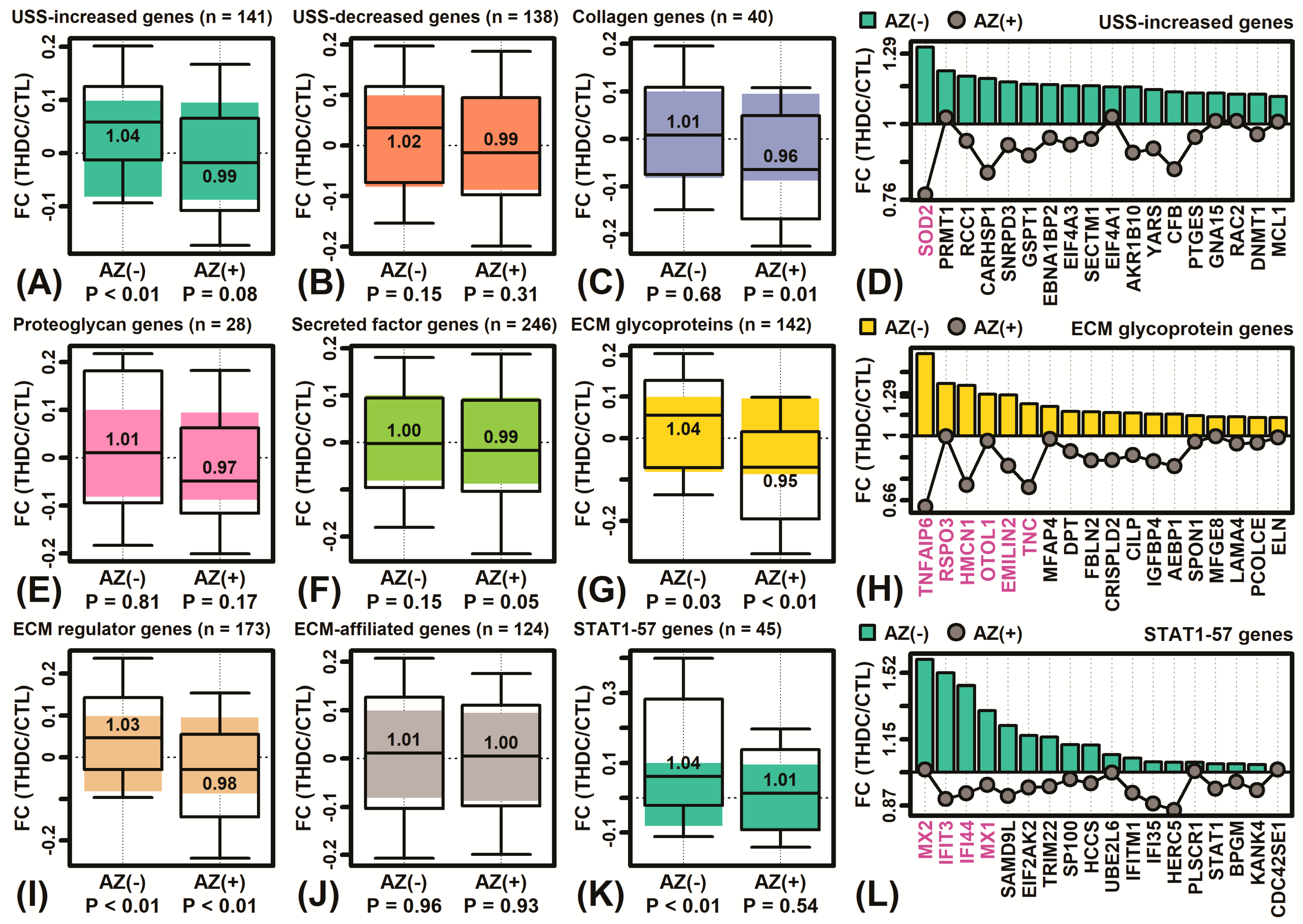
2.6. The THDC + AZ Combination Triggers Gene Expression Shifts Similar to Those Seen during KC Differentiation
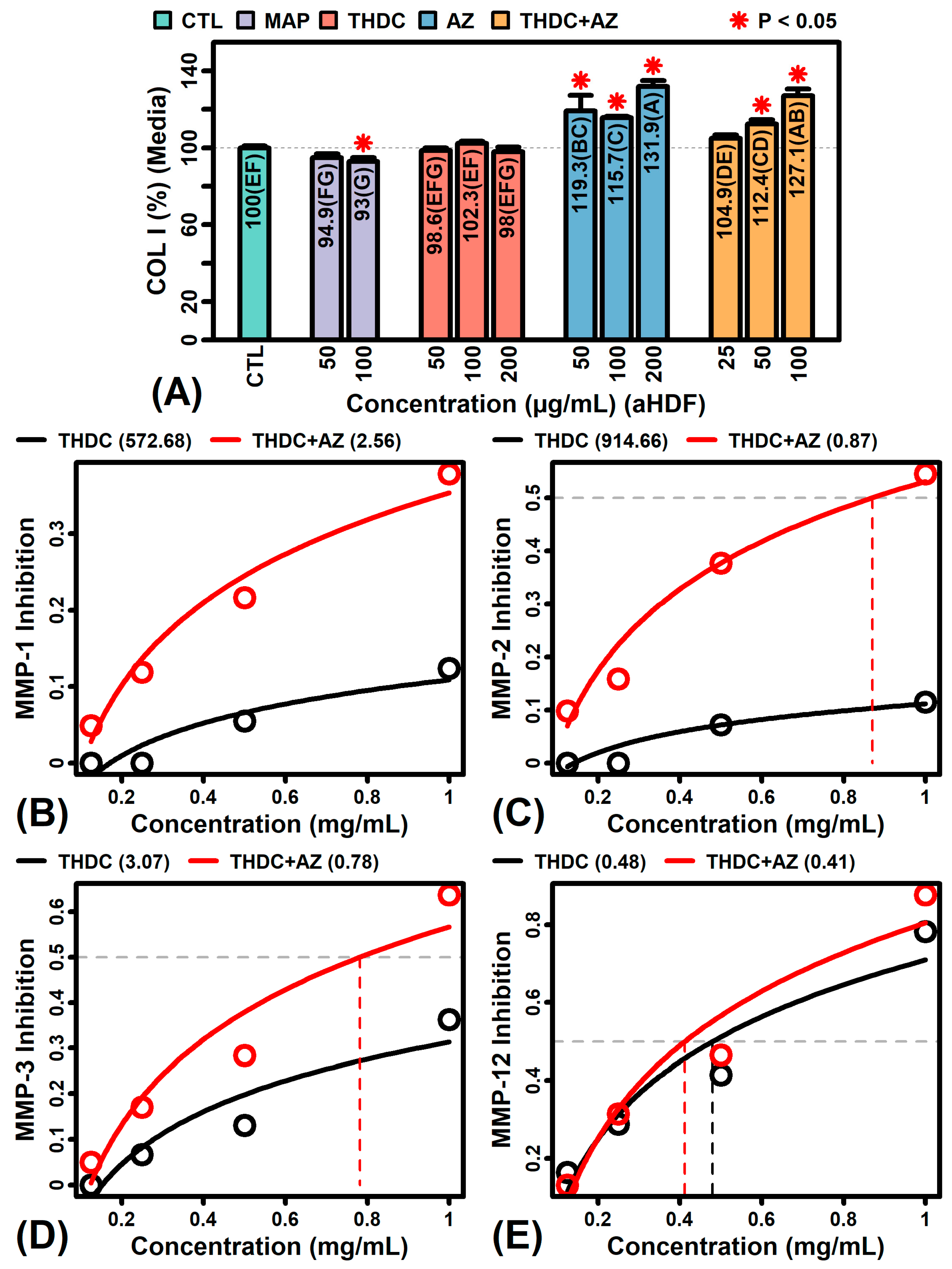
2.7. The Addition of AZ to THDC Augments Collagen Protein and Inhibits MMP Expression and Activity
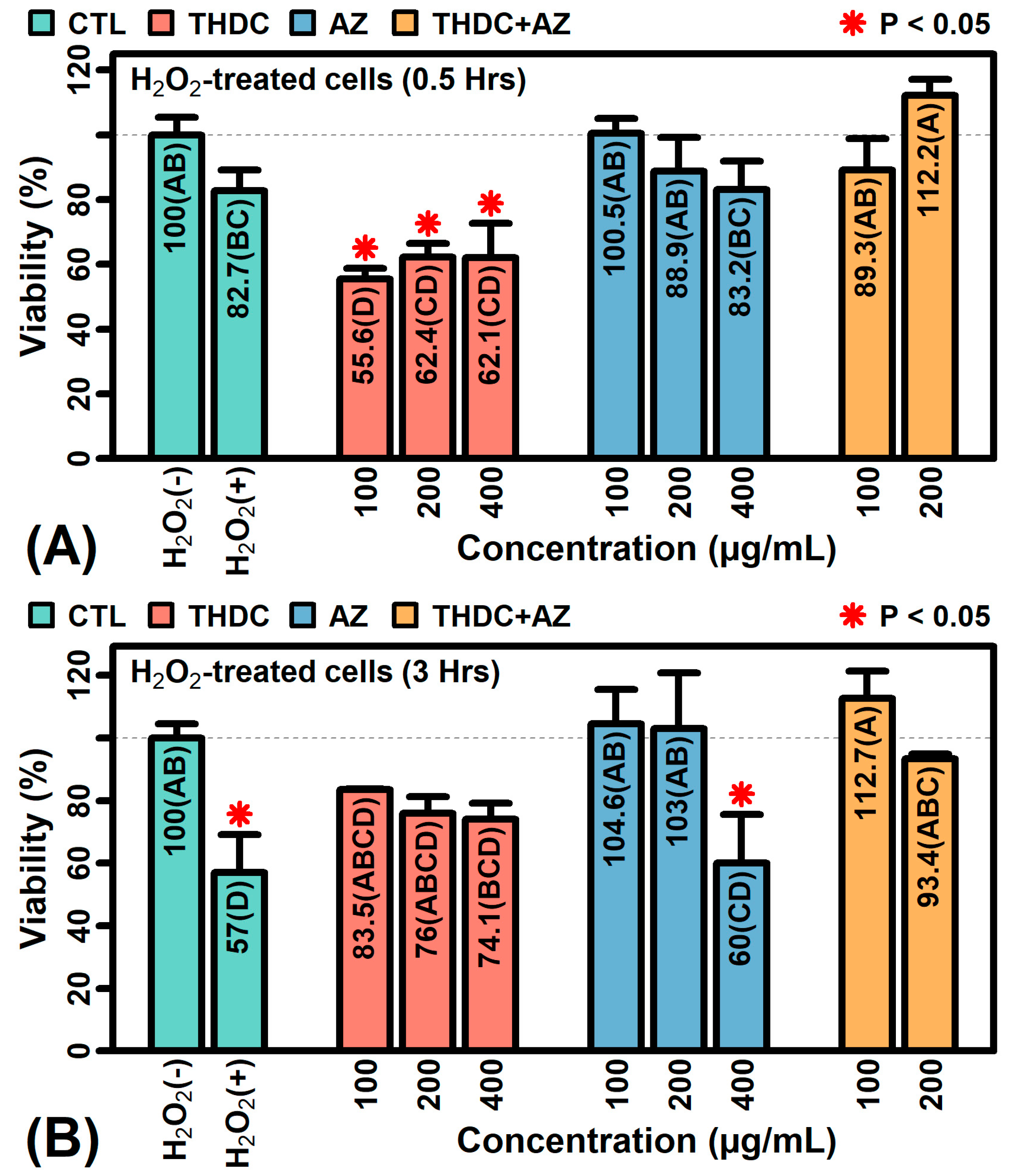
2.8. The Combination AZ + THDC Improves Survival of KCs Treated with Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. THDC Antioxidant Capacity
4.2. Effects of THDC and AZ on Lipid Peroxidation
4.3. THDC Degradation under Singlet Oxygen
4.4. Stability of AZ under Photooxidation
4.5. H2O2 Scavenging Activity
4.6. THDC Fatty Acid Ester Hydrolysis
4.7. THDC HPLC Analysis
4.8. Microarray Profiling of THDC, AZ, and THDC + AZ Expression Responses
4.9. Differential Expression Analyses
4.10. Real-Time Quantitative PCR
4.11. Effects of THDC (±AZ) on Collagen Production
4.12. Effect of THDC (±AZ) on MMP Activity
4.13. Effect of THDC (±AZ) on Survival of H2O2-Stressed KCs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | L-Ascorbicacid |
| AAPH | 2,2′-Azobis(2-amidino-propane)dihydrochloride |
| aHDF | adulthumanadultdermalfibroblasts |
| AZ | acetylzingeroneBP |
| CTL | control |
| DEG | differentiallyexpressedgene |
| GO | geneontology |
| HE | hydroethidine |
| HORAC | hydroxylradicalscavengingcapacity |
| HPLC | high-performanceliquidchromatography |
| IC50 | half-maximalinhibitoryconcentration |
| KC | keratinocyte |
| KEGG | kyotoEncyclopediaofgenesandgenomes |
| MDA | malonaldehyde |
| MMP | matrixmetallopeptidase |
| nHDF | neonatalhumanadultdermalfibroblasts |
| NORAC | peroxynitritescavengingcapacity |
| NUSE | normalizedunscaledstandarderrors |
| ORAC | oxygenradicalabsorbancecapacity |
| RHE | reconstitutedhumanepidermis |
| RLE | relativelogexpression |
| SOAC | singletoxygenscavengingcapacity |
| SORAC | superoxideanionscavengingcapacity |
| THDC | tetrahexyldecylascorbate |
| UVA | ultravioletAlight |
| UVB | ultravioletBlight |
References
- Thomas, J.M.; Burtson, K.M. Scurvy: A Case Report and Literature Review. Cureus 2021, 13, e14312. [Google Scholar] [PubMed]
- Pan, T.; Hennrikus, E.F.; Hennrikus, W.L. Modern Day Scurvy in Pediatric Orthopaedics: A Forgotten Illness. J. Pediatr. Orthop. 2021, 41, e279–e284. [Google Scholar] [CrossRef]
- Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991, 13, S2–S7. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Saito, N.; Kurita, K.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.L.; Cooke, M.S.; Jones, G.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free. Radic. Biol. Med. 2009, 46, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Steiling, H.; Longet, K.; Moodycliffe, A.; Mansourian, R.; Bertschy, E.; Smola, H.; Mauch, C.; Williamson, G. Sodium-dependent vitamin C transporter isoforms in skin: Distribution, kinetics, and effect of UVB-induced oxidative stress. Free. Radic. Biol. Med. 2007, 43, 752–762. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Rossi, A.; Duranti, G.; Melino, G.; Avigliano, L. Characterization of keratinocyte differentiation induced by ascorbic acid: Protein kinase C involvement and vitamin C homeostasis. J. Investig. Dermatol. 2002, 118, 372–379. [Google Scholar] [CrossRef]
- Pasonen-Seppänen, S.; Suhonen, T.M.; Kirjavainen, M.; Suihko, E.; Urtti, A.; Miettinen, M.; Hyttinen, M.; Tammi, M.; Tammi, R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 2001, 116, 287–297. [Google Scholar] [CrossRef]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mulder, A.; Gooris, G.S.; Bouwstra, J.; Mommaas, A.M. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Investig. Dermatol. 1997, 109, 348–355. [Google Scholar] [CrossRef]
- Sauermann, K.; Jaspers, S.; Koop, U.; Wenck, H. Topically applied vitamin C increases the density of dermal papillae in aged human skin. BMC Dermatol. 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-ethyl-l-ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.H.B.; Frame, J.; Zhang, J.; Najlah, M. Comparative Study on the Outcome of Periorbital Wrinkles Treated with Laser-Assisted Delivery of Vitamin C or Vitamin C Plus Growth Factors: A Randomized, Double-blind, Clinical Trial. Aesthetic Plast. Surg. 2021, 45, 1020–1032. [Google Scholar] [CrossRef]
- Darr, D.; Combs, S.; Dunston, S.; Manning, T.; Pinnell, S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br. J. Dermatol. 1992, 127, 247–253. [Google Scholar] [CrossRef]
- Doi, N.; Togari, H.; Minagi, K.; Iwaoka, Y.; Tai, A.; Nakaoji, K.; Hamada, K.; Tatsuka, M. 2-O-Octadecylascorbic acid represses RhoGDIβ expression and ameliorates DNA damage-induced abnormal spindle orientations. J. Cell. Biochem. 2021, 122, 739–751. [Google Scholar] [CrossRef]
- Gęgotek, A.; Łuczaj, W.; Skrzydlewska, E. Effects of Natural Antioxidants on Phospholipid and Ceramide Profiles of 3D-Cultured Skin Fibroblasts Exposed to UVA or UVB Radiation. Antioxidants 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Yun, I.S.; Yoo, H.S.; Kim, Y.O.; Rah, D.K. Improved scar appearance with combined use of silicone gel and vitamin C for Asian patients: A comparative case series. Aesthetic Plast. Surg. 2013, 37, 1176–1181. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Lakra, R.; Kiran, M.S.; Korrapati, P.S. Effect of magnesium ascorbyl phosphate on collagen stabilization for wound healing application. Int. J. Biol. Macromol. 2021, 166, 333–341. [Google Scholar] [CrossRef]
