Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer
Abstract
:1. Introduction
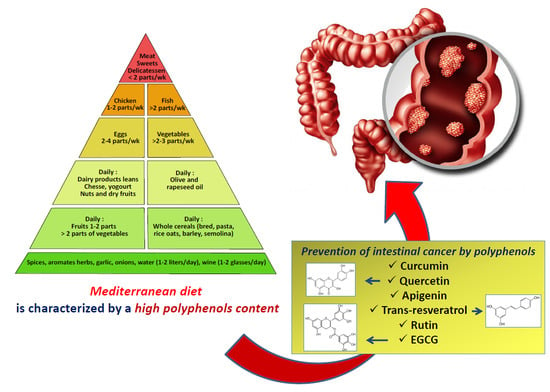
2. Benefits of the Mediterranean Diet in Preventing or Treating Cancer
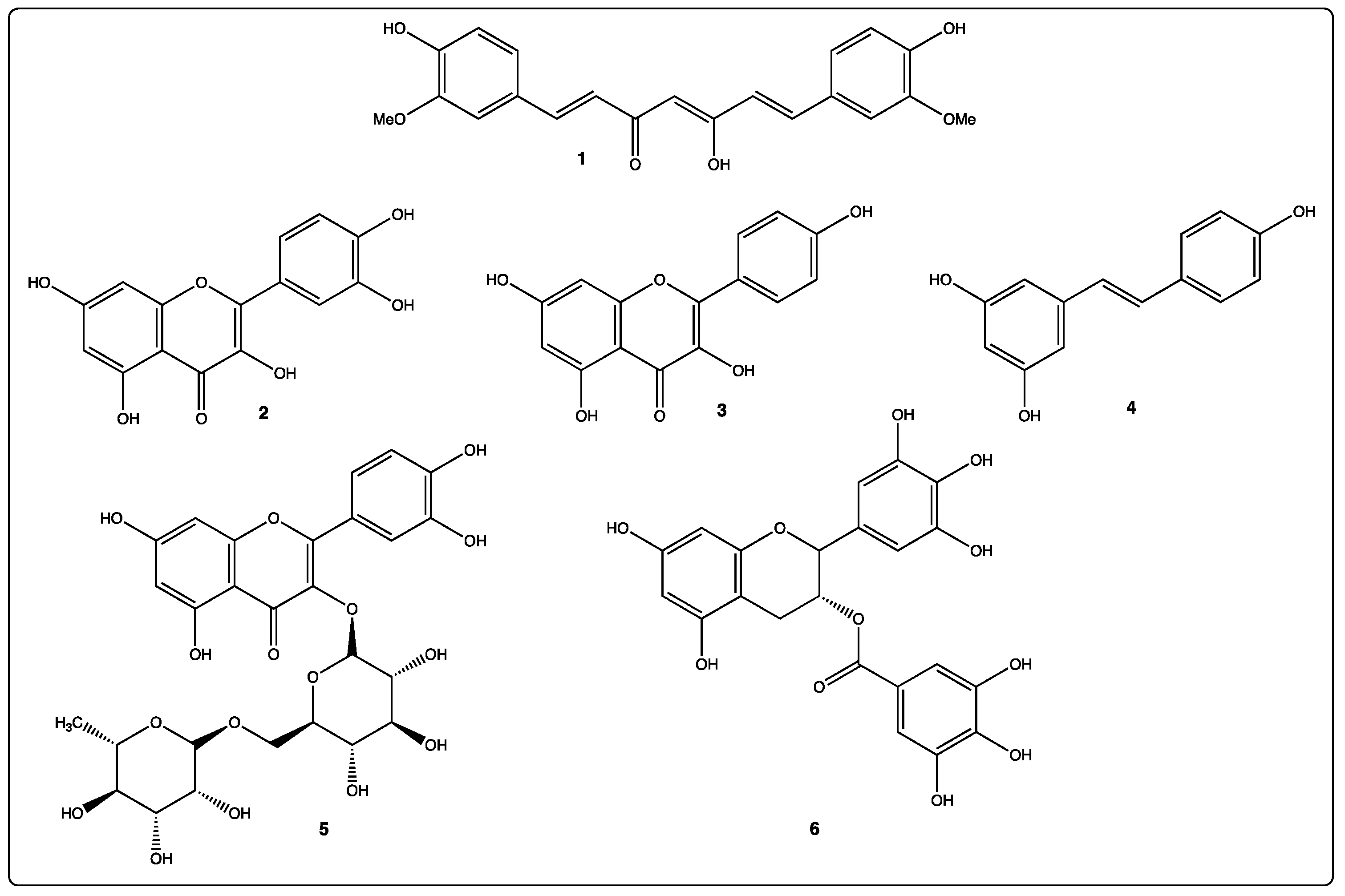
3. Key Selected Polyphenols
4. Metabolism and Microbiota Metabolites
4.1. Metabolism
4.1.1. Apigenin
4.1.2. Curcumin
4.1.3. EGCG
4.1.4. Quercetin
4.1.5. Resveratrol
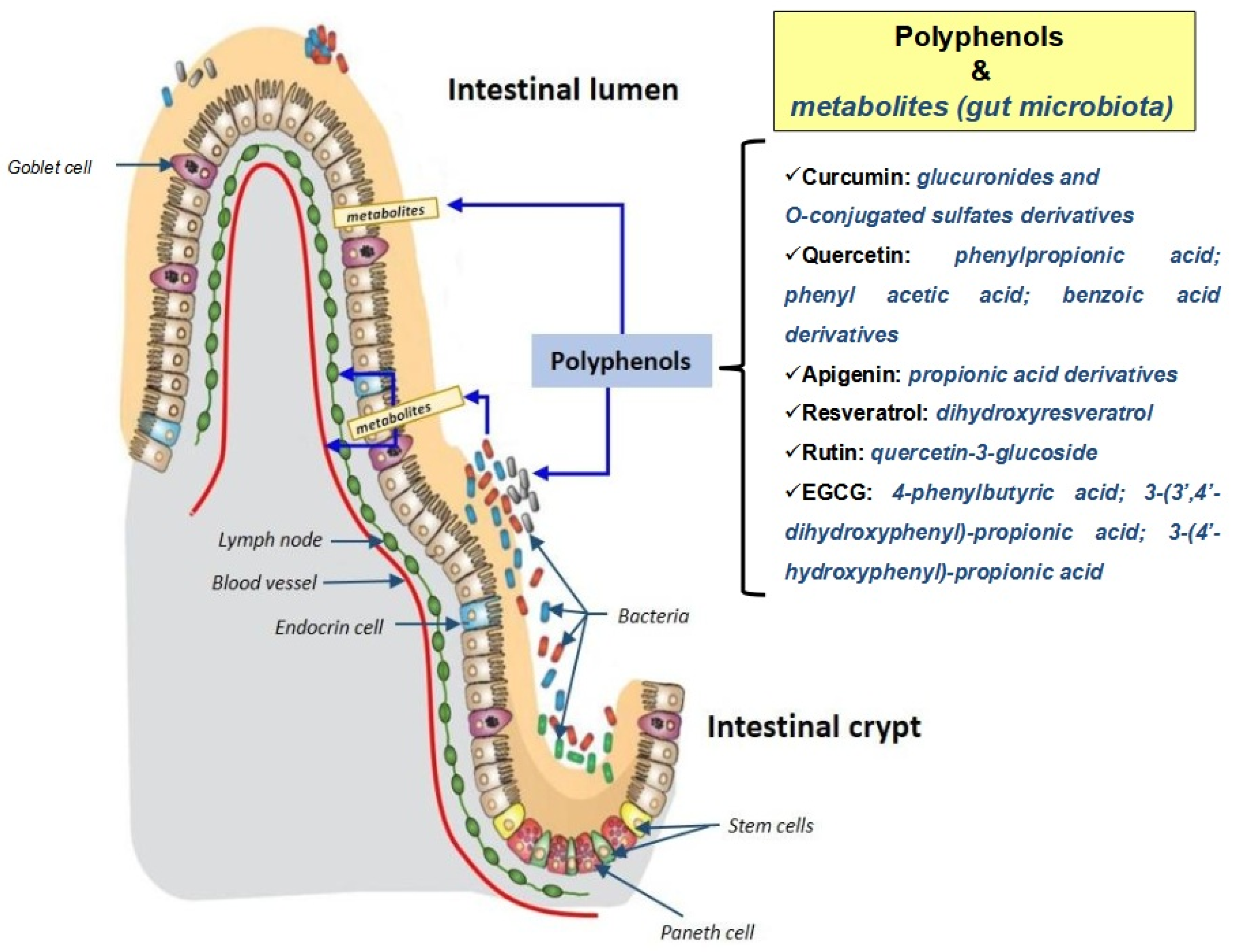
4.2. Microbiota Metabolites
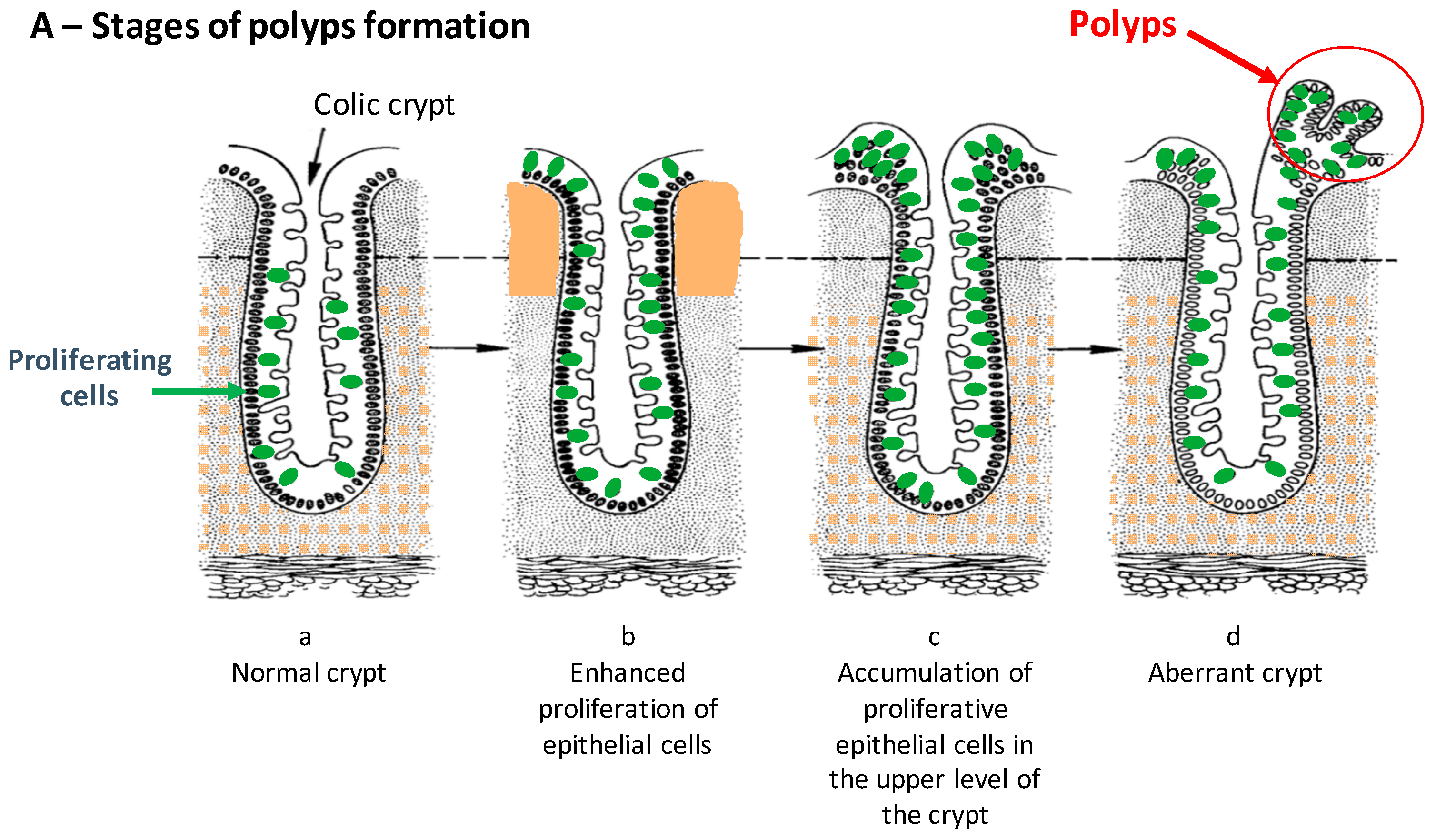
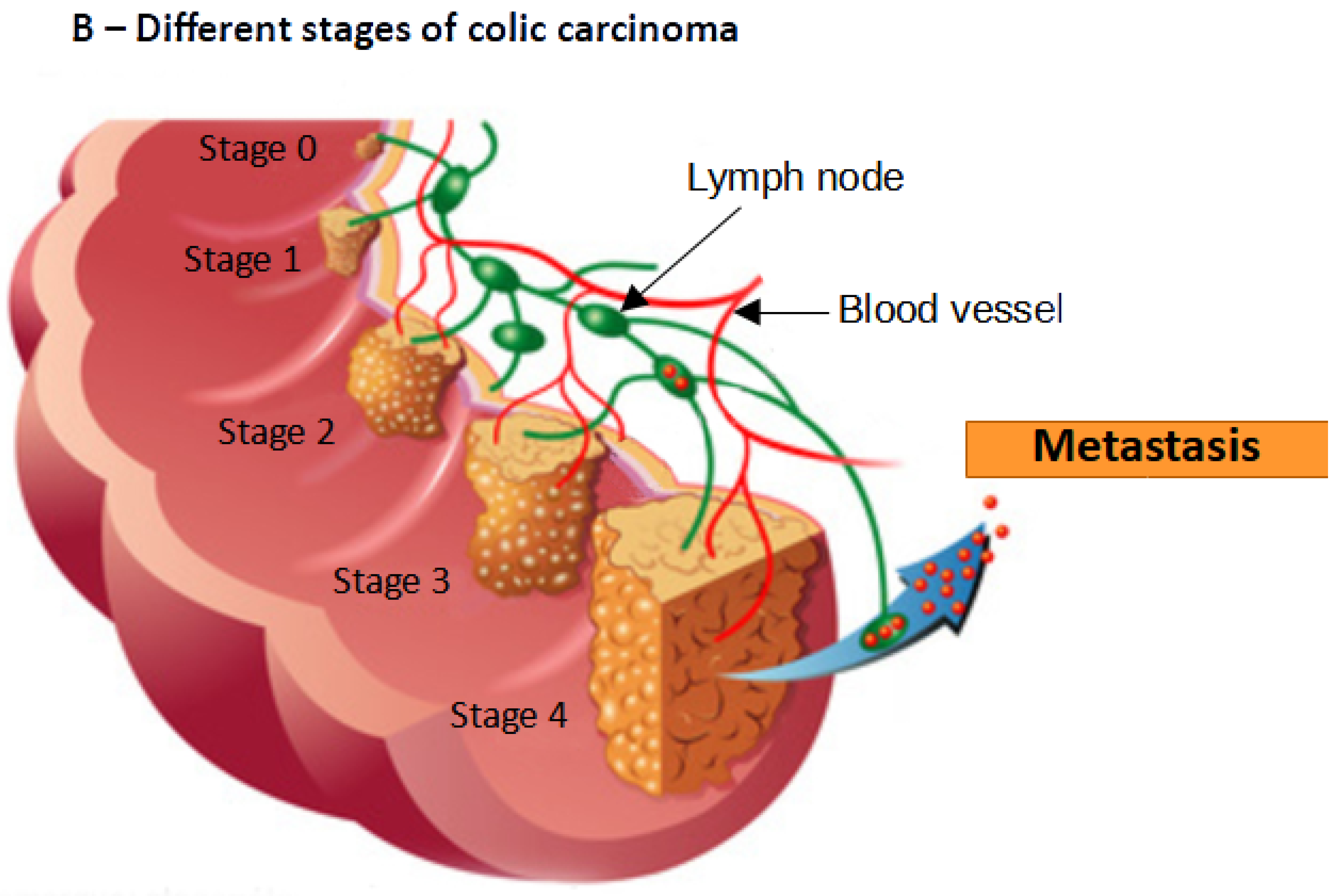
5. Antitumor Efficiency
5.1. Apigenin
5.2. Curcumin
5.3. EGCG
5.4. Quercetin
5.5. Rutin
5.6. Resveratrol
5.7. Synergistic Effects of Polyphenol Mixtures
6. Perspectives
6.1. Improvements
6.2. Nanoformulation Improvement
6.3. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [Green Version]
- Debbabi, M.; Karym, E.M.; Camus, E.; Durand, P.; Cherkaoui-Malki, M.; Prost, M.; Jabrane, A.; Nasser, B.; Hammami, M.; Lizard, G. Etude comparative des profils anti-oxydants et de la composition en polyphénols de fruits et de légumes associés au régime méditerranéen. In Wine, Mediterranean Diet and Health; Latruffe, N., Ed.; EUD: Dijon, France, 2017; pp. 63–70. Available online: eud.u-bourgogne.fr (accessed on 7 June 2021). (In French)
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 14025–14065. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.; Eckert, G.P.; Schmitt-Schillig, S.; Müller, W.E. Plant foods and brain aging: A critical appraisal. Forum Nutr. 2006, 59, 86–115. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Alicja Kuban Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Villarini, M.; Lanari, C.; Nucci, D.; Gianfredi, V.; Marzulli, T.; Berrino, F.; Borgo, A.; Bruno, E.; Gargano, G.; Moretti, M.; et al. Community-based participatory research to improve life quality and clinical outcomes of patients with breast cancer (DianaWeb in Umbria pilot study). BMJ Open 2016, 6, e009707. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Biondi, A.; Galvano, F.; Mistretta, A.; Marventano, S.; Buscemi, S.; Drago, F.; Basile, F. Factors associated with colorectal cancer in the context of the Mediterranean diet: A case-control study. Nutr. Cancer 2014, 66, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Salvatore Benito, A.; Valero Zanuy, M.Á.; Alarza Cano, M.; Ruiz Alonso, A.; Alda Bravo, I.; Rogero Blanco, E.; Maíz Jiménez, M.; León Sanz, M. Adherence to Mediterranean diet: A comparison of patients with head and neck cancer and healthy population. Endocrinol. Diabetes Nutr. 2019, 66, 417–424. [Google Scholar] [CrossRef]
- Melander, O.; Antonini, P.; Ottosson, F.; Brunkwall, L.; Gallo, W.; Nilsson, P.M.; Orho-Melander, M.; Pacente, G.; D’Arena, G.; Di Somma, S. Comparison of cardiovascular disease and cancer prevalence between Mediterranean and north European middle-aged populations (The Cilento on Ageing Outcomes Study and The Malmö Offspring Study). Intern. Emerg. Med. 2021. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [Green Version]
- Boccardi, V.; Esposito, A.; Rizzo, M.R.; Marfella, R.; Barbieri, M.; Paolisso, G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS ONE 2013, 8, e62781. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Hoang, T.; Sidney, S.; Steffen, L.M.; Jacobs, D.R., Jr.; Shikany, J.M.; Wilkins, J.T.; Yaffe, K. Dietary patterns during adulthood and cognitive performance in midlife: The CARDIA study. Neurology 2019, 92, e1589–e1599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, D.Y. Docking Characterization and in vitro Inhibitory Activity of Flavan-3-ols and Dimeric Proanthocyanidins against the Main Protease Activity of SARS-Cov-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef] [PubMed]
- Nadtochiy, S.M.; Redman, E.K. Mediterranean diet and cardioprotection: The role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 2011, 27, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor Targeted Curcumin Delivery by Folate-Modified MPEG-PCL Self-Assembly Micelles for Colorectal Cancer Therapy. Int. J. Nanomed. 2020, 15, 1239–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Dorandeu, C.; Muller, R.H.; Begu, S. Dermal quercetin smartCrystals: Formulation development, antioxidant activity and cellular safety. Eur. J. Pharm. Biopharm. 2016, 102, 51–63. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Wein, S.; Beyer, B.; Zimmermann, B.F.; Blank, R.H.; Wolffram, S. Bioavailability of Quercetin from Onion Extracts after Intraruminal Application in Cows. J. Agric. Food Chem. 2018, 66, 10188–10192. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Balata, G.F.; Essa, E.A.; Shamardl, H.A.; Zaidan, S.H.; Abourehab, M.A.S. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des. Dev. Ther. 2016, 10, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; Van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Raal, A.; Pokk, P.; Arend, A.; Aunapuu, M.; Jogi, J.; Okva, K.; Pussa, T. Trans-resveratrol alone and hydroxystilbenes of rhubarb (Rheum rhaponticum L.) root reduce liver damage induced by chronic ethanol administration: A comparative study in mice. Phytoter. Res. 2009, 23, 525–532. [Google Scholar] [CrossRef]
