Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Materials and Equipment
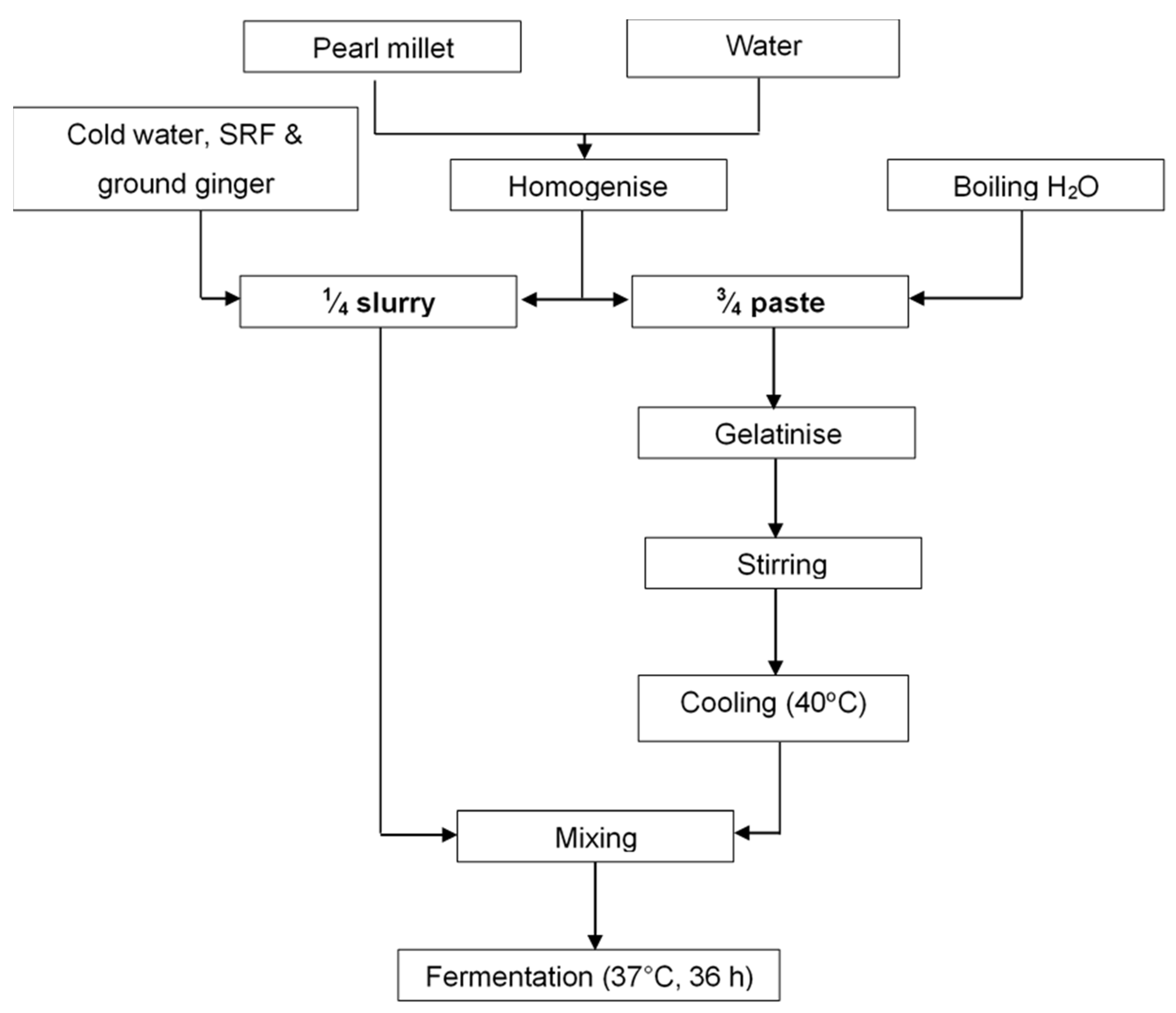
2.2. Production of Pearl Millet Slurry and Fermentation
2.3. Physicochemical Analysis of Pearl Millet Slurry during Fermentation
2.4. Enumeration of Bacteria in Pearl Millet Slurry during Fermentation
2.5. Isolation and Identification of Lactic Acid Bacteria in Pearl Millet Slurry during Fermentation
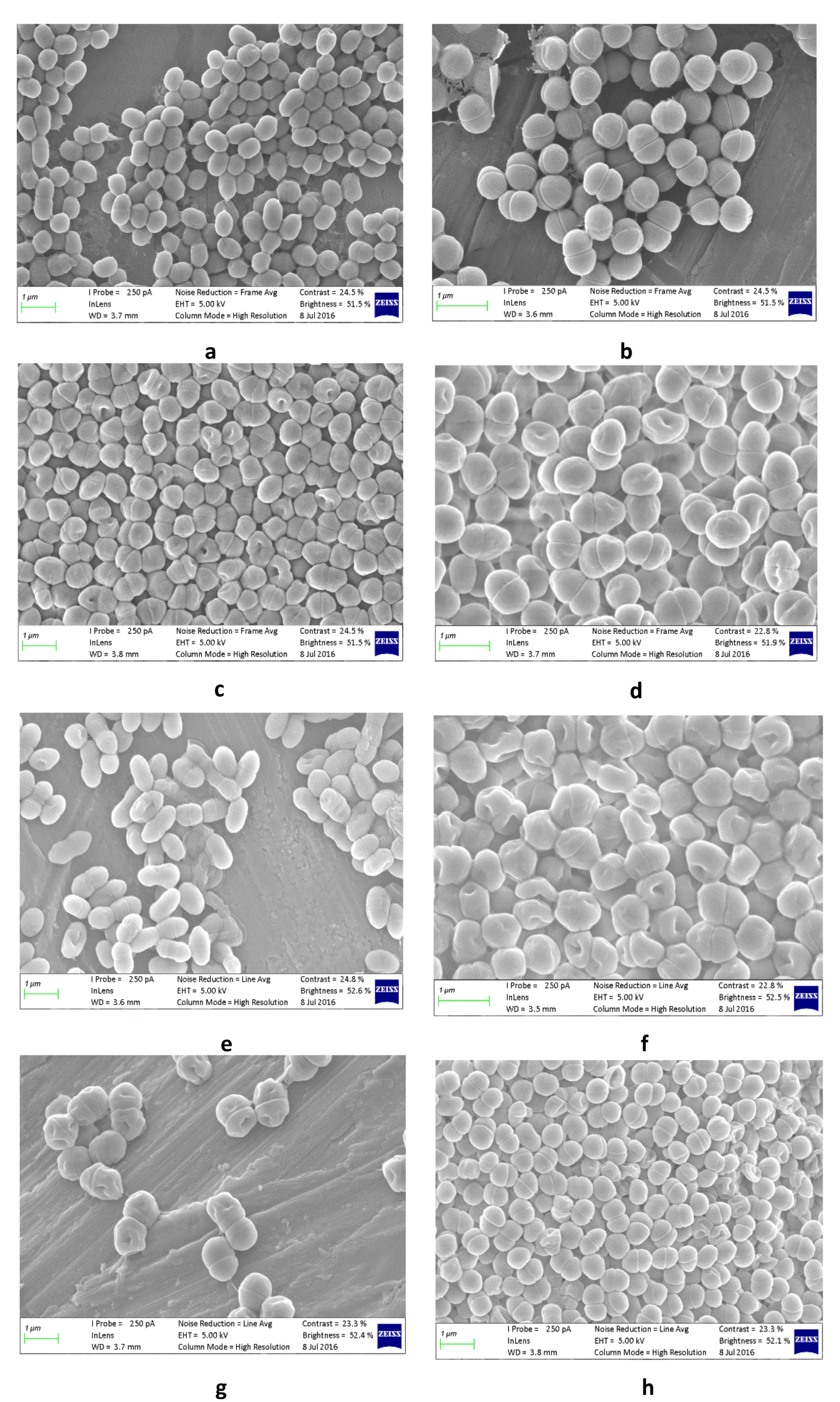
2.6. Lactic Acid Bacteria Preparation for Scanning Electron Microscope Imaging
2.7. Experimental Design for the Effect of Bioburden Lactic Acid Bacteria on Pearl Millet Extract
2.8. Effect of L. mesenteroides and P. pentosaceus on the pH, Total Titratable Acidity (TTA) and Viscosity of the Pearl Millet Extract
2.9. Production of Optimal Non-Alcoholic Pearl Millet Beverage
2.10. Determination of the Viscosity of Non-Alcoholic Pearl Millet Beverage
2.11. Data Analysis
3. Results and Discussion
3.1. Effect of Fermentation Time on the pH and Total Titratable Acidity (TTA) of Pearl Millet Slurry
3.2. Soluble Sugar Kinetics of Pearl Millet Slurry during Fermentation
3.3. Kinetics of Lactic Acid Bacteria and Total Viable Microbes in Pearl Millet Slurry during Fermentation
3.4. Lactic Acid Bacteria Associated with Pearl Millet Slurry Fermentation
3.5. Effect of Isolated Bioburden Lactic Acid Bacteria on the pH, Total Titratable Acidity and Viscosity of Pearl Millet Extract
3.6. Effect of Different Purified Lactic Acid Bacteria on the Viscosity of Pearl Millet Extract
3.7. Non-Alcoholic Pearl Millet Beverage (NAPMB) Produced Using Pure Cultures of Lactic Acid Bacteria (LAB)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional Fermented Foods and Beverages from a Microbiological and Nutritional Perspective: The Colombian Heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Emanuilova, M.; Petrov, K. Amylolytic Lactobacillus Strains from Bulgarian Fermented Beverage Boza. Z. Nat. Sect. C J. Biosci. 2010, 65, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Diversity of bacteriocinogenic lactic acid bacteria isolated from boza, a cereal-based fermented beverage from Bulgaria. Food Control 2010, 21, 1011–1021. [Google Scholar] [CrossRef]
- Botes, A.; Todorov, S.D.; von Mollendorff, J.W.; Botha, A.; Dicks, L.M.T. Identification of lactic acid bacteria and yeast from boza. Process Biochem. 2007, 42, 267–270. [Google Scholar] [CrossRef]