- Basketter, D.A.; White, I.R.; Kullavanijaya, P.; Tresukosol, P.; Wichaidit, M.; McFadden, J.P. Influence of vitamin C on the elicitation of allergic contact dermatitis to p-phenylenediamine. Contact Dermat. 2016, 74, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Kelm, R.C.; Zahr, A.S.; Kononov, T.; Ibrahim, O. Effective lightening of facial melasma during the summer with a dual regimen: A prospective, open-label, evaluator-blinded study. J. Cosmet. Dermatol. 2020, 19, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Hseu, Y.C.; Gowrisankar, Y.V.; Chung, Y.T.; Zhang, Y.Z.; Way, T.D.; Yang, H.L. The anti-melanogenic effects of 3-O-ethyl ascorbic acid via Nrf2-mediated α-MSH inhibition in UVA-irradiated keratinocytes and autophagy induction in melanocytes. Free. Radic. Biol. Med. 2021, 173, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; John, A.M.; Tam, A.; Firoz, B.F. The Tam formula: A pilot study for a new treatment for melasma. J. Cosmet. Dermatol. 2021, 20, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Laughter, M.R.; Anderson, J.B.; Sadeghpour, M. Emerging topical therapies to treat pigmentary disorders: An evidence-based approach. J. Dermatol. Treat. 2021, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021. [Google Scholar] [CrossRef]
- Shin, J.; Kim, Y.J.; Kwon, O.; Kim, N.I.; Cho, Y. Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis. Nutr. Res. Pract. 2016, 10, 398–403. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.; Jo, H.; Go, C.; Jeong, Y.; Jang, Y.; Kang, D.; Park, K.; Kim, Y.S.; Kang, J.S. The Anti-Inflammatory Effect of Aptamin C on House Dust Mite Extract-Induced Inflammation in Keratinocytes via Regulation of IL-22 and GDNF Production. Antioxidants 2021, 10, 945. [Google Scholar] [CrossRef]
- La Padula, S.; Hersant, B.; Pizza, C.; Chesné, C.; Jamin, A.; Mosbah, I.B.; Errico, C.; D’Andrea, F.; Rega, U.; Persichetti, P.; et al. Striae Distensae: In Vitro Study and Assessment of Combined Treatment with Sodium Ascorbate and Platelet-Rich Plasma on Fibroblasts. Aesthetic Plast. Surg. 2021, 45, 1282–1293. [Google Scholar] [CrossRef]