- Gullon, B.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Fahra, A.K.; Gian, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.T.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Hu, J.M.; Huang, Y.W.; Wu, X.Y.; Zi, C.T.; Wang, X.J.; Sheng, J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiki, H. Green tea: Health benefits as cancer preventive for humans. Chem. Record 2005, 5, 119–132. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C.; Kromhout, D. Chocolate as a source of tea flavonoids. Lancet 1999, 354, 488. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, L.; Mai, X.; Wang, H.; Liu, S.; Zeng, H.; Chen, T.; Li, J. Use of UHPLC-QTOF-MS/MS with combination of in silico approach for distributions and metabolites profile of flavonoids after oral administration of Niuhuang Shangqing tablets in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1114–1115, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoehle, S.I.; Pfeiffer, E.; Sólyom, A.M.; Metzler, M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lançon, A.; Delmas, D.; Osman, H.; Thenot, J.P.; Jannin, B.; Latruffe, N. Human hepatic cell uptake of resveratrol: Involvement of both carrier mediaten and diffusion process. Biochem. Biophys. Res. Commun. 2004, 316, 1132–1137. [Google Scholar] [CrossRef]
- Jannin, B.; Menzel, M.; Berlot, J.P.; Delmas, D.; Lançon, A.; Latruffe, N. Interactions of resveratrol, a chemopreventive agent, with serum albumin. Biochem. Pharmacol. 2004, 68, 1113–1118. [Google Scholar] [CrossRef]
- Latruffe, N.; Menzel, M.; Delmas, D.; Buchet, R.; Lancçon, A. Compared binding properties between resveratrol and of other polyphenols to plasmatic albumin. Consequences for the health protecting effect of dietary plant microcomponents. Molecules 2014, 19, 17066–17077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, W.C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; et al. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch. Biochem. Biophys. 2020, 694, 108589. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazue, F.; Ghiringhelli, F.; Latruffe, N. Transport and stability of Resveratrol condition its biological activity. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Lançon, A.; Hanet, N.; Jannin, B.; Delmas, D.; Heydel, J.M.; Chagnon, M.C.; Lizard, G.; Artur, Y.; Latruffe, N. Resveratrol in human hepatoma HepG2 cells: Metabolism and inducibility of detoxifying enzymes. Drug. Metab. Dispos. 2007, 35, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Colin, D.; Lançon, A.; Delmas, D.; Abrossimow, J.; Kahn, E.; Lizard, G.; Jannin, B.; Latruffe, N. Comparative study of the cell uptake and the antiproliferative effects of resveratrol, epsilon-viniferin and their acetates. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S. Microbiomics: Were we all wrong before? Periodontology 2000, 85, 8–11. [Google Scholar] [CrossRef]
- Hardman, W.E. Diet components can suppress inflammation and reduce cancer risk. Nutr. Res. Pract. 2014, 8, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Seyed Hameed, A.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef] [PubMed]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Chalons, P.; Courtaut, F.; Limagne, E.; Chalmin, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schini-Kerth, V.; Ghiringhelli, F.; et al. Red Wine Extract Disrupts Th17 Lymphocyte Differentiation in a Colorectal Cancer Context. Mol. Nutr. Food Res. 2020, 64, e1901286. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A review on flavonoid apigenin: Dietary intake, adme, antimicrobial effects, and interactions with human gut microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin impacts the growth of the gut microbiota and alters the gene expression of enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual two-way interactions of curcumin and gut microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between Epigallocatechin-3-gallate (EGCG) and Human Gut Microbiota In vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The human microbial metabolism of quercetin in different formulations: An in vitro evaluation. Foods 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Firrman, J.; Liu, L.; Zhang, L.; Arango Argoty, G.; Wang, M.; Tomasula, P.; Kobori, M.; Pontious, S.; Xiao, W. The effect of quercetin on genetic expression of the commensal gut microbes Bifidobacterium catenulatum, Enterococcus caccae and Ruminococcus gauvreauii. Anaerobe 2016, 42, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Mitchell, D.; Gold, B.; Pour, P.; Pinch, H.C. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997, 17, 85–91. [Google Scholar]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Yin, F.; Wakino, S.; Liu, Z.; Kim, S.; Hsueh, W.A.; Collins, A.R.; Van Herle, A.J.; Law, R.E. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem. Biophys. Res. Commun. 2001, 286, 916–922. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell. Int. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Banerjee, S.; Mandal, M. Enhanced chemotherapeutic efficacy of apigenin liposomes in colorectal cancer based on flavone-membrane interactions. J. Colloid Interface Sci. 2017, 491, 98–110. [Google Scholar] [CrossRef]
- Li, J.; Yu, B.; Deng, P.; Cheng, Y.; Yu, Y.; Kevork, K.; Ramadoss, S.; Ding, X.; Li, X.; Wang, C.Y. KDM3 epigenetically controls tumorigenic potentials of human colorectal cancer stem cells through Wnt/β-catenin signalling. Nat. Commun. 2017, 8, 15146. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Van Wie, P.G.; Fai, L.Y.; Kim, D.; Wang, L.; Poyil, P.; Luo, J.; Zhang, Z. Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicol. Appl. Pharmacol. 2016, 311, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Krisanapun, C.; Lee, S.H.; Nualsanit, T.; Sams, C.; Peungvicha, P.; Baek, S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chunhua, L.; Donglan, L.; Xiuqiong, F.; Lihua, Z.; Qin, F.; Yawei, L.; Liang, Z.; Ge, W.; Linlin, J.; Ping, Z.; et al. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J. Nutr. Biochem. 2013, 24, 1766–1775. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, S103–S127. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Söderlund, S.; Brandt, L.; Lapidus, A.; Karlén, P.; Broström, O.; Löfberg, R.; Ekbom, A.; Askling, J. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 2009, 136, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Dempe, J.S.; Scheerle, R.K.; Pfeiffer, E.; Metzler, M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 1543–1549. [Google Scholar] [CrossRef]