- Muyanja, C.M.B.K.; Narvhus, J.A.; Treimo, J.; Langsrud, T. Isolation, characterisation and identification of lactic acid bacteria from bushera: A Ugandan traditional fermented beverage. Int. J. Food Microbiol. 2003, 80, 201–210. [Google Scholar] [CrossRef]
- Adelekan, A.O.; Alamu, A.E.; Arisa, N.U.; Adebayo, Y.O.; Dosa, A.S. Nutritional, Microbiological and Sensory Characteristics of Malted Soy-Kunu Zaki: An Improved Traditional Beverage. Adv. Microbiol. 2013, 3, 389–397. [Google Scholar] [CrossRef]
- Obadina, A.O.; Oyewole, O.B. Awojobi Effect of steeping time of milled grains on the quality of Kunnu-Zaki (A Nigerian beverage). Afr. J. Food Sci. 2008, 2, 33–36. [Google Scholar]
- Liu, J.-R.; Tanner, R.S.; Schumann, P.; Weiss, N.; McKenzie, C.A.; Janssen, P.H.; Seviour, E.M.; Lawson, P.A.; Allen, T.D.; Seviour, R.J. Emended description of the genus Trichococcus, description of Trichococcus collinsii sp. nov., and reclassification of Lactosphaera pasteurii as Trichococcus pasteurii comb. nov. and of Ruminococcus palustris as Trichococcus palustris comb. nov. in the low-G+C gram-positive bacteria. Int. J. Syst. Evol. Microbiol. 2002, 52, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Oguntoyinbo, F.A.; Narbad, A. Molecular characterization of lactic acid bacteria and in situ amylase expression during traditional fermentation of cereal foods. Food Microbiol. 2012, 31, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Aka, S.; Dridi, B.; Bolotin, A.; Yapo, E.A.; Koussemon-Camara, M.; Bonfoh, B.; Renault, P. Characterization of lactic acid bacteria isolated from a traditional Ivoirian beer process to develop starter cultures for safe sorghum-based beverages. Int. J. Food Microbiol. 2020, 322, 108547. [Google Scholar] [CrossRef] [PubMed]
- Temitope, O.S.; Taiyese, O.B. Quality assessment of “oti-oka” like beverage produced from pearl millet. J. Appl. Biosci. 2012, 51, 3608–3617. [Google Scholar]
- Abegaz, K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr. J. Biotechnol. 2007, 6, 1469–1478. [Google Scholar] [CrossRef]
- Kivanç, M.; Yilmaz, M. Isolation and identifi cation of lactic acid bacteria from boza, and their microbial activity against several reporter strains. Turk. J. Biol. 2011, 35, 313–324. [Google Scholar] [CrossRef]