- Pernice, C.; Murri, D.; Valli, R.; Crocetta, F.M.; Iori, M.; Asti, M.; Ghidini, A.; Capponi, P.C. Complete response of cutaneous SCC to topical treatment with ascorbic acid solution: A case report. Clin. Case Rep. 2021, 9, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Holló, P.; Jókai, H.; Hársing, J.; Soós, G.; Kárpáti, S.; Németh, K. Topically applied ascorbic acid solution for the treatment of basal cell carcinoma (BCC). J. Am. Acad. Dermatol. 2016, 75, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Coerdt, K.M.; Goggins, C.A.; Khachemoune, A. Vitamins A, B, C, and D: A Short Review for the Dermatologist. Altern. Ther. Health Med. 2021, 27, 41–49. [Google Scholar]
- Kitahata, K.; Matsuo, K.; Hara, Y.; Naganuma, T.; Oiso, N.; Kawada, A.; Nakayama, T. Ascorbic acid derivative DDH-1 ameliorates psoriasis-like skin lesions in mice by suppressing inflammatory cytokine expression. J. Pharmacol. Sci. 2018, 138, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; de Azevedo, J.R.; Buri, M.V.; Bolzinger, M.A.; Chevalier, Y.; Riske, K.A.; Leonardi, G.R. Stabilization of vitamin C in emulsions of liquid crystalline structures. Int. J. Pharm. 2021, 592, 120092. [Google Scholar] [CrossRef] [PubMed]
- Colven, R.M.; Pinnell, S.R. Topical vitamin C in aging. Clin. Dermatol. 1996, 14, 227–234. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Stamford, N.P. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kramarenko, G.G.; Hummel, S.G.; Martin, S.M.; Buettner, G.R. Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem. Photobiol. 2006, 82, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.L.; Almeida, G.M.; Jones, G.D. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol. Lett. 2007, 170, 57–65. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takehana, M.; Itoh, S.; Ogata, E. Protective effect of magnesium-L-ascorbyl-2 phosphate against skin damage induced by UVB irradiation. Photochem. Photobiol. 1996, 64, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Shokri, J.; Ranjkesh, M.; Hamed, S.A.; Monajjemzadeh, F. Comparative physicochemical stability and clinical anti-wrinkle efficacy of transdermal emulgel preparations of 5% sodium ascorbyl phosphate and or ascorbic acid on human volunteers. J. Cosmet. Dermatol. 2021, 20, 174–180. [Google Scholar] [CrossRef]
- Tran, V.V.; Hoang, V.K.B.; Chun, H.S.; Moon, J.Y.; Lee, Y.C. Novel Calcium Aminoclay (CaAC)-Vitamin C Hybrid: In-Vitro Cytotoxicity Assessment in HaCaT Cells and In-Vivo Embryotoxicity Assay in Zebrafish. J. Nanosci. Nanotechnol. 2021, 21, 3667–3672. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K.; Yoshida, Y.; Suzuki, R.; Yoshizawa, K.; Mori, K.; Itakura, S.; Takayama, K.; Todo, H. Physical Properties of an Ionic Liquid Composed of Two Water-Soluble Vitamins and Enhanced Skin Permeation of Both Vitamins. Pharmaceutics 2020, 12, 427. [Google Scholar] [CrossRef]