- Kuttan, G.; Kumar, K.B.; Guruvayoorappan, C.; Kuttan, R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 173–184. [Google Scholar] [CrossRef]
- Bartik, L.; Whitfield, G.K.; Kaczmarska, M.; Lowmiller, C.L.; Moffet, E.W.; Furmick, J.K.; Hernandez, Z.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Curcumin: A novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J. Nutr. Biochem. 2010, 21, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta. Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Silvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Q.; Ives, K.L.; Evers, B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin. Cancer Res. 2006, 12, 5346–5355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE 2013, 8, e57218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; He, L.J.; Ye, H.Z.; Liu, D.F.; Zhu, Y.B.; Miao, D.D.; Zhang, S.P.; Chen, Y.Y.; Jia, Y.W.; Shen, J.; et al. Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT-8/5-Fu human colorectal cancer cell line. Mol. Med. Rep. 2018, 18, 5409–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas, I.; Sánchez-Fidalgo, S.; de la Lastra, C.A. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol. Nutr. Food. Res. 2011, 55, 259–267. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Li, Q.S.; Liang, Y.R. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Cromie, M.M.; Gao, W. Epigallocatechin-3-gallate enhances the therapeutic effects of leptomycin B on human lung cancer a549 cells. Oxid. Med. Cell. Longev. 2015, 2015, 217304. [Google Scholar] [CrossRef] [Green Version]
- Kumazoe, M.; Takai, M.; Hiroi, S.; Takeuchi, C.; Yamanouchi, M.; Nojiri, T.; Onda, H.; Bae, J.; Huang, Y.; Takamatsu, K.; et al. PDE3 inhibitor and EGCG combination treatment suppress cancer stem cell properties in pancreatic ductal adenocarcinoma. Sci. Rep. 2017, 7, 1917. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; La Vecchia, C.; Turati, F. Green tea and liver cancer. Hepatobiliary Surg. Nutr. 2017, 6, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Shirakami, Y.; Shimizu, M.; Tsurumi, H.; Hara, Y.; Tanaka, T.; Moriwaki, H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol. Med. Rep. 2008, 1, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, G.; Magaribuchi, T.; Shingu, K.; Suga, S.; Tamai, S.; Nakao, K.; Mori, K. Positive end-expiratory pressure ventilation decreases plasma atrial and brain natriuretic peptide levels in humans. Anesth. Analg. 1993, 77, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. Biomed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Hara, T.; Shimizu, M.; Nagano, J.; Ohno, T.; Hoshi, M.; Ito, H.; Tsurumi, H.; Saito, K.; Seishima, M.; et al. (-)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncol. Lett. 2012, 4, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Li, M.C.; Wang, F.R.; Mackenzie, G.G.; Oteiza, P.I. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem. Pharmacol. 2020, 175, 113923. [Google Scholar] [CrossRef]
- Jin, H.; Gong, W.; Zhang, C.; Wang, S. Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. Onco Targets Ther. 2013, 6, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Md Nesran, Z.N.; Shafie, N.H.; Ishak, A.H.; Mohd Esa, N.; Ismail, A.; Md Tohid, S.F. Induction of Endoplasmic Reticulum Stress Pathway by Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Activation of PERK/p-eIF2a/ATF4 and IRE1a. Biomed. Res. Int. 2019, 2019, 3480569. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, T.; Murata, S.; Nakayama, K.; Sano, N.; Ogawa, K.; Nowatari, T.; Tamura, T.; Nozaki, R.; Fukunaga, K.; Ohkohchi, N. (-)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol. Rep. 2014, 31, 625–633. [Google Scholar] [CrossRef]
- Hu, F.; Wei, F.; Wang, Y.; Wu, B.; Fang, Y.; Xiong, B. EGCG synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells. J. Pharmacol. Sci. 2015, 128, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-gallate Enhances Radiation Sensitivity in Colorectal Cancer Cells Through Nrf2 Activation and Autophagy. Anticancer Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef]
- Kim, W.K.; Bang, M.H.; Kim, E.S.; Kang, N.E.; Jung, K.C.; Cho, H.J.; Park, J.H. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer 2013, 65, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Han, H.; Ma, L.; Zhou, H.; Zhao, C. The chemosensitization effect of quercetin on cisplatin induces the apoptosis of human colon cancer HT-29 cell line. Int. J. Clin. Med. 2016, 9, 2285–2292. [Google Scholar]
- Psahoulia, F.H.; Drosopoulos, K.G.; Doubravska, L.; Andera, L.; Pintzas, A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 2007, 6, 2591–2599. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, S.K.; Kim, B.S.; Lee, S.H.; Park, Y.S.; Park, B.K.; Kim, S.J.; Kim, J.; Choi, C.; Kim, J.S.; et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J. Basic Med. Sci. 2015, 18, 635–643. [Google Scholar]