- Nwachukwu, E.; Achi, O.K.; Ijeoma, I.O. Lactic acid Bacteria in Fermentation of Cereals for the Production of Indigenous Nigerian Foods. Afr. J. Food Sci. Technol. 2010, 1, 21–26. [Google Scholar]
- Osuntogun, B.; Aboaba, O.O. Microbiological and Physico-chemical Evaluation of Some Non-alcoholic Beverages. Pak. J. Nutr. 2004, 3, 188–192. [Google Scholar]
- Miller, P.H.; Wiggs, L.S.; Miller, J.M. Evaluation of AnaeroGen system for growth of anaerobic bacteria. J. Clin. Microbiol. 1995, 33, 2388–2391. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Lücke, F.-K. Identification of lactobacilli from meat and meat products. Food Microbiol. 1987, 4, 199–208. [Google Scholar] [CrossRef]
- Nonhoff, C.; Rottiers, S.; Struelens, M.J. Evaluation of the Vitek 2 system for identification and antimicrobial susceptibility testing of Staphylococcus spp. Clin. Microbiol. Infect. 2005, 11, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Epa Ocspp, U. Standard Operating Procedure for VITEK 2 Compact: Use, Maintenance and Quality Control Procedures Manaual; SOP Number: QC-22-04; US Environmental Protection Agency Office of Pesticide Programs: Ft. Meade, MD, USA, 2016. [Google Scholar]
- Kandil, S.; El Soda, M. Influence of Freezing and Freeze Drying on Intracellular Enzymatic Activity and Autolytic Properties of Some Lactic Acid Bacterial Strains. Adv. Microbiol. 2015, 05, 371–382. [Google Scholar] [CrossRef]
- Katongole, J.N. The Microbial Succession in Indigenous Fermented Maize Products. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2008. [Google Scholar]
- Mavhungu, J. Isolation and Characterization of Lactic Acid Bacteria from “Ting” in the Northern Province of South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2005. [Google Scholar]
- Zvauya, R.; Mygochi, T.; Parawira, W. Microbial and Biochemical Changes Occurring during Production of Masvusvu and Mangisi, Traditional Zimbabwean Beverages. Plant Foods Hum. Nutr. 1997, 51, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J. Saudi Soc. Agric. Sci. 2011, 10, 1–6. [Google Scholar] [CrossRef]
- Pushparaj, F.S.; Urooj, A. Antioxidant Activity in Two Pearl Millet (Pennisetum typhoideum) Cultivars as Influenced by Processing. Antioxidants 2014, 3, 55–66. [Google Scholar] [CrossRef]