- Fitzpatrick, R.E.; Rostan, E.F. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol. Surg. 2002, 28, 231–236. [Google Scholar]
- Herndon, J.H. Jr.; Jiang, L.; Kononov, T.; Fox, T. An Open Label Clinical Trial of a Multi-Ingredient Anti-Aging Moisturizer Designed to Improve the Appearance of Facial Skin. J. Drugs Dermatol. 2015, 14, 699–704. [Google Scholar]
- Makino, E.T.; Mehta, R.C.; Banga, A.; Jain, P.; Sigler, M.L.; Sonti, S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J. Drugs Dermatol. 2013, 12, s16–s20. [Google Scholar] [PubMed]
- Swindell, W.R.; Bojanowski, K.; Chaudhuri, R.K. A Zingerone Analog, Acetyl Zingerone, Bolsters Matrisome Synthesis, Inhibits Matrix Metallopeptidases, and Represses IL-17A Target Gene Expression. J. Investig. Dermatol. 2020, 140, 602–614.e15. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Meyer, T.; Premi, S.; Brash, D. Acetyl zingerone: An efficacious multifunctional ingredient for continued protection against ongoing DNA damage in melanocytes after sun exposure ends. Int. J. Cosmet. Sci. 2020, 42, 36–45. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Rybak, I.; Pourang, A.; Burney, W.; Haas, K.; Sandhu, S.; Crawford, R.; Sivamani, R. Randomized double-blind vehicle controlled study of the effects of topical acetyl zingerone on photoaging. J. Cosmet. Dermatol. 2021, 20, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Sintara, M.; Chang, T. Multi-radical (ORAC MR5) antioxidant capacity of selected berries and effects of food processing. J. Berry Res. 2016, 6, 159–173. [Google Scholar] [CrossRef]
- Zhu, Q.G.; Hu, J.H.; Liu, J.Y.; Lu, S.W.; Liu, Y.X.; Wang, J. Stereoselective characteristics and mechanisms of epidermal carboxylesterase metabolism observed in HaCaT keratinocytes. Biol. Pharm. Bull. 2007, 30, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.J.; Robinson, M.K.; Sherrill, J.D.; Schnell, D.J.; Xu, J. Analysis of gene expression profiles of multiple skin diseases identifies a conserved signature of disrupted homeostasis. Exp. Dermatol. 2018, 27, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Johnston, A.; Xing, X.; Voorhees, J.J.; Elder, J.T.; Gudjonsson, J.E. Modulation of epidermal transcription circuits in psoriasis: New links between inflammation and hyperproliferation. PLoS ONE 2013, 8, e79253. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nocchi, S.; Björklund, S.; Svensson, B.; Engblom, J.; Ruzgas, T. Electrochemical monitoring of native catalase activity in skin using skin covered oxygen electrode. Biosens. Bioelectron. 2017, 93, 9–13. [Google Scholar] [CrossRef]
