Simultaneous Pretreatment of Aspirin and Omega-3 Fatty Acid Attenuates Nuclear Factor-κB Activation in a Murine Model with Ventilator-Induced Lung Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Ventilator-Induced Lung Injury Model
2.3. Anti-Inflammatory Compounds
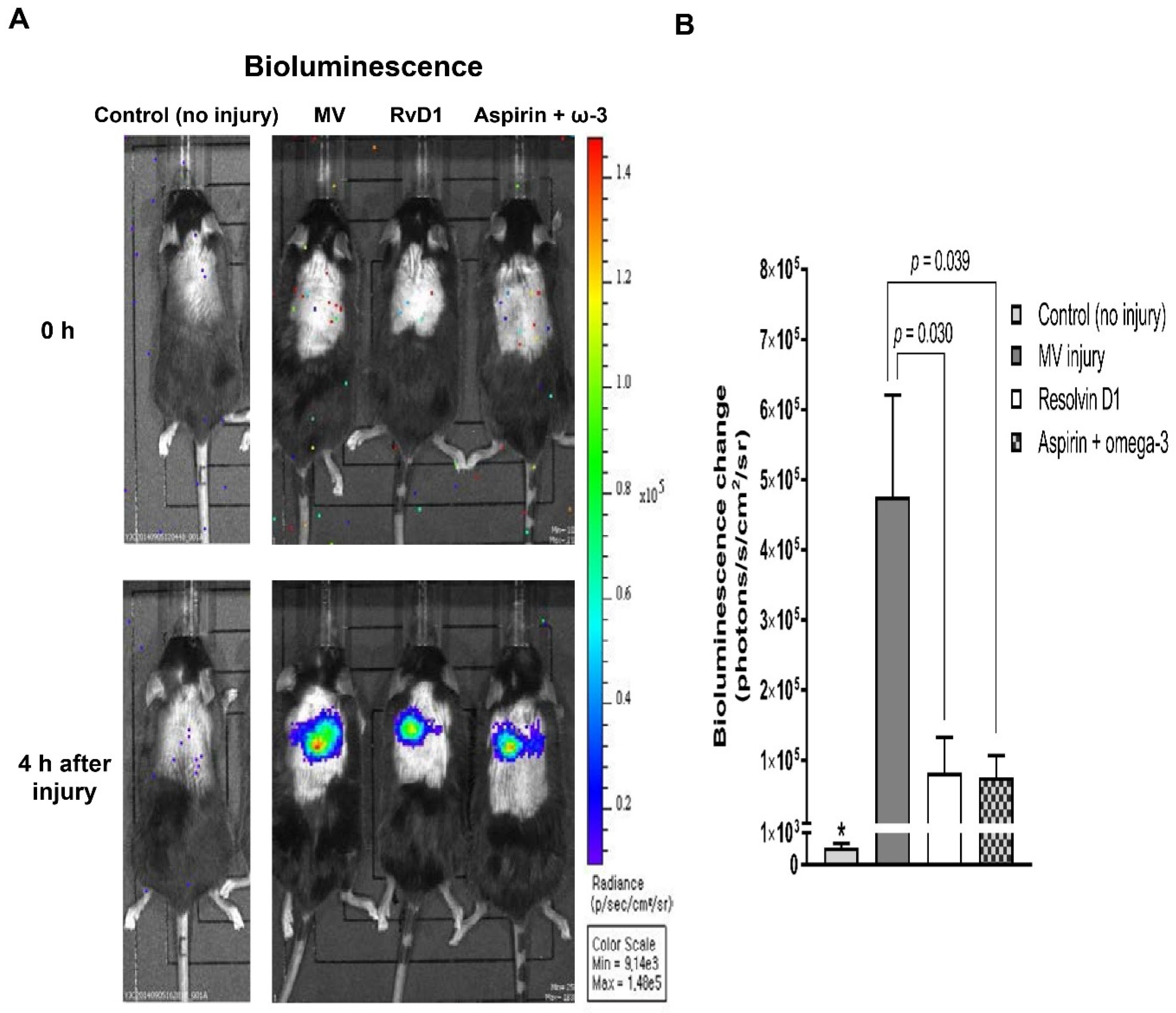
2.4. In Vivo Imaging to Evaluate NF-κB-Luciferase Assay
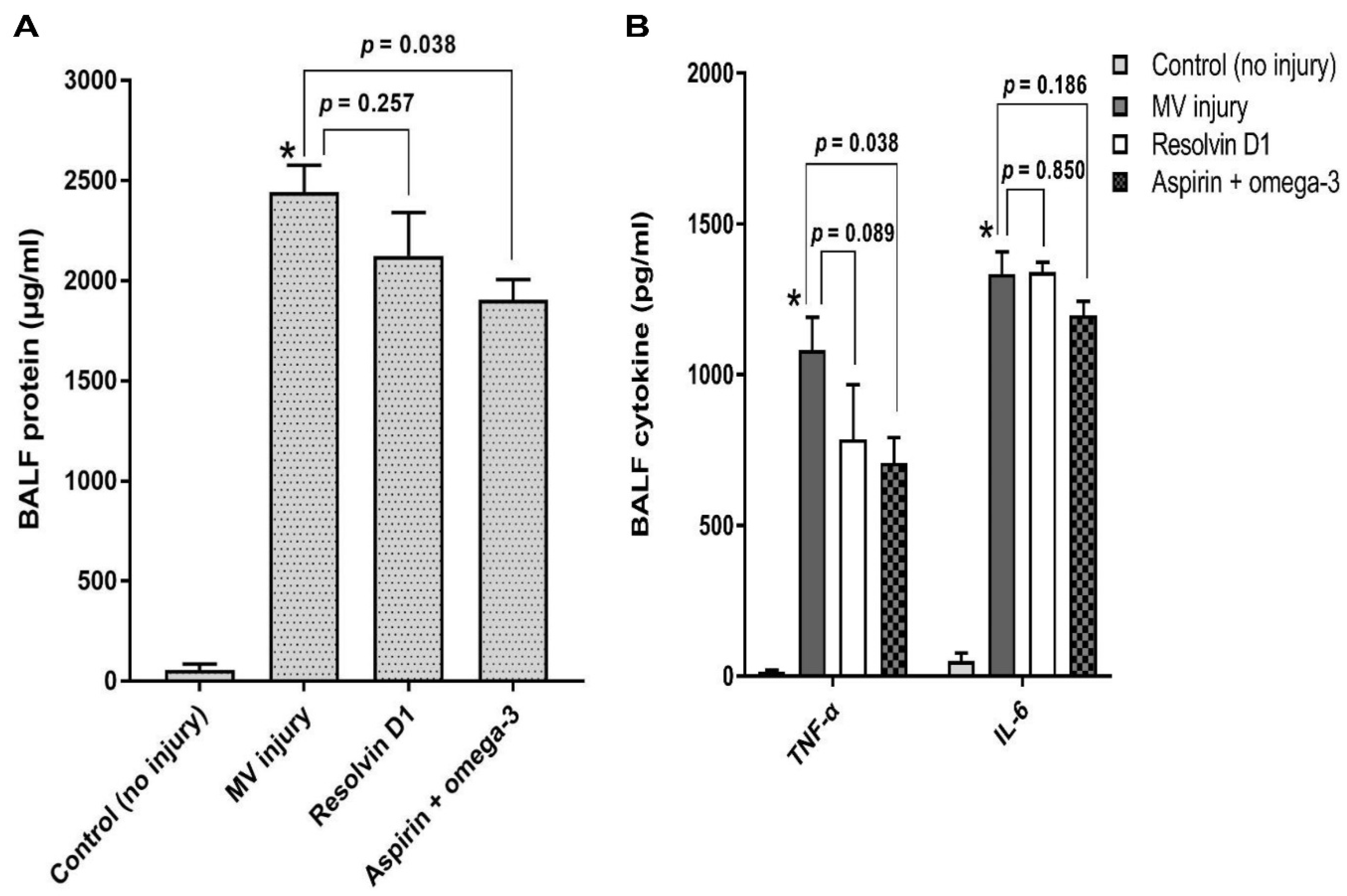
2.5. Bronchoalveolar Lavage Fluid Analysis and Enzyme-Linked Immunosorbent Assays (ELISAs)
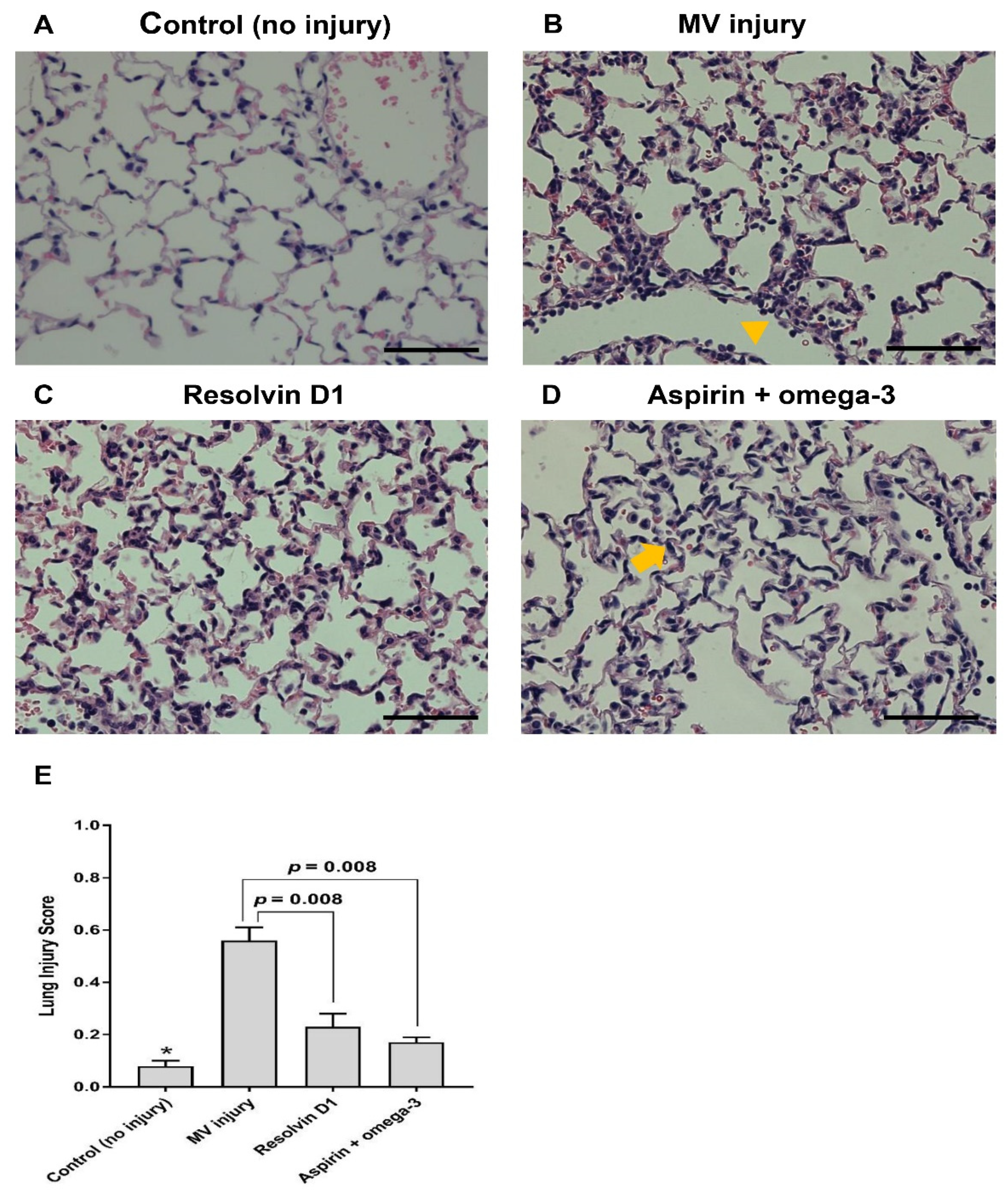
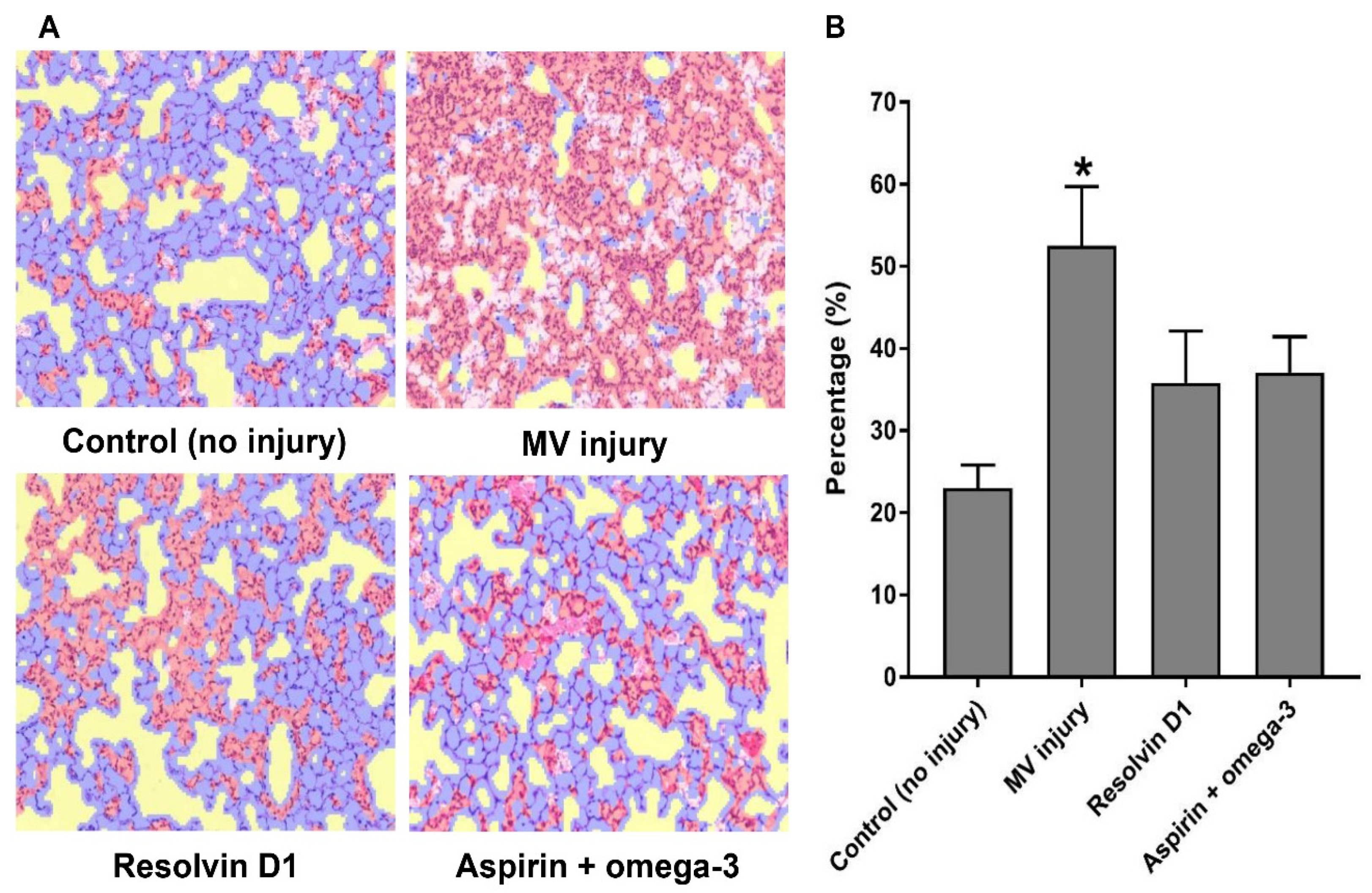
2.6. Histopathological Evaluation and Quantitative Estimation of Inflammation Using Machine Learning Software
2.7. Statistical Analyses
3. Results
3.1. Setting of VILI Model
3.2. Effect of RvD1 Pretreatment and Aspirin and Omega-3 Fatty Acid Pretreatment on ALI
3.2.1. In Vivo Imaging of NF-κB Activation
3.2.2. Total Protein and Pro-Inflammatory Cytokines Analysis in the Bronchoalveolar Lavage Fluid
3.2.3. Histopathological Examination
3.2.4. A Machine Learning Algorithm for the Interpretation of the Pathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, C.C.; Slutsky, A.S. Invited review: Mechanisms of ventilator-induced lung injury: A perspective. J. Appl. Physiol. 2000, 89, 1645–1655. [Google Scholar] [CrossRef]