- Ibegbulem, C.O.; Chikezie, P.C. Biochemical Indices and Sensory Scores of Kunu-zaki Beverages Produced from Sprouted and Unsprouted Guinea Corn and Their Correlations. Am. J. Food Technol. 2014, 9, 56–62. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K.J.A.E.M. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Mwale, M.M. Microbiological Quality and Safety of the Zambian Fermented Cereal Beverage: Chibwantu. Ph.D. Thesis, The University of the Free State, Bloemfontein, South Africa, 2014. [Google Scholar]
- Yates, G.T.; Smotzer, T. On the lag phase and initial decline of microbial growth curves. J. Theor. Biol. 2007, 244, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Omemu, A.M. Fermentation dynamics during production of ogi, a Nigerian fermented cereal porridge. Rep. Opin. 2011, 3, 1–17. [Google Scholar]
- Schutte, L.M. Isolation and Identification of the Microbial Consortium Present in Fermented Milks from Sub-Saharan Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2013. [Google Scholar]
- Dimic, G. Characteristics of the Leuconostoc mesenteroides subsp. mesenteroides strains from fresh vegetables. APTEFF. 2006, 37, 3–11. [Google Scholar] [CrossRef]
- Achi, O.K.; Asamudo, N.U. Cereal-Based Fermented Foods of Africa as Functional Foods. Ref. Ser. Phytochem. 2019, 2, 1527–1558. [Google Scholar] [CrossRef]
- Meslier, V.; Loux, V.; Renault, P. Genome Sequence of Leuconostoc pseudomesenteroides Strain 4882, Isolated from a Dairy Starter Culture. J. Bacteriol. 2012, 194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doulgeraki, A.I.; Pramateftaki, P.; Argyri, A.A.; Nychas, G.-J.E.; Tassou, C.C.; Panagou, E.Z. Molecular characterization of lactic acid bacteria isolated from industrially fermented Greek table olives. LWT 2013, 50, 353–356. [Google Scholar] [CrossRef]
- Nuraida, L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci. Hum. Wellness 2015, 4, 47–55. [Google Scholar] [CrossRef]
- Facklam, R. What Happened to the Streptococci: Overview of Taxonomic and Nomenclature Changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef]
- Giménez-Pereira, M.L. Enterococci in Milk Products. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2005. [Google Scholar]
- Araújo, T.F.; Ferreira, C.L.L.F. The genus Enterococcus as probiotic: Safety concerns. Braz. Arch. Biol. Technol. 2013, 56, 457–466. [Google Scholar] [CrossRef]
- De Castro, A.; Montaño, A.; Casado, F.J.; Sánchez, A.-H.; Rejano, L. Utilization of Enterococcus casseli£avus and Lactobacillus pentosus as starter cultures for Spanish-style green olive fermentation. Food Microbiol. 2002, 19, 637–644. [Google Scholar] [CrossRef]