- Maresca, V.; Flori, E.; Briganti, S.; Camera, E.; Cario-André, M.; Taïeb, A.; Picardo, M. UVA-induced modification of catalase charge properties in the epidermis is correlated with the skin phototype. J. Investig. Dermatol. 2006, 126, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, L.; Corstjens, H.; Neven, A.; Declercq, L.; Maes, D. Antioxidant enzyme activity in human stratum corneum shows seasonal variation with an age-dependent recovery. J. Investig. Dermatol. 2003, 120, 434–439. [Google Scholar] [CrossRef]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef]
- Farris, P.K. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–817. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Dijkhoff, I.M.; Petracca, B.; Prieux, R.; Valacchi, G.; Rothen-Rutishauser, B.; Eeman, M. Cultivating a Three-dimensional Reconstructed Human Epidermis at a Large Scale. J. Vis. Exp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef]
- Boyce, S.T.; Supp, A.P.; Swope, V.B.; Warden, G.D. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J. Investig. Dermatol. 2002, 118, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Sarkar, M.K.; Liang, Y.; Xing, X.; Gudjonsson, J.E. Cross-Disease Transcriptomics: Unique IL-17A Signaling in Psoriasis Lesions and an Autoimmune PBMC Signature. J. Investig. Dermatol. 2016, 136, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Martinez, C.G.; West, P. Oxidative stress enhances mitochondrial DNA-dependent type I interferon responses. J. Immunol. 2018, 200, 169.11. [Google Scholar]
- Yamamura, Y.; Morizane, S.; Yamamoto, T.; Wada, J.; Iwatsuki, K. High calcium enhances the expression of double-stranded RNA sensors and antiviral activity in epidermal keratinocytes. Exp. Dermatol. 2018, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Banno, T.; Adachi, M.; Mukkamala, L.; Blumenberg, M. Unique keratinocyte-specific effects of interferon-gamma that protect skin from viruses, identified using transcriptional profiling. Antivir. Ther. 2003, 8, 541–554. [Google Scholar]
- Luo, W.W.; Lian, H.; Zhong, B.; Shu, H.B.; Li, S. Krüppel-like factor 4 negatively regulates cellular antiviral immune response. Cell. Mol. Immunol. 2016, 13, 65–72. [Google Scholar] [CrossRef]
- Eggenberger, J.; Blanco-Melo, D.; Panis, M.; Brennand, K.J.; tenOever, B.R. Type I interferon response impairs differentiation potential of pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 1384–1393. [Google Scholar] [CrossRef]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef]