- Perros, F.; Lambrecht, B.N.; Hammad, H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir. Res. 2011, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, H.S.; Seo, K.H.; Na, J.O.; Kim, Y.H.; Uh, S.T.; Park, C.S.; Oh, M.H.; Lee, S.H.; Kim, Y.T. The effect of post-treatment N-acetylcysteine in LPS-induced acute lung injury of rats. Tuberc. Respir. Dis. 2012, 73, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, I.; Aguirre, A.; Gonzalez-Lopez, A.; Fernandez, A.F.; Amado-Rodriguez, L.; Astudillo, A.; Batalla-Solis, E.; Albaiceta, G.M. Impairment of autophagy decreases ventilator-induced lung injury by blockade of the NF-kappaB pathway. Am. J. Physiol. 2013, 304, L844–L852. [Google Scholar] [CrossRef][Green Version]
- Calder, P.C. n-3 fatty acids, inflammation, and immunity--relevance to postsurgical and critically ill patients. Lipids 2004, 39, 1147–1161. [Google Scholar] [CrossRef]
- Pontes-Arruda, A.; Martins, L.F.; de Lima, S.M.; Isola, A.M.; Toledo, D.; Rezende, E.; Maia, M.; Magnan, G.B. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in the early treatment of sepsis: Results from a multicenter, prospective, randomized, double-blinded, controlled study: The intersept study. Crit. Care 2011, 15, R144. [Google Scholar] [CrossRef]
- Chen, W.; Janz, D.R.; Bastarache, J.A.; May, A.K.; O’Neal, H.R., Jr.; Bernard, G.R.; Ware, L.B. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: A propensity-adjusted analysis. Crit. Care Med. 2015, 43, 801–807. [Google Scholar] [CrossRef]