- Oladipo, I.C.; Sanni, A.; Chakraborty, W.; Chakravorty, S.; Jana, S.; Rudra, D.S.; Gacchui, R.; Swarnakar, S. Malaysian Journal of Microbiology Technological properties of strains of Enterococcus gallinarum isolated from selected Nigerian traditional fermented foods. Malays. J. Microbiol. 2015, 11, 1–13. [Google Scholar] [CrossRef][Green Version]
- Menconi, A.; Kallapura, G.; Latorre, J.D.; Morgan, M.J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Identification and Characterization of Lactic Acid Bacteria in a Commercial Probiotic Culture. Biosci. Microbiota Food Health 2014, 33, 25–30. [Google Scholar] [CrossRef]
- Mcdonald, L.C.; Fleming, H.P.; Hassan, H.M. Acid Tolerance of Leuconostoc mesenteroides and Lactobacillus plantarumt. Appl. Environ. Microbiol. 1990, 56, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Battcock, M.; Azam-Ali, S. Fermented Frutis and Vegetables. A Global Perspective. Table of Contents. Available online: http://www.fao.org/3/x0560e/x0560e00.htm (accessed on 8 April 2021).
- Kohajdová, Z.; Karovičová, J. Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51–57. [Google Scholar]
- Basinskiene, L.; Juodeikiene, G.; Vidmantiene, D.; Tenkanen, M.; Makaravicius, T.; Bartkiene, E. Non-Alcoholic Beverages from Fermented Cereals with Increased Oligosaccharide Content. Food Technol. Biotechnol. 2016, 54, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hayta, M.; Alpaslan, M.; Kose, E. The effect of fermentation on viscosity and protein solubility of Boza, a traditional cereal-based fermented Turkish beverage. Eur. Food Res. Technol. 2001, 213, 335–337. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology Practical Applications and Procedures, 2nd ed.; Springer Science and Business Media, LLC: New York, NY, USA, 2007. [Google Scholar]
- Oh, Y.J.; Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT 2015, 63, 437–444. [Google Scholar] [CrossRef]
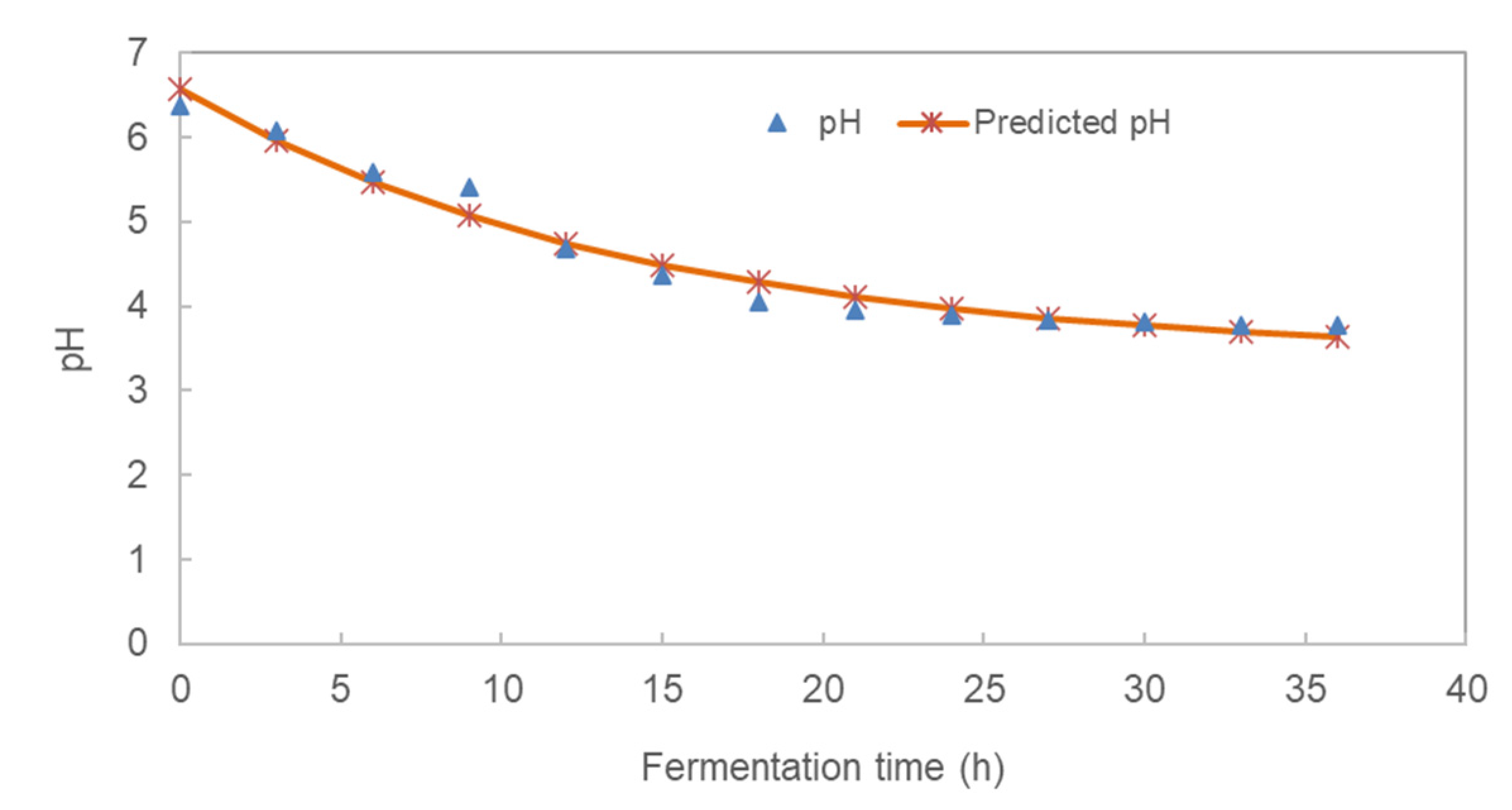
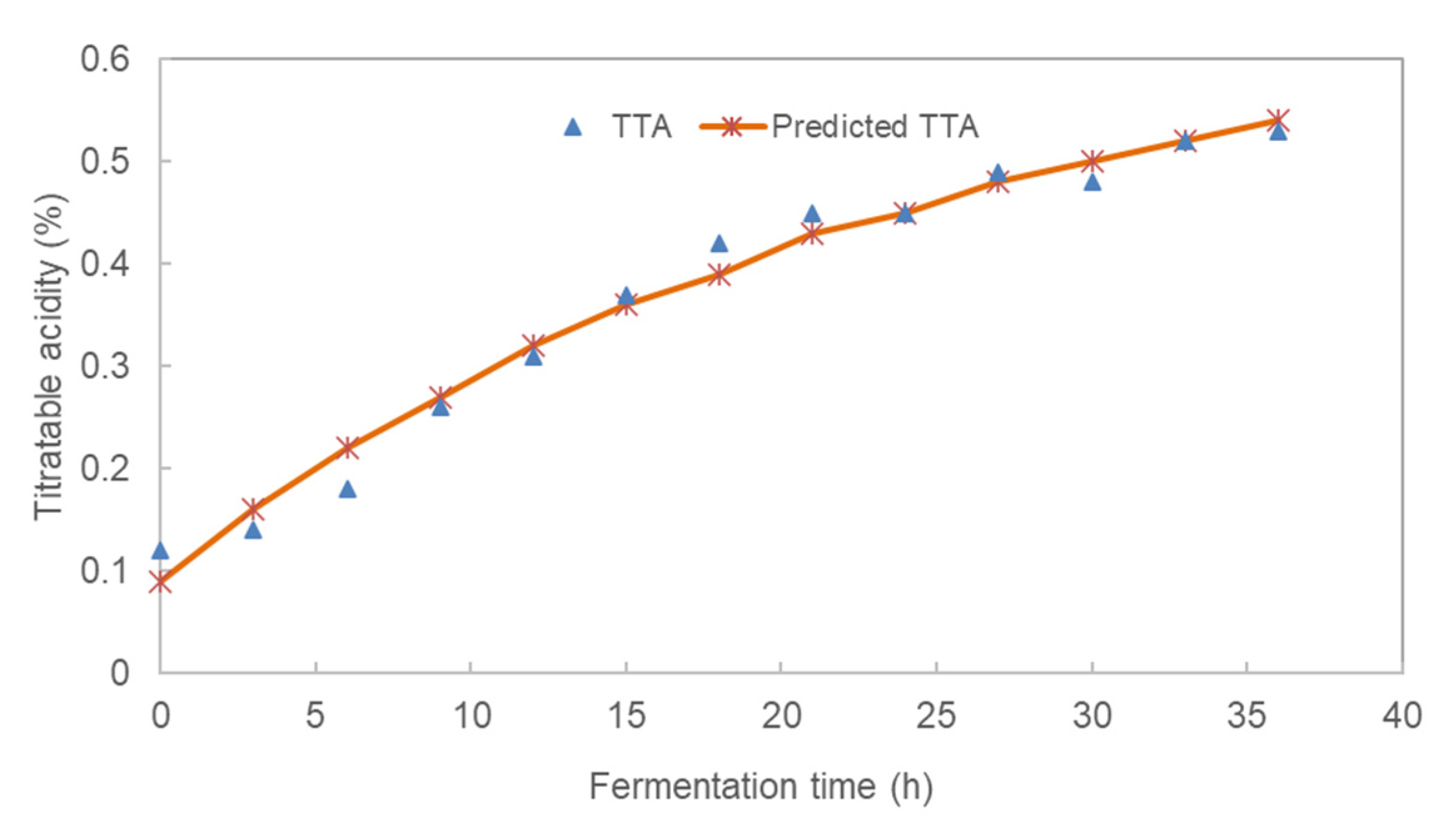
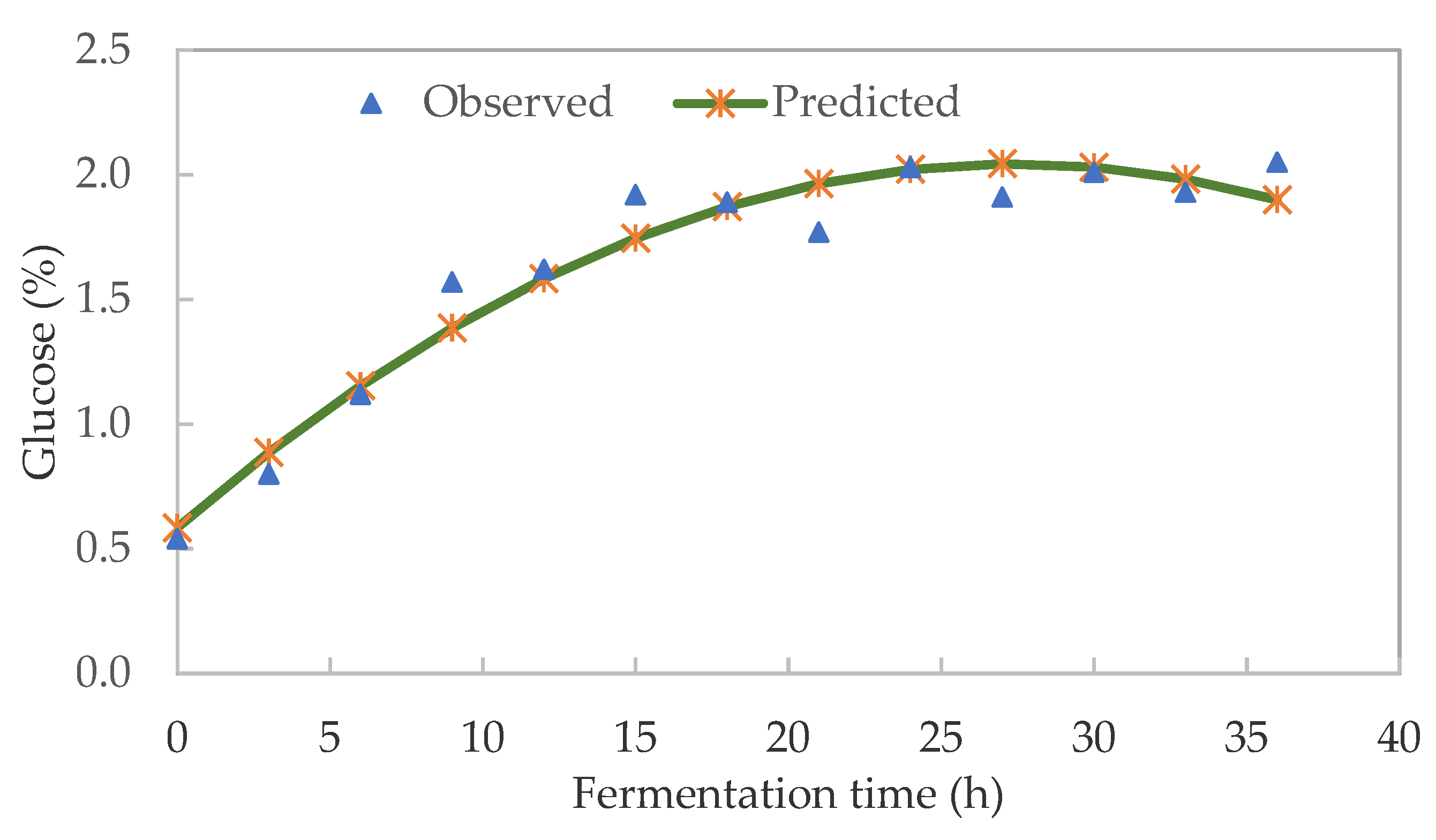

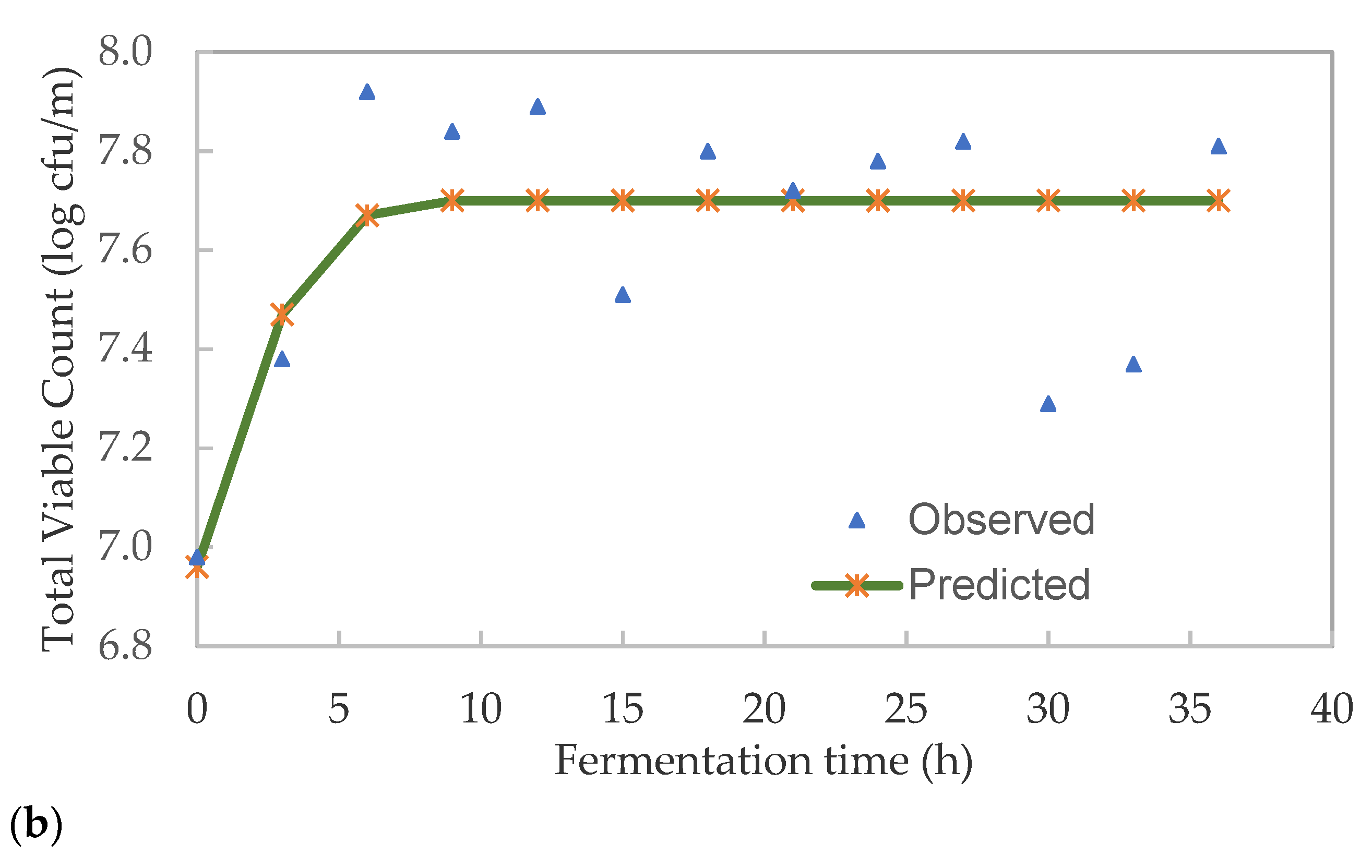
| Fermentation Time | pH | TTA (%) | Glucose (%) | LAB (log CFU/mL) | TVC (log CFU/mL) |
|---|---|---|---|---|---|
| 0 | 6.37 ± 0.15 | 0.12 ± 0.01 | 0.55 ± 0.10 | 7.04 ± 0.95 | 6.98 ± 0.05 |