- van Erk, M.J.; Roepman, P.; van der Lende, T.R.; Stierum, R.H.; Aarts, J.M.; van Bladeren, P.J.; van Ommen, B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur. J. Nutr. 2005, 44, 143–156. [Google Scholar] [CrossRef]
- Han, M.; Song, Y.; Zhang, X. Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells Through Regulating Toll-like Receptor 4/Nuclear Factor-kappa B Pathway. Pharm. Mag. 2016, 12, S237–S244. [Google Scholar]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharm. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.E.; Wang, M.X.; Li, R.Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Investig. 2009, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Ebermann, R.; Marian, B. Quercetin-induced apoptosis in colorectal tumor cells: Possible role of EGF receptor signaling. Nutr. Cancer 1999, 34, 88–99. [Google Scholar] [CrossRef]
- Özsoy, S.; Becer, E.; Kabaday, H.; Vatansever, H.S.; Yücecan, S. Quercetin-Mediated Apoptosis and Cellular Senescence in Human Colon Cancer. Anticancer Agents Med. Chem. 2020, 20, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.Y.; Han, Y.H.; Kim, D.S.; Mun, J.G.; Park, J.; Jeong, M.Y.; Um, J.Y.; Hong, S.H. Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Komatsu, H.; Enya, T.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: Structure-activity relationship. Jpn. J. Cancer Res. 2000, 91, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef]
- Pampaloni, B.; Palmini, G.; Mavilia, C.; Zonefrati, R.; Tanini, A.; Brandi, M.L. In vitro effects of polyphenols on colorectal cancer cells. World J. Gastrointest. Oncol. 2014, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Jin, J.; Zhu, S.X.; He, G.Q.; Li, S.H.; Wang, J.; Cai, Y. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 2020, 523, 947–953. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.Y.; Fang, M.Z.; Wang, X.F.; Chen, H.; Huang, S.L.; Kong, D.S.; Li, M.; Zhang, X.; Sun, Y.; et al. Epigallocatechin gallate inhibits dimethylhydrazine-induced colorectal cancer in rats. World J. Gastroenterol. 2020, 26, 2064–2081. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020, 253, 117584. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, J.; Li, Y.; Luo, J.; Zhang, C.; Ou, S.; Zhang, G.; Yang, X.; Peng, X. Alternating consumption of β-glucan and quercetin reduces mortality in mice with colorectal cancer. Food Sci. Nutr. 2019, 7, 3273–3285. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic Interaction of Rutin and Silibinin on Human Colon Cancer Cell Line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Singh, M.; Gautam, S.; Rawat, J.K.; Saraf, S.A.; Kaithwas, G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Fideles, L.S.; de Miranda, J.A.L.; Martins, C.D.S.; Barbosa, M.L.L.; Pimenta, H.B.; Pimentel, P.V.S.; Teixeira, C.S.; Scafuri, M.A.S.; Façanha, S.O.; Barreto, J.E.F.; et al. Role of Rutin in 5-Fluorouracil-Induced Intestinal Mucositis: Prevention of Histological Damage and Reduction of Inflammation and Oxidative Stress. Molecules 2020, 25, 2786. [Google Scholar] [CrossRef]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Vervandier-Fasseur, D.; Latruffe, N. Cancer prevention potential of resveratrol. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [Green Version]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Ferrucci, L.; Bartali, B.; Urpí-Sarda, M.; Zamora-Ros, R.; Sun, K.; Cherubini, A.; Bandinelli, S.; Andres-Lacueva, C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern. Med. 2014, 174, 1077–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provinciali, M.; Re, F.; Donnini, A.; Orlando, F.; Bartozzi, B.; Di Stasio, G.; Smorlesi, A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer 2005, 115, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Ametller, E.; Fuster, G.; Olivan, M.; Raab, V.; Argilés, J.M.; López-Soriano, F.J. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007, 245, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Limagne, E.; Cotte, A.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergizes with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.K.; Khandelwal, N.; Hintzsche, H.; Stopper, H. Antigenotoxic effects of resveratrol: Assessment of in vitro and in vivo response. Mutagenesis 2016, 31, 27–33. [Google Scholar] [PubMed] [Green Version]
- Delmas, D.; Rébé, C.; Lacour, S.; Filomenko, R.; Athias, A.; Gambert, P.; Cherkaoui-Malki, M.; Jannin, B.; Dubrez-Daloz, L.; Latruffe, N.; et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J. Biol. Chem. 2003, 278, 41482–41490. [Google Scholar] [CrossRef] [Green Version]
- Aires, V.; Colin, D.J.; Agnès, A.; Di Pietro, A.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E.; et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tili, E.; Michaille, J.-J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.; Yang, Y.C.S.; Chin, Y.T.; Chou, S.Y.; Chen, Y.R.; Shih, Y.J.; Whang-Peng, J.; Changou, C.A.; Liu, H.L.; Lin, S.J.; et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food Chem. Toxicol. 2018, 120, 346–355. [Google Scholar] [CrossRef]
- Frazzi, R.; Valli, R.; Tamagnini, I.; Casali, B.; Latruffe, N.; Merli, F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int. J. Cancer 2013, 132, 1013–1021. [Google Scholar] [CrossRef]