- Shibayama, H.; Hisama, M.; Matsuda, S.; Ohtsuki, M.; Iwaki, M. Effect of a novel ascorbic derivative, disodium isostearyl 2-O-L-ascorbyl phosphate on human dermal fibroblasts: Increased collagen synthesis and inhibition of MMP-1. Biol. Pharm. Bull. 2008, 31, 563–568. [Google Scholar] [CrossRef][Green Version]
- Hantke, B.; Lahmann, C.; Venzke, K.; Fischer, T.; Kocourek, A.; Windsor, L.J.; Bergemann, J.; Stäb, F.; Tschesche, H. Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochem. Photobiol. Sci. 2002, 1, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Effect of a nutrient mixture on matrix metalloproteinase-9 dimers in various human cancer cell lines. Int. J. Oncol. 2014, 44, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Orbe, J.; Rodríguez, J.A.; Arias, R.; Belzunce, M.; Nespereira, B.; Pérez-Ilzarbe, M.; Roncal, C.; Páramo, J.A. Antioxidant vitamins increase the collagen content and reduce MMP-1 in a porcine model of atherosclerosis: Implications for plaque stabilization. Atherosclerosis 2003, 167, 45–53. [Google Scholar] [CrossRef]
- Pfeffer, F.; Casanueva, E.; Kamar, J.; Guerra, A.; Perichart, O.; Vadillo-Ortega, F. Modulation of 72-kilodalton type IV collagenase (Matrix metalloproteinase-2) by ascorbic acid in cultured human amnion-derived cells. Biol. Reprod. 1998, 59, 326–329. [Google Scholar] [CrossRef][Green Version]
- Rashidbenam, Z.; Jasman, M.H.; Tan, G.H.; Goh, E.H.; Fam, X.I.; Ho, C.C.K.; Zainuddin, Z.M.; Rajan, R.; Rani, R.A.; Nor, F.M.; et al. Fabrication of Adipose-Derived Stem Cell-Based Self-Assembled Scaffold under Hypoxia and Mechanical Stimulation for Urethral Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 3350. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free. Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Swindell, W.R.; Bojanowski, K.; Chaudhuri, R.K. A standardized Terminalia chebula fruit extract alters the expression of genes associated with skin architecture and barrier formation. Eur. J. Dermatol. 2020, 30, 469–492. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. Hydrogen Peroxide Scavenging Activity of Novel Coumarins Synthesized Using Different Approaches. PLoS ONE 2015, 10, e0132175. [Google Scholar] [CrossRef] [PubMed]
- Quinney, S.K.; Sanghani, S.P.; Davis, W.I.; Hurley, T.D.; Sun, Z.; Murry, D.J.; Bosron, W.F. Hydrolysis of capecitabine to 5’-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J. Pharmacol. Exp. Ther. 2005, 313, 1011–1016. [Google Scholar] [CrossRef]
- Heber, S.; Sick, B. Quality assessment of Affymetrix GeneChip data. Omics 2006, 10, 358–368. [Google Scholar] [CrossRef]