- Kumar, K.V.; Rao, S.M.; Gayani, R.; Mohan, I.K.; Naidu, M.U. Oxidant stress and essential fatty acids in patients with risk and established ARDS. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 298, 111–120. [Google Scholar] [CrossRef]
- Panka, B.A.; de Grooth, H.J.; Spoelstra-de Man, A.M.; Looney, M.R.; Tuinman, P.R. Prevention or treatment of ards with aspirin: A review of preclinical models and meta-analysis of clinical studies. Shock 2017, 47, 13–21. [Google Scholar] [CrossRef]
- Spindler, S.R.; Mote, P.L.; Flegal, J.M. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age 2014, 36, 9659. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, D.; Hod, E.A.; Stellari, F.; Kim, J.B.; Lim, E.; Roskey, M.; Francis, K.P.; Singh, R.; Zhang, N. Imaging pulmonary NF-kappaB activation and therapeutic effects of MLN120B and TDZD-8. PLoS ONE 2011, 6, e25093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Fujioka, H.; Kano, H.; Egerstedt, M.; Martin, C.F. Smoothing spline curves and surfaces for sampled data. In Proceedings of Proceedings of the 36th ISCIE International Symposium on Stochastic Systems Theory and Its Applications, Saitama, Japan, 3–4 November 2004; pp. 289–296. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Merched, A.J.; Ko, K.; Gotlinger, K.H.; Serhan, C.N.; Chan, L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008, 22, 3595–3606. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef]