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jäger, W. Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Baarine, M.; Thandapilly, S.J.; Louis, X.L.; Mazué, F.; Yu, L.; Delmas, D.; Netticadan, T.; Lizard, G.; Latruffe, N. Pro-apoptotic versus anti-apoptotic properties of dietary resveratrol on tumoral and normal cardiac cells. Genes Nutr. 2011, 6, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Marel, A.K.; Lizard, G.; Izard, J.-C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Cilibrasi, C.; Riva, G.; Romano, G.; Cadamuro, M.; Bazzoni, R.; Butta, V.; Paoletta, L.; Dalprà, L.; Strazzabosco, M.; Lavitrano, M.; et al. Resveratrol Impairs Glioma Stem Cells Proliferation and Motility by Modulating the Wnt Signaling Pathway. PLoS ONE 2017, 12, e0169854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In vitro and In vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jordá, L.; Estrela, J.M. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef]
- Mazué, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014, 5, 663–670. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Kotfis, K.; Zaid, S.; Terpilowska, S.; Robba, C.; Siwicki, A.K. The brain-gut axis—where are we now and how can we modulate these connections? Curr. Neuropharmacol. 2020. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols with Management of Psychiatric Mood Disorders. Front Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, L.; Zhang, X.; Wu, Z.; Weng, P. The interaction between tea polyphenols and host intestinal microorganisms: An effective way to prevent psychiatric disorders. Food Funct. 2021, 12, 952–962. [Google Scholar] [CrossRef]
- Matarazzo, I.; Toniato, E.; Robuffo, I. Psychobiome Feeding Mind: Polyphenolics in Depression and Anxiety. Curr. Top. Med. Chem. 2018, 18, 2108–2115. [Google Scholar] [CrossRef]
- Chung, J.Y.; Jeong, J.H.; Song, J. Resveratrol Modulates the Gut-Brain Axis: Focus on Glucagon-Like Peptide-1, 5-HT, and Gut Microbiota. Front. Aging Neurosci. 2020, 12, 588044. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F. Nanoparticle formulations to enhance tumor targeting of poorly soluble polyphenols with potential anticancer properties. Semin. Cancer Biol. 2017, 46, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Escribano-Ferrer, E.; Coma-Cros, E.M.; Biosca, A.; Lantero, E.; Fernandez-Busquets, X.; Fadda, A.M.; Caddeo, C. Nanoformulation of curcumin-loaded eudragit-nutriosomes to counteract malária infection by a dual strategy: Improving anti-oxidant intestinal activity and systemic efficacy. Int. J. Pharm. 2019, 556, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of bioactive quercetin in oncotherapy : From nutrition to nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Chen, G.; Chen, X.; Sun, Z.; Ma, X.; Su, W.; Deng, Z.; Ma, L.; Ran, Y.; Tong, Q.; et al. Molecular assembly of versatile nanoparticles with epigallocatechin gallate. ACS Sustain. Chem. Eng. 2020, 8, 9833–9845. [Google Scholar] [CrossRef]
- Dutta, D.; Chakraborty, A.; Mukherjee, B.; Gupta, S. Aptamer-conjugated apigenin nanoparticles to target colorectal carcinoma: A promising safe alternative of colorectal cancer chemotherapy. ACS Appl. Bio Mater. 2018, 1, 1538–1556. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.L.; Wei, X.W.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.L.; Huang, M.; Guo, G.; et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7021–7030. [Google Scholar] [CrossRef]
- Xu, G.; Shi, H.; Ren, L.; Gou, H.; Gong, D.; Gao, X.; Huang, N. Enhancing the anti-colon cancer activity of quercetin by self-assembled micelles. Int. J. Nanomed. 2015, 10, 2051–2063. [Google Scholar]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Feng, M.; Zhong, L.X.; Zhan, Z.Y.; Huang, Z.H.; Xiong, J.P. Enhanced antitumor efficacy of resveratrol-loaded nanocapsules in colon cancer cells: Physicochemical and biological characterization. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 375–382. [Google Scholar]
- Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Parekh, H.S.; Popat, A. Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J. Colloid Interface Sci. 2016, 462, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and its nanoformulation attenuate growth and the angiogenesis of xenograft and orthotopic colon cancer models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin Nanoformulations for Colorectal Cancer: A Review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Chun, J.; Li, R.J.; Cheng, M.S.; Kim, Y.S. Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells. Cancer Lett. 2015, 357, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shen, L.; Li, X.; Song, W.; Liu, Y.; Huang, L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano 2019, 13, 12511–12524. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.V.; Athira, K.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Lotfi-Attari, J.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Alipour, S.; Farajzadeh, R.; Javidfar, S.; Zarghami, N. Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutr. Cancer 2017, 69, 1290–1299. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- Patial, V.; Mahesh, S.; Sharma, S.; Pratap, K.; Singh, D.; Padwad, Y.S. Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2015, 40, 445–452. [Google Scholar] [CrossRef]
- Milincic, D.D.; Popovic, D.A.; Levic, S.M.; Kostic, A.Z.; Tesic, Z.L.; Nedovic, V.A.; Pesic, M.B. Application of polyphenol-loaded nanoparticles in food chemistry. Nanomater 2019, 9, 1629. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.A. Phenolics: Prooxidants or antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds–Implications for health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef]
 resveratrol;
resveratrol;  glucurono resveratrol;
glucurono resveratrol;  sulfo resveratrol.
sulfo resveratrol.
 resveratrol;
resveratrol;  glucurono resveratrol;
glucurono resveratrol;  sulfo resveratrol.
