Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
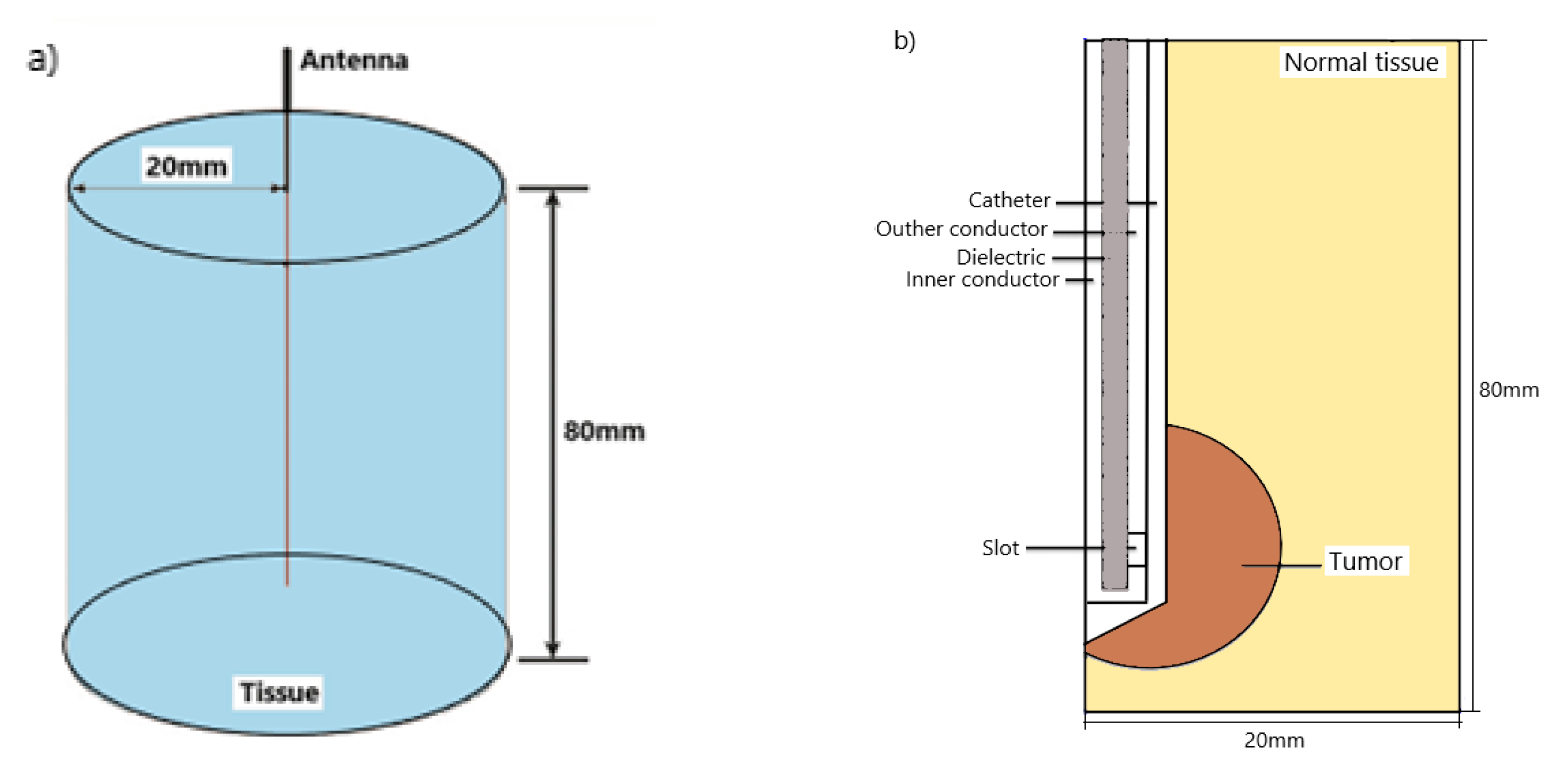
2. Numerical Method and Simulation Conditions
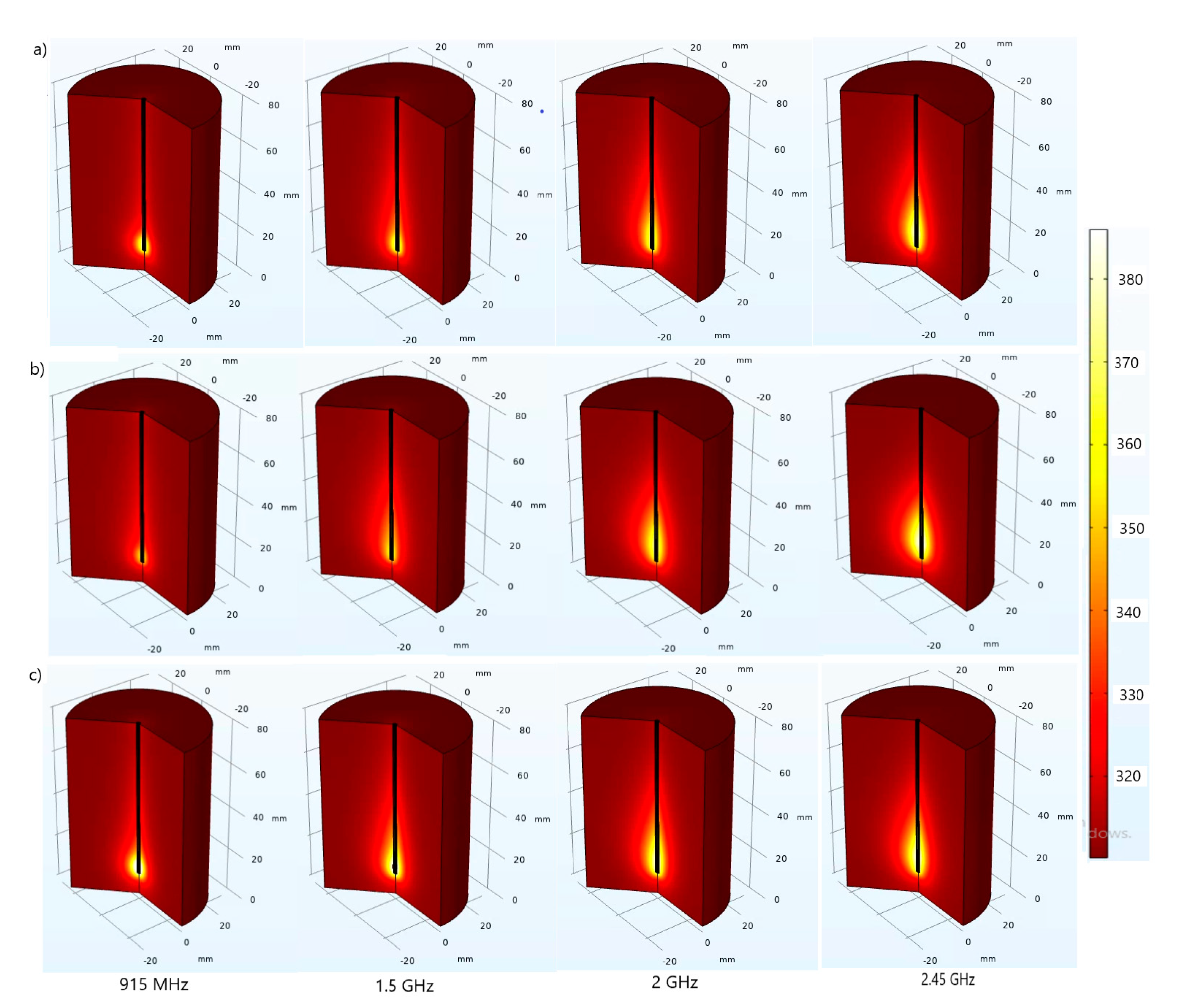
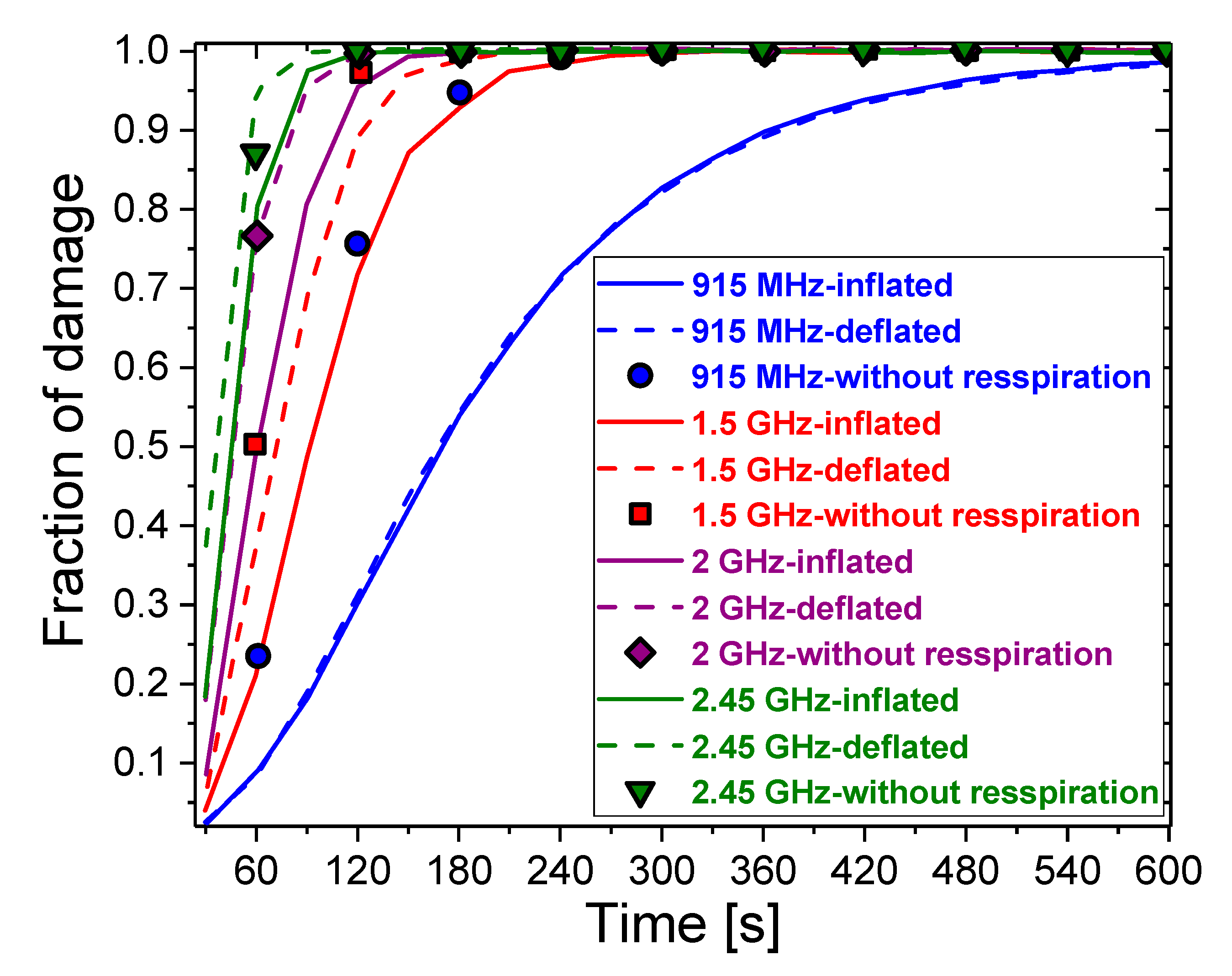
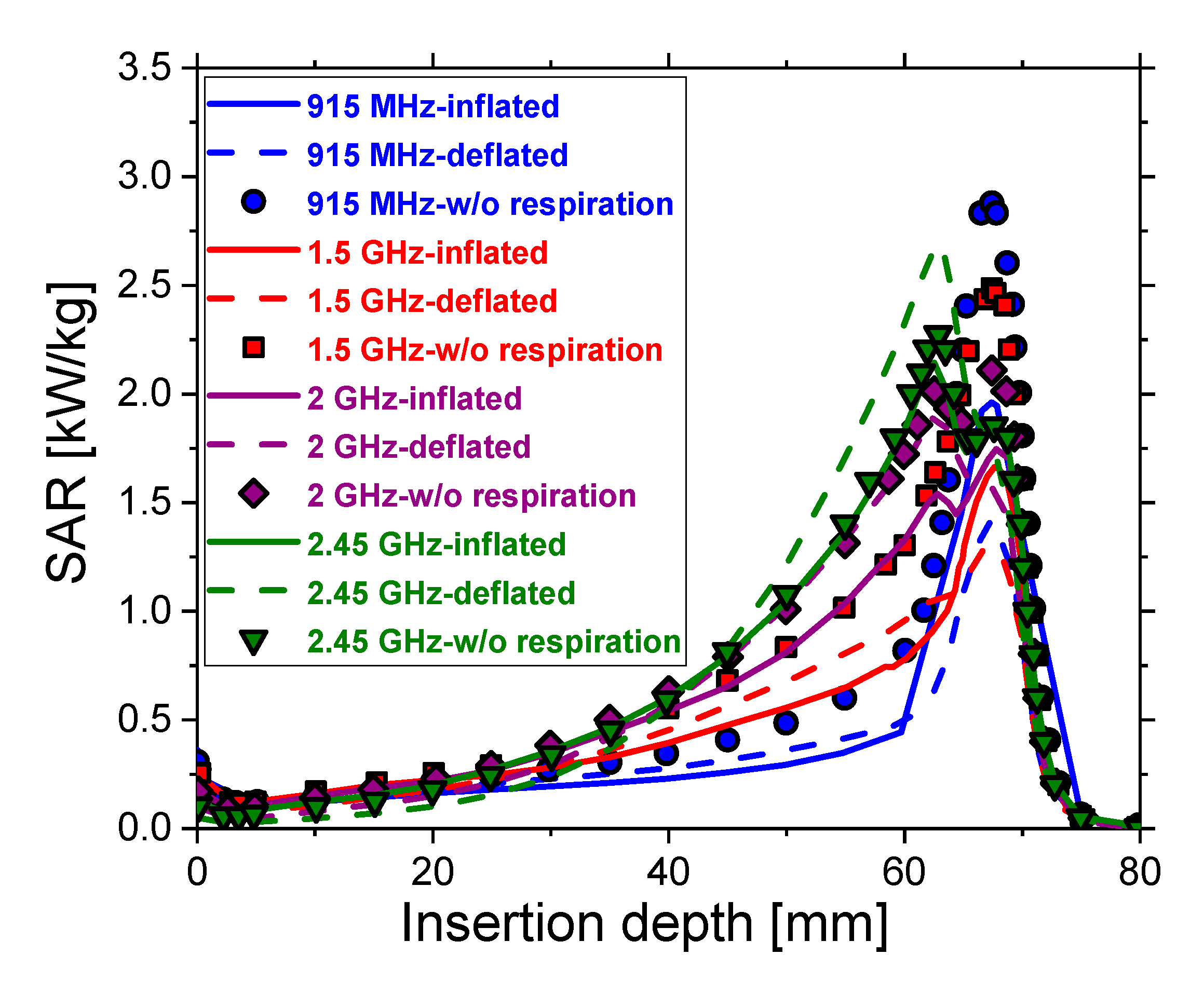
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cagle, P.T.; Allen, T.C.; Olsen, R.J. Lung cancer biomarkers: Present status and future developments. Arch. Pathol. Lab. Med. 2013, 137, 1191–1198. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandi, A.; Mamo, G.; Getachew, D.; Lemessa, F.; Kalappan, V.M.; Dhiravidamani, S. A Brief Review on Lung Cancer. Int. J. Pharma Res. Health Sci. 2016, 4, 907–914. [Google Scholar]
- Mustafa, M.; Jamalul Azizi, A.R.; IIIzam, E.L.; Nazirah, A.; Sharifa, A.M.; Abbas, S.A. Lung Cancer: Risk Factors, Management, And Prognosis. IOSR J. Dent. Med. Sci. 2016, 15, 94–101. [Google Scholar] [CrossRef]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, R.R.; Call, S.; Dooms, C.; Obiols, C.; Sánchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Respir. J. 2018, 51, 1800190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, R.D.; Sun, F.; Emery, J.D. Lung cancer. BMJ 2019, 365, l1725. [Google Scholar] [CrossRef] [PubMed]
- Pallari, E.; Eriksson, M.; Billhult, A.; Billhult, T.; Aggarwal, A.; Lewison, G.; Sullivan, R. Lung cancer research and its citation on clinical practice guidelines. Lung Cancer 2021, 154, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; Eberth, J.M. Lung cancer screening utilization: A behavioral risk factor surveillance system analysis. Am. J. Prev. Med. 2019, 57, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N. Lung Canser: An Overview. 2021. Available online: https://www.dssimage.com/blog/lung-cancer-an-overview/ (accessed on 14 March 2021).