| 3 | 6.09 ± 0.13 | 0.14 ± 0.04 | 0.80 ± 0.07 | 6.73 ± 0.46 | 7.38 ± 0.40 |
| 6 | 5.59 ± 0.09 | 0.18 ± 0.01 | 1.12 ± 0.10 | 7.74 ± 0.47 | 7.92 ± 0.14 |
| 9 | 5.41 ± 0.07 | 0.26 ± 0.03 | 1.57 ± 0.07 | 6.76 ± 0.02 | 7.84 ± 0.34 |
| 12 | 4.68 ± 0.09 | 0.31 ± 0.01 | 1.62 ± 0.03 | 7.87 ± 0.34 | 7.89 ± 0.19 |
| 15 | 4.36 ± 0.17 | 0.37 ± 0.03 | 1.92 ± 0.05 | 8.1 ± 1.01 | 7.51 ± 0.04 |
| 18 | 4.06 ± 0.06 | 0.42 ± 0.01 | 1.89 ± 0.03 | 7.79 ± 0.25 | 7.80 ± 0.26 |
| 21 | 3.96 ± 0.03 | 0.45 ± 0.01 | 1.77 ± 0.06 | 8.00 ± 0.56 | 7.72 ± 0.19 |
| 24 | 3.9 ± 0.05 | 0.45 ± 0.03 | 2.03 ± 0.03 | 7.99 ± 0.40 | 7.78 ± 0.16 |
| 27 | 3.84 ± 0.06 | 0.49 ± 0.02 | 1.91 ± 0.05 | 8.01 ± 0.28 | 7.82 ± 0.08 |
| 30 | 3.81 ± 0.04 | 0.48 ± 0.02 | 2.01 ± 0.02 | 7.97 ± 0.43 | 7.29 ± 0.27 |
| 33 | 3.78 ± 0.03 | 0.52 ± 0.02 | 2.12 ± 0.09 | 7.92 ± 1.24 | 7.37 ± 0.24 |
| 36 | 3.77 ± 0.01 | 0.53 ± 0.03 | 2.05 ± 0.03 | 7.68 ± 0.60 | 7.81 ± 0.17 |
| Variable | Model Parameters | R2 | ||
|---|---|---|---|---|
| a | b | c | ||
| pH | 3.382 ± 0.213 | −3.188 ± 0.203 | 0.071 ± 0.013 | 0.971 |
| TTA (%) | 0.663 ± 0.065 | 0.575 ± 0.058 | 0.042 ± 0.009 | 0.981 |
| Parameter | Count (CFU/mL) | |
|---|---|---|
| Lactic Acid Bacteria | Total Viable Count | |
| K | 6.971 | 6.911 |
| A | 0.092 | 0.790 |
| µmax | 0.092 | 0.202 |
| λ (h) | 3.903 | 0.000 |
| E% | 0.70 | 2.01 |
| pH and lactic Acid Bacteria | Fermentation Time (h) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 | |
| pH * | 6.37 ± 0.15 | 6.09 ± 0.13 | 5.59 ± 0.09 | 5.41 ± 0.07 | 4.68 ± 0.09 | 4.36 ± 0.17 | 4.06 ± 0.06 | 3.96 ± 0.03 | 3.9 ± 0.05 | 3.84 ± 0.06 | 3.81 ± 0.04 | 3.78 ± 0.03 | 3.77 ± 0.01 |
| Leuconostoc mesenteroides ssp. dextranicum | x | ||||||||||||
| Leuconostoc pseudomesenteroides | x | x | x | ||||||||||
| Pediococcus pentosaceus | x | x | x | x | |||||||||
| Streptococcus thoraltensis; | x | x | x | ||||||||||
| Enterococcus gallinarum | X | x | x | x | x | ||||||||
| Enterococcus casseliflavus | x | x | |||||||||||
| Enterococcus faecium | X | x | x | x | x | x | |||||||
| Enterococcus faecalis | x | ||||||||||||
| Enterococcus avium | x | ||||||||||||
| Enterococcus durans | x | ||||||||||||
| Lactic Acid Bacteria | Gram Reaction | Catalase Test | Morphology | Hot-Loop Test | 4 °C | 10 °C | 45 °C | 6.5% NaCl |
|---|---|---|---|---|---|---|---|---|
| L. mesenteroides ssp. dextranicum | + | − | Cocci, groups forming chains | − | − | + | − | + |