sulfo resveratrol.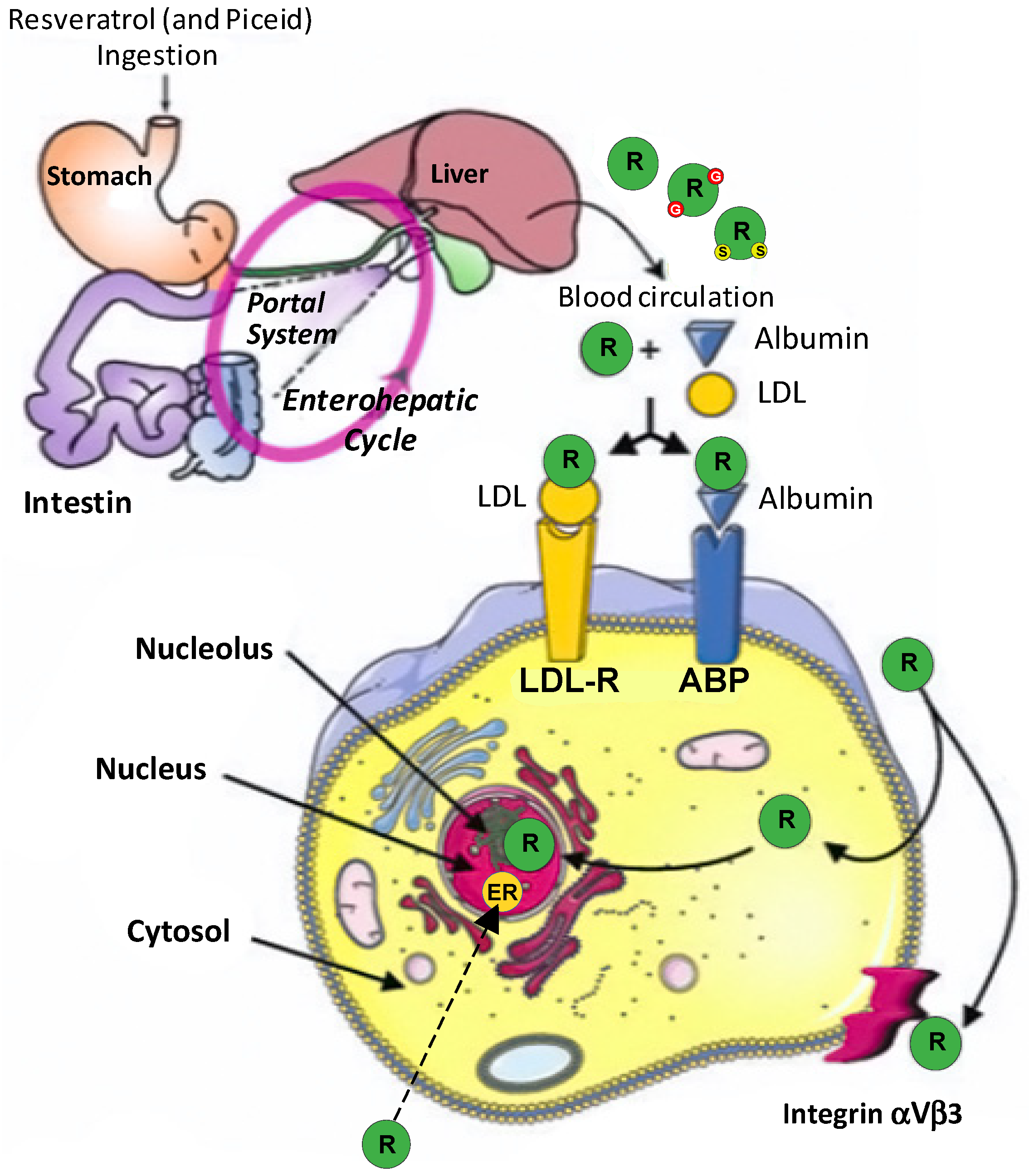
| Fruits | Mean of Total Polyphenol Content (mg of GAE/100g Fresh Edible Portion) | Vegetables | Mean of Total Polyphenol Content (mg of GAE/100 g Fresh Edible Portion) |
|---|---|---|---|
| Strawberry | 263.8 | Artichoke heart | 321.3 |
| Lychee | 222.3 | Parsley | 280.2 |
| Grape | 195.5 | Brussels sprout | 257.1 |
| Apricot | 179.8 | Shallot | 104.1 |
| Apple | 1179.1 | Broccoli | 98.9 |
| Date | 99.3 | Celery | 84.7 |
| Cherry | 94.3 | Onion | 79.1 |
| Fig | 92.5 | Eggplant | 65.6 |
| Pear | 69.2 | Garlic | 59.4 |
| White nectarine | 72.7 | Turnip | 54.7 |
| Passion fruit | 71.8 | Celeriac | 39.8 |
| Mango | 68.1 | Radish | 38.4 |
| Yellow/white peach | 59.3–44.2 | Pea | 36.7 |
| Banana | 51.5 | Leek | 32.7 |
| Pineapple | 47.2 | Red bell pepper | 26.8 |
| Lemon | 45 | Cherry tomato | 26.6 |
| Grape fruit | 43.5 | Potato | 23.1 |
| Orange | 31 | Zucchini | 18.8 |
| Clementine | 30.6 | Green bell pepper | 18.2 |
| Lime | 30.6 | Chicory | 14.7 |
| Kiwi | 28.1 | Asparagus | 14.5 |
| Watermelon | 11.6 | Tomato | 13.7 |
| melon | 7.8 | Fennel | 13 |
| Cauliflower | 12.5 | ||
| Carrot | 10.1 | ||
| French string bean | 10 | ||
| Avocado | 3.6 |
| Extracts | Polyphenols Expressed in (mg/100 g of Fresh Matter) |
|---|---|
| Muscat grape | Gallic acid (1.7), epigallocatechin (10.8), catechin (21.8), epicatechin (5.3), quercetin-3-O-beta-d-glucuronide (10.8) |
| White grape | Gallic acid (1.1), caftaric acid (10.8), epigallocatechin (4.4), catechin (10.6), epicatechin (6.0), quercetin-3-O-beta-d-glucuronide (1.7) |
| Strawberry | Epigallocatechin (6.3), catechin (7.1), epicatechin (4.1), epicatechin gallate (1.3), quercetin-3-O-beta-d-glucuronide (15.4), kaempferol-3-O-glucoside (2.6) |
| Raspberry | Rutin (1.6), quercetin-3-O-beta-d-glucuronide (1.7) |
| Beans | Catechin (1.0), myricetin-3-O-glucoside (12.0), rutin (0.8), kaempferol-3-rutinoside (8.1) |
| Tomato | Chlorogenic acid (2.4), myricetin-3-O-glucoside (0.3), rutin (1.3), kaempferol-3-O-glucoside (0.1), quercetin (0.4) |
| Celery | Chlorogenic acid (75.3), quercetin-3,4′-diglucoside (2.3), rutin (368.9), quercetin-3-beta-glucoside (5.7), kaempferol-3-rutinoside (22.1) |
| Radish | Catechin (2.7) |
| Polyphenol Family | Mol.Formula Mol. Weight | Water Solubility (mg/L) | Main Dietary and Geographic Sources | Ref. |
|---|---|---|---|---|
| 1: Curcumin Curcuminoid | C21H20O6 368.35 g/mol | 0.125 mg/L | Rhizomes of Curcuma longa (turmeric) India | [18,19] |
| 2: Quercetin Flavonoid | C15H10O7 302.20 g/mol | 0.48 mg/L (MilliQ water) | Red grape, onion, broccoli, tomato, lettuce | [20,21,22,23] |
| 3: Apigenin Flavonoid | C15H10O5 270.21 g/mol | 1.43 mg/L (pH = 1) | Chamomile (Europe, Western Asia) | [24,25] |
| 4: Resveratrol Stilbenoid | C14H12O3 228.24 g/mol | 30 mg/L | Grapes and red wine, Peanuts, blueberries, rhubarb | [26,27,28,29,30] |
| 5: Rutin Flavonoid | C27H30O16 610.44 g/mol | 130 mg/L | Red grape, citrus, apple, fig, asparagus, onion, mulberry, tea | [21,31,32] |
| 6: EGCG Flavonoid | C22H18O11 358.32 g/mol | 5733.12 mg/L | Green tea from leaves and buds of Camellia sinensis Japan, Chocolate, Red grape | [21,33,34,35] |
| APIGENIN | ||||
|---|---|---|---|---|
| Experimental Models | Concentration Range | Biological Response | Pathway/Genes/Proteins Involved | Refs. |
| Cells and Cell Lines | ||||
| HCT-15 | 43.28 µM | ↑ Cell cytotoxicity ↑ Apoptosis ↑ Cell cycle arrest G2/M | ↑ p21, ↑ cyclin B1 | [76] |
| SW480 | 40 µM | ↓ Proliferation ↓ Invasion ↓ Migration | ↓ Wnt/β-catenin | [78] |
| ↓ Cell migration, ↓ Invasion, ↓ Metastasis | ↓ FAK, Src, crk-L, AKT | [79] | ||
| ↓ Proliferation | ↓ NEDD9 | |||
| HCT-116 | 25 µM | ↓ Proliferation, ↓ Apoptosis ↓ Autophagy | ↓ Cyclin B1, ↓Cdc2, Cdc25c, ↑ PPAR cleavage, ↑ LC3-II | [80] |
| 20 μM and 40 μM | ↑ Autophagy/Apoptosis ↓ Cell grouth, cell cycle arrest G2/M | ↓ PI3K/AKT/Mtor | [81] | |
| 10 µM | ↑ Apoptosis, ↓ Trascriptional level | PKCδ/ATM kinase, ↓ NAG-1, ↓ p53, ↓ p21 | [82] | |
| LoVo | 1–10 µM | ↑ Apoptosis | ↓ NAG-1, ↓ p53, ↓ p21 | |
| DLD-1 | 40 µM | ↓ Cell migration, ↓ Invasion, ↓ Migration | ↓ NEDD9, ↓ FAK, Src, crk-L, AKT, ↑ TAGLN, ↓ MMP-9 | [79] |
| ↓ Cell migration, ↓ Invasion, | ↑ TAGLN, ↓ MMP-9 | [83] | ||
| HT-29 | 45.96 µM | ↑ cell cytotoxicity ↑ Apoptosis | ↑ p21, ↑ cyclin B1 | [76] |
| Animal models | ||||
| Athymic nude mice | 20 mg/kg (I.P) | ↓ Cell migration, ↓ Invasio ↓ proliferation n | ↓ FAK, Src, crk-L, AKT | [79] |
| ↓ NEDD9 | ||||
| 50 mg/kg (I.P) | ↓ Angigenesis ↓ proliferation | ↓ CD-31, ↓ Ki-67 | [76] | |