- Uddin, M.; Seumois, G.; Lau, L.C.; Rytila, P.; Davies, D.E.; Djukanovic, R. Enhancement of neutrophil function by the bronchial epithelium stimulated by epidermal growth factor. Eur. Respir. J. 2008, 31, 714–724. [Google Scholar] [CrossRef]
- Pontes-Arruda, A.; Aragao, A.M.; Albuquerque, J.D. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006, 34, 2325–2333. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; de Boisblanc, B.P.; Steingrub, J.; Rock, P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011, 306, 1574–1581. [Google Scholar] [CrossRef]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Haworth, O.; Croze, R.; Oh, S.F.; Uddin, M.; Carlo, T.; Pfeffer, M.A.; Priluck, R.; Serhan, C.N.; Levy, B.D. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 2012, 189, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Eickmeier, O.; Seki, H.; Haworth, O.; Hilberath, J.N.; Gao, F.; Uddin, M.; Croze, R.H.; Carlo, T.; Pfeffer, M.A.; Levy, B.D. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013, 6, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Yan, C.; Petasis, N.A.; Serhan, C.N.; Gao, H. Protective actions of aspirin-triggered (17R) resolvin D1 and its analogue, 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester, in C5a-dependent IgG immune complex-induced inflammation and lung injury. J. Immunol. 