| L. pseudomesenteroides | + | − | Cocci, groups forming chains | − | − | + | − | + |
| P. pentosaceus | + | − | Cocci, groups forming chains | − | − | + | − | + |
| S. thoraltensis; | + | − | Cocci, strepto forming chains | − | − | + | − | + |
| E. gallinarum | + | − | Cocci, groups forming chains | − | − | + | − | + |
| E. casseliflavus | + | − | Cocci, single, pairs, tetracocci forming small chains | − | − | − | − | − |
| E. faecium | + | − | Cocci, groups forming chains | − | − | + | + | + |
| E. faecalis | + | − | Cocci, groups forming chains | − | − | + | − | + |
| E. avium | + | − | Cocci, single, pairs, groups forming chains | − | − | − | − | − |
| E. durans | + | − | Cocci, groups forming chains | − | − | + | − | + |
| Independent Variable | Dependent Variable | ||||
|---|---|---|---|---|---|
| L. mesenteroides | P. pentosaceus | E. gallinarum | pH | Titratable Acidity (%) | Viscosity (mPa.s) |
| 0.050 | 0.050 | 0.050 | 3.58 ± 0.15 | 0.59 ±0.06 | 6.68 ± 4.42 |
| 0.050 | 0.050 | 0.100 | 3.57 ± 0.16 | 0.61 ± 0.09 | 1.32 ± 1.76 |
| 0.050 | 0.100 | 0.050 | 3.63 ± 0.18 | 0.59 ± 0.080 | 6.48 ± 4.01 |
| 0.050 | 0.100 | 0.100 | 3.63 ± 0.20 | 0.56 ± 0.02 | 5.72 ± 5.96 |
| 0.075 | 0.075 | 0.075 | 3.57 ± 0.16 | 0.59 ± 0.04 | 6.71 ± 1.71 |
| 0.100 | 0.050 | 0.050 | 3.61 ± 0.17 | 0.55 ± 0.03 | 2.11 ± 0.84 |
| 0.100 | 0.050 | 0.100 | 3.76 ± 0.11 | 0.54 ± 0.03 | 5.17 ± 3.98 |
| 0.100 | 0.100 | 0.050 | 3.52 ± 0.14 | 0.61 ± 0.02 | 4.62 ± 4.84 |
| 0.100 | 0.100 | 0.100 | 3.64 ± 0.20 | 0.59 ± 0.06 | 10.67 ± 0.81 |
| Parameter | Coefficient (β) | Std, Error | 95% Wald Confidence Interval | Significance | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Linear coefficient effect | |||||
| Intercept | 3.44 | 0.06 | 3.32 | 3.57 | 0.000 |
| Main coefficient effect | |||||
| L. mesentoroides (X1) | 1.28 | 0.53 | 0.24 | 2.31 | 0.016 |
| P. pentosaceus (X2) | 4.41 | 0.53 | 3.37 | 5.45 | 0.000 |
| E. gallinarum (X3) | −2.73 | 0.53 | −3.76 | −1.69 | 0.000 |
| Interactive coefficient effect | |||||
| L. mesenteroides × P. pentosaceus | −63.00 | 4.85 | −72.51 | −53.49 | 0.000 |
| L. mesenteroides × E. gallinarum | 55.00 | 4.85 | 45.49 | 64.51 | 0.000 |
| P. pentosaceus × E. gallinarum | −2.33 | 4.85 | −11.85 | 7.18 | 0.631 |
| (Scale) | 0.027 | ||||
| Parameter | Coefficient (β) | Std, Error | 95% Wald Confidence Interval | Significance | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Linear coefficient effect | |||||