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Luo, L.; Wampfler, J.A.; Wang, Y.; Liu, D.; Chen, Y.M.; Adjei, A.A.; Midthun, D.E.; Yang, P. 5-year overallsurvival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, P1098–P1108. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann. Oncol. 2017, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J. Your Chances of Surviving Lung Cancer. 2019. Available online: https://www.webmd.com/lung-cancer/guide/lung-cancer-survival-rates (accessed on 22 February 2021).
- Eriksson, M.; Billhult, A.; Billhult, T.; Pallari, E.; Lewison, G. A new database of the references on international clinical practice guidelines: A facility for the evaluation of clinical research. Scientometrics 2020, 122, 1221–1235. [Google Scholar] [CrossRef] [Green Version]
- Moya-Horno, I.; Viteri, S.; Karachaliou, N.; Rosell, R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther. Adv. Med. Oncol. 2018, 10, 1758834017745012. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.; Gillaspie, E.A.; Osmundson, E.C.; Horn, L. Neoadjuvant approaches to locally advanced non-small cell lung cancer. Front. Oncol. 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- El-Turk, N.; Chou, M.S.H.; Ting, N.C.H.; Girgis, A.; Vinod, S.K.; Bray, V.; Dobler, C.C. Treatment burden experienced by patients with lung cancer. PLoS ONE 2021, 16, e0245492. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.; Gruber-Rouh, T.; Koitka, K.; Lehnert, T.; Nour-Eldin, N.E. Microwave ablation therapy: Clinical utility in treatment of pulmonary metastases. Radiology 2011, 261, 643–651. [Google Scholar] [CrossRef]
- Belfiore, G.; Ronza, F.; Belfiore, M.P.; Serao, N.; di Ronza, G.; Grassi, R.; Rotondo, A. Patients’ survival in lung malignancies treated by microwave ablation: Our experience on 56 patients. Eur. J. Radiol. 2013, 82, 177–181. [Google Scholar] [CrossRef]
- Carrafiello, G.; Mangini, M.; Fontana, F.; Ierardi, A.M.; De Marchi, G.; Rotolo, N.; Chini, C.; Cuffari, S.; Fugazzola, C. Microwave ablation of lung tumors: Single-centre preliminary experience. Radiol. Med. 2014, 119, 75–82. [Google Scholar] [CrossRef]
- Zheng, A.; Ye, X.; Yang, X.; Huang, G.; Gai, Y. Local efficacy and survival after microwave ablation of lung tumors: A retrospective study in 183 patients. J. Vasc. Interv. Radiol. 2016, 27, 1806–1814. [Google Scholar] [CrossRef]
- Pusceddu, C.; Melis, L.; Sotgia, B.; Guerzoni, D.; Porcu, A.; Fancellu, A. Usefulness of percutaneous microwave ablation for large non-small cell lung cancer: A preliminary report. Oncol. Lett. 2019, 18, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Gala, K.B.; Shetty, N.S.; Patel, P.; Kulkarni, S.S. Microwave ablation: How we do it? Indian J. Radiol. Imaging 2020, 30, 206–213. [Google Scholar] [PubMed]
- El-Zein, R.A.; Abdel-Rahman, S.; Santee, K.J.; Yu, R.; Shete, S. Identification of Small and Non-Small Cell Lung Cancer Markers in Peripheral Blood Using Cytokinesis-Blocked Micronucleus and Spectral Karyotyping Assays. Cytogenet. Genome Res. 2017, 152, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Shock, S.A.; Meredith, K.; Warner, T.F.; Sampson, L.A.; Wright, A.S.; Winter, T.C.; Mahvi, D.M.; Fine, J.P.; Lee, F.T., Jr. Microwave ablation with loop antenna: In vivo porcine liver model. Radiology 2004, 231, 143–149. [Google Scholar] [CrossRef]