2014, 193, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Cox, R., Jr.; Phillips, O.; Fukumoto, J.; Fukumoto, I.; Parthasarathy, P.T.; Arias, S.; Cho, Y.; Lockey, R.F.; Kolliputi, N. Enhanced resolution of hyperoxic acute lung injury as a result of aspirin triggered resolvin D1 treatment. Am. J. Respir. Cell Mol. Biol. 2015, 53, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Anthony, D.; Yatmaz, S.; Wijburg, O.; Satzke, C.; Levy, B.; Vlahos, R.; Bozinovski, S. Aspirin-triggered resolvin D1 reduces pneumococcal lung infection and inflammation in a viral and bacterial coinfection pneumonia model. Clin. Sci. 2017, 131, 2347–2362. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Dier, U.; Calderonartero, P.; Shearer, G.C.; Kakinami, L.; Larson, M.K.; Harris, W.S.; Georas, S.; Mousa, S.A. The Effects of EPA+DHA and Aspirin on Inflammatory Cytokines and Angiogenesis Factors. World J. Cardiovasc. Dis. 2012, 2, 14–19. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Yang, Z.H.; Vaisman, B.L.; Thacker, S.; Yu, Z.X.; Sampson, M.; Serhan, C.N.; Remaley, A.T. Addition of aspirin to a fish oil-rich diet decreases inflammation and atherosclerosis in ApoE-null mice. J. Nutr. Biochem. 2016, 35, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Yull, F.E.; Chen, C.L.; Venkatakrishnan, A.; Blackwell, T.R.; Hicks, D.J.; Lancaster, L.H.; Christman, J.W.; Kerr, L.D. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am. J. Respir. Crit. Care Med. 2000, 162, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwack, W.-G.; Lee, Y.-J.; Eo, E.-Y.; Chung, J.-H.; Lee, J.-H.; Cho, Y.-J. Simultaneous Pretreatment of Aspirin and Omega-3 Fatty Acid Attenuates Nuclear Factor-κB Activation in a Murine Model with Ventilator-Induced Lung Injury. Nutrients 2021, 13, 2258. https://doi.org/10.3390/nu13072258
Kwack W-G, Lee Y-J, Eo E-Y, Chung J-H, Lee J-H, Cho Y-J. Simultaneous Pretreatment of Aspirin and Omega-3 Fatty Acid Attenuates Nuclear Factor-κB Activation in a Murine Model with Ventilator-Induced Lung Injury. Nutrients. 2021; 13(7):2258. https://doi.org/10.3390/nu13072258
Chicago/Turabian StyleKwack, Won-Gun, Yoon-Je Lee, Eun-Young Eo, Jin-Haeng Chung, Jae-Ho Lee, and Young-Jae Cho. 2021. "Simultaneous Pretreatment of Aspirin and Omega-3 Fatty Acid Attenuates Nuclear Factor-κB Activation in a Murine Model with Ventilator-Induced Lung Injury" Nutrients 13, no. 7: 2258. https://doi.org/10.3390/nu13072258
APA StyleKwack, W.-G., Lee, Y.-J., Eo, E.-Y., Chung, J.-H., Lee, J.-H., & Cho, Y.-J. (2021). Simultaneous Pretreatment of Aspirin and Omega-3 Fatty Acid Attenuates Nuclear Factor-κB Activation in a Murine Model with Ventilator-Induced Lung Injury. Nutrients, 13(7), 2258. https://doi.org/10.3390/nu13072258