| (Intercept) | 0.686 | 0.035 | 0.616 | 0.755 | 0.000 |
| Main coefficient effect | |||||
| L. mesentoroides (X1) | −2.383 | 0.311 | −2.993 | −1.774 | 0.000 |
| P. pentosaceus (X2) | −1.15 | 0.311 | −1.759 | −0.541 | 0.000 |
| E. gallinarum (X3) | 1.05 | 0.311 | 0.441 | 1.659 | 0.000 |
| Interactive coefficient effect | |||||
| L. mesenteroides × P. pentosaceus | 32 | 2.853 | 26.408 | 37.59 | 0.000 |
| L. mesenteroides × E. gallinarum | −4.667 | 2.853 | −10.26 | 0.925 | 0.102 |
| P. pentosaceus × E. gallinarum | −12.667 | 2.853 | −18.26 | −7.075 | 0.000 |
| (Scale) | 0.003 | ||||
| Parameter | Coefficient (β) | Std. Error | 95% Wald Confidence Interval | Significance | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Linear coefficient effect | |||||
| (Intercept) | 34.44 | 2.41 | 29.71 | 39.16 | 0.000 |
| Main coefficient effect | |||||
| L. mesenteroides (X1) | −347.18 | 30.41 | −406.79 | −287.58 | 0.000 |
| P. pentosaceus (X2) | −183.45 | 30.41 | −243.05 | −123.85 | 0.000 |
| E. gallinarum (X3) | −400.45 | 30.41 | −460.05 | −340.85 | 0.000 |
| Interactive coefficient effect | |||||
| L. mesenteroides and P. pentosaceus | 1742.13 | 384.66 | 988.22 | 0.000 | |
| L. mesenteroides and E. gallinarum | 4020.87 | 384.66 | 3266.95 | 4774.78 | 0.000 |
| P. pentosaceus and E. gallinarum | 2494.20 | 384.66 | 1740.29 | 3248.11 | 0.000 |
| L. mesenteroides,P. pentosaceus and E. gallinarum | −13,017.33 | 4865.56 | −22,553.67 | −3481.00 | 0.007 |
| (Scale) | 12.49 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jideani, V.A.; Ratau, M.A.; Okudoh, V.I. Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum. Foods 2021, 10, 1447. https://doi.org/10.3390/foods10071447
Jideani VA, Ratau MA, Okudoh VI. Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum. Foods. 2021; 10(7):1447. https://doi.org/10.3390/foods10071447
Chicago/Turabian StyleJideani, Victoria A., Mmaphuti A. Ratau, and Vincent I. Okudoh. 2021. "Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum" Foods 10, no. 7: 1447. https://doi.org/10.3390/foods10071447
APA StyleJideani, V. A., Ratau, M. A., & Okudoh, V. I. (2021). Non-Alcoholic Pearl Millet Beverage Innovation with Own Bioburden: Leuconostoc mesenteroides, Pediococcus pentosaceus and Enterococcus gallinarum. Foods, 10(7), 1447. https://doi.org/10.3390/foods10071447