- Brace, C.L.; Laeseke, P.F.; Sampson, L.A.; Frey, T.M.; van der Weide, D.W.; Lee, F.T., Jr. Microwave ablation with a single small-gauge triaxial antenna: In vivo porcine liver model. Radiology 2007, 242, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Ni, X.; Gao, Y.; Shao, Q.; Liu, L.; Liang, P. Comparison of ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: Results in in vivo porcine livers. AJR Am. J. Roentgenol. 2009, 192, 511–514. [Google Scholar] [CrossRef]
- Macchi, M.; Belfiore, M.P.; Floridi, C.; Serra, N.; Belfiore, G.; Carmignani, L.; Grasso, R.F.; Mazza, E.; Pusceddu, C.; Brunese, L.; et al. Radiofrequency versusmicrowave ablation for treatment of the lung tumors: LUMIRA (lung microwave radiofrequency) randomized trial. Med. Oncol. 2014, 34, 96. [Google Scholar] [CrossRef]
- Palussière, J.; Catena, V.; Buy, X. Percutaneous thermal ablation of lung tumors-Radiofrequency, microwave and cryotherapy: Where are we going? Diagn. Interv. Imaging 2017, 98, 619–625. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Yang, M.; Yan, Q.; You, F.; Fu, F.; Wang, T.; Huo, X.; Dong, X.; Shi, X. Dielectric properties of human liver from 10Hz to 100MHz: Normal liver, hepatocellular carcinoma, hepatic fibrosis and liver hemangioma. Bio-Med. Mater. Eng. 2014, 24, 2725–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahat, N.; Zhadobov, M.; Sauleau, R.R.A. Human skin permittivity models for millimetre-wave range. IET Electron. Lett. 2011, 47, 427–428. [Google Scholar] [CrossRef]
- Zhekov, S.S.; Franek, O.; Pedersen, G.F. Dielectric Properties of Human Hand Tissue for Handheld Devices Testing. IEEE Access 2019, 7, 61949–61959. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Kim, S. The Interaction of Radio-Frequency Fields with Dielectric Materials at Macroscopic to Mesoscopic Scales. J. Res. Natl. Inst. Stand. Technol. 2012, 117, 1–60. [Google Scholar] [CrossRef] [PubMed]
- O’rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric properties of human normal, malignant and cirrhotic liver tissue: In vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007, 52, 4707–4719. [Google Scholar] [CrossRef] [Green Version]
- Farsaci, F.; Russo, A.; Ficarra, S.; Tellone, E. Dielectric properties of human normal and malignant liver tissue: A nonequilibrium thermodynamics approach. Open Access Libr. J. 2015, 2, e1395. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Hamad, A.M.; Sayed, F.A.; Abdelaziz, Y.S.; Mustafa, A.B.E.; Mohammed, M.A.; Saadeldin, A.M. Dielectric spectroscopy signature for cancer diagnosis: A review, Microwave and optical technology letters. Microw. Opt. Technol. Lett. 2020, 62, 1–15. [Google Scholar] [CrossRef]
- Nopp, P.; Rapp, E.; Pfützner, H.; Nakesch, H.; Ruhsam, C. Dielectric properties of lung tissue as a function of air content. Phys. Med. Biol. 1993, 38, 699–716. [Google Scholar] [CrossRef]
- Yamashiro, T.; Ando, M.; Okazaki, Y.; Sasaguri, S. Dielectric behavior of pulmonary edema induced in the rat lung. Respir. Physiol. Neurobiol. 2005, 145, 91–100. [Google Scholar] [CrossRef]