| BALB/c-nude mice | 50 mg/kg (Per Os) | ↓ Cell migration, ↓ Invasion, ↓ Proliferation | ↑ TAGLN, ↓ MMP-9 | [83] |
| APCMin/+ mice | 50 mg/kg (I.P) | ↓ tumor volume, ↑ Apoptosis | ↑ p21, ↓ p53 | [82] |
| Curcumin | ||||
|---|---|---|---|---|
| Experimental Models | Concentration Range | Biological Response | Pathway/Genes/Proteins Involved | Refs. |
| Cells and Cell Lines | ||||
| HCT-116 | 10–25 µM | ↑ Apoptosis | ↓ AP-1, ↓ NF-κB, ↓ MMP-9 | [95] |
| 20 µM with 5-FU (5 µM) | ↑ Cell cycle arrest (S) ↑ Apoptosis ↓ Cell proliferation | ↓ caspase-3, ↓ caspase-8, ↓ caspase-9, Bax, ↓ PARP, ↑ Bcl-2 | [96] | |
| ↓ cyclin D1 | ||||
| 25 µM with Piperine (7 µM) | ↓ Cell proliferation ↑ Cell cycle arrest (G2/M ↑ Apoptosis | ↓ cyclin D1, ↑ caspase-3 | [97] | |
| HT29 | 41 µM | ↓ Oxydative stress ↓ Cell growth, ↓ Invasion, ↓ Metastasis | ↓ NF-E2, ↓ Nrf2 ↓ Bcl-2, ↓ Cyclin D1, ↓ IL6, ↓ Cox2 | [17] |
| HCT-8/5-Fu | 10 µM with 5-FU (10 mM) | ↑ Apoptosis, | ↑ Nrf2, ↑ Bcl-2, ↓ Bax | [98] |
| Animal models | ||||
| C57BL/6 | 300 mg/kg with DSS (5 mg/kg) I.P. | ↓ Disease activity index, ↓ neoplasic lesions | ↓ β-catenin, Cox2, iNOS | [99] |
| ↑ Apoptotosis | ↓ cyclinD1, ↓ cyclinD3, ↑ caspase-3, ↑ caspase-7, ↑ caspase-9, ↑ PARP | [92] | ||
| Oxaliplatin-resistant HCT116-xenograft | (1 g/kg) per os | ↑ Radiosensitivity | ↓ NF-κB, ↓ Ki-67, ↓ Notch-1 | [100] |
| Orthopically implanted CRC tumors (HC116) | (1 g/kg) per os | ↓ Cell growth, ↓ Metastasis | ↓ NF-κB | [91] |
| EGCG | ||||
|---|---|---|---|---|
| Experimental Models | Concentration Range | Biological Response | Pathway/Genes/Proteins Involved | Refs. |
| Cells and Cell Lines | ||||
| SW837 | 50–100 µM | ↓ Cell growth | ↑ IFN-γ, ↓ IDO, ↓ STAT1, ↓ JAK/STAT1, ↓ ISRE, ↓ GAS | [109] |
| 10 ng/mL | ↓ Cell proliferation, ↑ Apoptosis | ↑ CD133, CD44, ALDHA1, Oct-4, and Nanog, ↓ p-GSK3β, ↑ GSK3β, ↓ Wnt, ↓ β-catenin, ↓ Cyclin D1, ↓ PCNA, ↓ Bcl2, ↑ Bax, ↑ caspases 3, 8 and 9 | [110] | |
| 10–30 µM | ↓ Cell growth, ↑ Cell cycle arrest (G2/M), ↓ Proliferation, ↓ Cell invasion, ↓ Cell adhesion, ↑ Apoptosis | ↓ MMP2/9, ↑ caspases 3, 8 and 9, ↓ EGFR and IGF1R, ↓ MEK and ERK, ↓ PI3K and AKT, ↓ Bad | [111] | |
| 35 µg/mL | ↑ Cell cycle arrest (G0/G1) ↓ Cell proliferation, ↑ Apoptosis | [112] | ||
| LoVo | 35 µg/mL | ↓ Cell proliferation, ↑ Apoptosis, ↑ Cell cycle arrest (G0/G1) | [112] | |
| 10–30 µM | ↓ Cell growth, ↑ Cell cycle arrest (G2/M), ↓ Proliferation, ↓ Cell invasion, ↓ Cell adhesion, ↑ Apoptosis | ↓ MMP2 and 9, ↑ caspases 3, 8 and 9, ↓ EGFR and IGF1R, ↓ MEK and ERK, ↓ PI3K and AKT, ↓ Bad | [111] | |
| HT29 | 35 µg/mL | ↑ Cell cycle arrest (S) | [112] | |
| 88 μM 262 μM 190 μM/88 μM 262 μM/190 μM/88 μM 262 μM/190 μM/88 μM 88 μM | ↑ ER stress, ↑ Apoptosis | ↑ Bip ↑ p-eIF2α ↓ PERK, ATF4 ↑ IRE1α ↑ Caspases 3 and 7 ↑TfR | [113] | |
| 100 µM (with 20 µM csplatin or 20 µM oxaliplatin) | ↓ Cell viability, ↑ Autophagy | ↑ LC3II, ↓ IP3K | [113] | |
| HCT-8 | 35 µg/mL | ↑ Cell cycle arrest G2/M | [112] | |
| HCT116 | 12.5 µM | ↑ Radiosensitivity, ↑ Autophagy and Apoptosis | ↑ Nrf2, ↑ LC3, ↑ Caspase-9 | [114] |
| 50–100 µM | ↓ Cell proliferation | [115] | ||
| 50–100 µM | ↑ Apoptosis | ↓ VEGFR2, ↓ AKT, ↓ tumor growth, ↓ proliferation, ↓ migration and ↓ angiogenesis | [115] | |
| 10–30 µM | ↓ Cell growth, ↑ Cell cycle arrest (G2/M), ↓ Proliferation, ↓ Cell invasion, ↓ Cell adhesion, ↑ Apoptosis | ↓ MMP2 and 9, ↑ caspases 3, 8 and 9, ↓ EGFR and IGF1R, ↓ MEK and ERK, ↓ PI3K and AKT, ↓ Bad | [111] | |
| DLD-1, | 100 µM with (20 µM cisplatin or 20 µM oxaliplatin) | ↓ Cell viability, ↑ Autophagy | ↑ LC3II, ↓ IP3K | [116] |
| 10 ng/mL | ↓ Cell proliferation, ↑ Apoptosis | ↑ CD133, CD44, ALDHA1, Oct-4, and Nanog, ↓ p-GSK3β, ↑GSK3β, ↓ Wnt, ↓ β-catenin, ↓ Cyclin D1, ↓ PCNA, ↓ Bcl2, ↑Bax, ↑ caspases 3, 8 and 9 | [110] | |
| RKO | 50–100 µM | ↑ Apoptosis | ↑ p38 | [115] |
| Caco-2 | 10–30 µM | ↓ Cell growth, ↑ Cell cycle arrest G2/M, ↓ Proliferation, ↓ Cell invasion, ↓ Cell adhesion, ↑ Apoptosis | ↓ MMP2 and 9, ↑ caspases 3, 8 and 9, ↓ EGFR and IGF1R, ↓ MEK and ERK, ↓ PI3K and AKT, ↓ Bad | [111] |
| Animal Models | ||||
| Male ICR mice | 0.1% with (AOM 10 mg/kg body weight I.P followed by 2% (w/v) DSS) | ↓ Weight, ↓ Inflammation | ↓ COX2, ↓ mRNA (TNFα, IFN δ, IL6, IL12, IL18) | [105] |
| Eighty SPF Wistar rats | 200 mg/kg with (DMH 40 mg/kg, s.c) | ↓ Tumor volume, ↑ Apoptosis | ↓ p53, PI3K-Akt, ↓ I-kappaB kinase/NF-kappaB, ↓ MAPK | [117] |
| QUERCETIN | ||||
|---|---|---|---|---|
| Experimental Models | Concentration Range | Biological Response | Pathway/Genes/Proteins Involved | Refs. |
| Cells and cell lines | ||||