- McCall, M.N.; Murakami, P.N.; Lukk, M.; Huber, W.; Irizarry, R.A. Assessing affymetrix GeneChip microarray quality. BMC Bioinform. 2011, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Chandola, V.; Banerjee, A.; Kumar, V. Anomaly detection: A survey. ACM Comput. Surv. 2009, 41, 1–58. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dobak, J.; Grzybowski, J.; Liu, F.T.; Landon, B.; Dobke, M. 1,25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J. Dermatol. Sci. 1994, 8, 18–24. [Google Scholar] [CrossRef]
- Zhao, H.; Alexeev, A.; Chang, E.; Greenburg, G.; Bojanowski, K. Lycium barbarum glycoconjugates: Effect on human skin and cultured dermal fibroblasts. Phytomed. Int. J. Phytother. Phytopharm. 2005, 12, 131–137. [Google Scholar] [CrossRef]
- Voigt, W. Sulforhodamine B assay and chemosensitivity. Methods Mol. Med. 2005, 110, 39–48. [Google Scholar] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Ochiai, Y.; Kaburagi, S.; Obayashi, K.; Ujiie, N.; Hashimoto, S.; Okano, Y.; Masaki, H.; Ichihashi, M.; Sakurai, H. A new lipophilic pro-vitamin C, tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J. Dermatol. Sci. 2006, 44, 37–44. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swindell, W.R.; Randhawa, M.; Quijas, G.; Bojanowski, K.; Chaudhuri, R.K. Tetrahexyldecyl Ascorbate (THDC) Degrades Rapidly under Oxidative Stress but Can Be Stabilized by Acetyl Zingerone to Enhance Collagen Production and Antioxidant Effects. Int. J. Mol. Sci. 2021, 22, 8756. https://doi.org/10.3390/ijms22168756
Swindell WR, Randhawa M, Quijas G, Bojanowski K, Chaudhuri RK. Tetrahexyldecyl Ascorbate (THDC) Degrades Rapidly under Oxidative Stress but Can Be Stabilized by Acetyl Zingerone to Enhance Collagen Production and Antioxidant Effects. International Journal of Molecular Sciences. 2021; 22(16):8756. https://doi.org/10.3390/ijms22168756
Chicago/Turabian StyleSwindell, William R., Manpreet Randhawa, Geovani Quijas, Krzysztof Bojanowski, and Ratan K. Chaudhuri. 2021. "Tetrahexyldecyl Ascorbate (THDC) Degrades Rapidly under Oxidative Stress but Can Be Stabilized by Acetyl Zingerone to Enhance Collagen Production and Antioxidant Effects" International Journal of Molecular Sciences 22, no. 16: 8756. https://doi.org/10.3390/ijms22168756
APA StyleSwindell, W. R., Randhawa, M., Quijas, G., Bojanowski, K., & Chaudhuri, R. K. (2021). Tetrahexyldecyl Ascorbate (THDC) Degrades Rapidly under Oxidative Stress but Can Be Stabilized by Acetyl Zingerone to Enhance Collagen Production and Antioxidant Effects. International Journal of Molecular Sciences, 22(16), 8756. https://doi.org/10.3390/ijms22168756