- Yang, D.; Cao, M. Effect of changes in lung physical properties on microwave ablation zone during respiration. Biomed. Eng. Lett. 2020, 10, 285–298. [Google Scholar] [CrossRef]
- Curto, S.; Taj-Eldin, M.; Fairchild, D.; Prakash, P. Microwave ablation at 915 MHz vs 2.45 GHz: A theoretical and experimental investigation. Med. Phys. 2015, 42, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Simo, K.A.; Tsirline, V.B.; Sindram, D.; McMillan, M.T.; Thompson, K.J.; Swan, R.Z.; McKillop, I.H.; Martinie, J.B.; Iannitti, D.A. Microwave ablation using 915-MHz and 2.45-GHz systems: What are the differences? HPB 2013, 15, 991–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comsol Multiphysics; COMSOL Inc.: Burlington, MA, USA, 1986; Available online: https://www.comsol.com/comsol-multiphysics (accessed on 24 February 2021).
- Heat Transfer Modeling Software for Analyzing Thermal Effects. Available online: https://www.comsol.com/heat-transfer-module (accessed on 24 February 2021).
- Selmi, M.; Dukhyil, A.A.B.; Belmabrouk, H. Numerical Analysis of Human Cancer Therapy Using Microwave Ablation. Appl. Sci. 2020, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Nwoye, E.O.; Aweda, M.A.; Oremosu, A.A.; Anunobi, C.C.; Akanmu, O.N.; Ibitoye, A.Z.; Adeneye, S.O.; Akpochafor, M.O. Finite Element Analysis of Single Slot Antenna for Microwave Tumor Ablation. IOSR J. Appl. Phys. 2014, 5, 55–62. [Google Scholar]
- Wongkedsada, T.; Phasukkit, P. Microwave Ablation Multi-Antennas Operation studying for Hepatic Cancer Microwave Ablation Treatment system using 3D-Finite Element Analysis. In Proceedings of the 12th Biomedical Engineering International Conference (BMEiCON2019), Ubon Ratchathani, Thailand, 19–22 November 2019. [Google Scholar]
- Neagu, V. A study of microwave ablation antenna optimization. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017. [Google Scholar]
- Towoju, O.; Ishola, F.; Sanni, T.; Olatunji, O. Investigation of Influence of Coaxial Antenna Slot Positioning on Thermal Efficiency in Microwave Ablation using COMSOL. In Journal of Physics: Conference Series; IOP: Bristol, UK, 2019; Volume 1378, p. 32066. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [Green Version]
- Brace, C.L. Assistant Scientist, Radiofrequency and microwave ablation of the liver, lung, kidney and bone: What are the differences. Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Rossmann, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmilović-Radjenović, M.; Sabo, M.; Prnova, M.; Šoltes, L.; Radjenović, B. Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment. Cancers 2021, 13, 3500. https://doi.org/10.3390/cancers13143500
Radmilović-Radjenović M, Sabo M, Prnova M, Šoltes L, Radjenović B. Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment. Cancers. 2021; 13(14):3500. https://doi.org/10.3390/cancers13143500
Chicago/Turabian StyleRadmilović-Radjenović, Marija, Martin Sabo, Marta Prnova, Lukaš Šoltes, and Branislav Radjenović. 2021. "Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment" Cancers 13, no. 14: 3500. https://doi.org/10.3390/cancers13143500
APA StyleRadmilović-Radjenović, M., Sabo, M., Prnova, M., Šoltes, L., & Radjenović, B. (2021). Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment. Cancers, 13(14), 3500. https://doi.org/10.3390/cancers13143500