| HT-29 | 25, 50, 100 µM | Apoptosis | ↓ Bcl-2, ↑ cleaved caspase-3, ↑ cleaved PARP, ↓ p-Akt, ↓ ErbB2/ErbB3 proteins | [117] |
| 5–30 µg/mL (with resveratrol, 1:1 ratio) | ↓ Oncogenic µmicroRNA-27a | ↓ Sp1, ↓ Sp3, ↓ Sp4, ↓ survivin mRNA and proteins | [118] | |
| 50, 100, 200 µM | Apoptosis S-phase arrest | ↓ p-Akt, ↓ CSN6, ↓ Myc, ↓ Bcl-2, ↑ p53, ↑ Bax proteins | [119] | |
| 50 μM (with cisplatin: 10 mg/L) | ↑ Cisplatin-induced Apoptosis | ↓ Activation of NF-κB protein expression | [120] | |
| 30 µM | ↑ TRAIL-induced Apoptosis | Redistribution of death receptors DR4 and DR5 into lipid rafts ↑ cleaved caspase-3 and ↑ cleaved Bid proteins,↑ release Cyt-C | [121] | |
| 50, 100 μM | Apoptosis G1-phase arrest | ↑ AMPK, ↑ p53, ↑ p21 proteins | [122] | |
| 50 µM (with dox: 250 nM) | ↑ Doxorubicin-induced cytotoxicity | ↓ Proliferation, ↑ apoptosis, and G2/M arrest for lower IC50 of Dox | [123] | |
| Caco-2 | 5–50 µM | ↓ Cell proliferation | ↓ CDC6, ↓ CDK4, ↓ cyclin D1 mRNA | [124] |
| 5–20 μM | Anti-migration Anti-invasion | ↓ MMP-2, ↓ MMP-9, ↓ TLR4, ↓ NF-κB, ↑ E-cadherin proteins ↓ TNF-α, ↓ COX-2, ↓ IL-6 production | [125] | |
| Caco-2 and SW-620 | 25–100 µM | Apoptosis | ↑ IκB-α, ↓ p-IκB-α, ↓ Bcl-2, ↑ Bax proteins | [126] |
| SW480 | 20–80 µM | Apoptosis | ↓ Cyclin D1, ↓ survivin mRNA, and proteins | [127] |
| 10 µM | Apoptosis S-phase arrest | ↓ EGF receptor phosphorylation | [128] | |
| Colo-320 and Colo-741 | 25 μg/mL | Apoptosis Senescence | ↑ p16, ↑ Lamin B1, ↑ cyclin B1, ↑ Bax, ↓ Bcl-2 proteins | [129] |
| CT26 and MC38 | 1–10 µM | Anti-metastasis | ↑ E-cadherin, ↓ N-cadherin, ↓ β-catenin, ↓ snail proteins ↓ MMP-2, ↓ MMP-9 activities | [130] |
| DLD-1 | 10.5 µM | Anticarcinogenesis | ↓ COX-2 transcription | [131] |
| CO115 and HCT15 | 12 µM (with 5-FU: 1 µM) | ↑ Fluorouracil-induced apoptosis | ↑ p53, ↑ cleaved caspase-9, ↑ cleaved caspase-3, ↑ cleaved PARP, ↓ Bcl-2 proteins | [132] |
| HCT8-β8 | 50 µM | ↓ Cell proliferation | ↑ ERβ mRNA, ↑ ER-responsive luciferase activity | [133] |
| Animal models | ||||
| HT-29 xenograft in Balb/C nude mice | 10 mg/kg/day (SC; 4 weeks) | ↑ Radiosensitivity | ↓ Jagged-1, ↓ Notch-1, ↓ Hes-1, ↓ Presenilin 1, ↓ Nicastrin proteins | [134] |
| RUTIN | ||||
|---|---|---|---|---|
| Experimental Models | Concentration Range | Biological Response | Pathway/Genes/Proteins Involved | Refs. |
| Cells and cell lines | ||||
| HT-29 | 100–200 µM | Apoptosis | ↑ Bax, ↓ Bcl-2, ↑ cleaved caspases-3, 8, 9, ↑ cleaved PARP proteins | [139] |
| 39 mM (with Silibinin: 76 mM) | Apoptosis | ↑ p53, ↓ Bcl-2, ↑ Bax, ↑ caspase 3, 8, 9 ↓ NFkB, ↓ IKK-α, ↓ IKK-β, ↑ p38MAPK, ↑ MK-2 proteins | [140] | |
| HT-29 and Caco-2 | 25–200 µM | ↓ Cell adhesion and Migration | ↓ ROS level, impairing attachment to fibronectin, disrupting cell–ECM interactions | [141] |
| 136 µM | ↓ Cell proliferation | ↓ Growth potency | [142] | |
| Animal models | ||||
| SW480 xenograft in nude mice | 1–20 mg/kg/day (I.P; 32 days) | Anti-tumor Anti-angiogenesis | ↑ Mean survival time, ↓ tumor volume, and weight, ↓ VEGF levels in serum | [143] |
| MTX-treated Wistar rats (Intestinal inflammation) | 50, 100 mg/kg/day (I.P; 1 week) | ↓ Oxidative stress ↓ Inflammation | ↓ COX-1, ↓ COX-2, and ↓ 15 LOX enzymatic activities, restoration of MDA, protein carbonyl, SOD, GSH levels, and catalase activity, ↓ free acidity and total acidity | [144] |
| 5-FU-treated Swiss mice (Intestinal Mucositis) | 50–200 mg/kg/day (Per os; 3 days) | ↓ Oxidative stress ↓ Inflammation | ↓ MDA, ↑ GSH concentrations, ↓ MPO activity, ↓ intestinal mastocytosis, ↓ COX-2 proteins | [145] |
| DSS-treated ICR mice (Colitis) | 0.6–6 mg/day (Per os; 2 weeks) | ↓ Inflammation | ↓ IL-1β, ↓ IL-6, ↓ GM-CSF, ↓ iNOS mRNA | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Lizard, G.; Latruffe, N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules 2021, 26, 3483. https://doi.org/10.3390/molecules26123483
Yammine A, Namsi A, Vervandier-Fasseur D, Mackrill JJ, Lizard G, Latruffe N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules. 2021; 26(12):3483. https://doi.org/10.3390/molecules26123483
Chicago/Turabian StyleYammine, Aline, Amira Namsi, Dominique Vervandier-Fasseur, John J. Mackrill, Gérard Lizard, and Norbert Latruffe. 2021. "Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer" Molecules 26, no. 12: 3483. https://doi.org/10.3390/molecules26123483
APA StyleYammine, A., Namsi, A., Vervandier-Fasseur, D., Mackrill, J. J., Lizard, G., & Latruffe, N. (2021). Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules, 26(12), 3483. https://doi.org/10.3390/molecules26123483