In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi
Abstract
1. Introduction
2. Results and Discussion
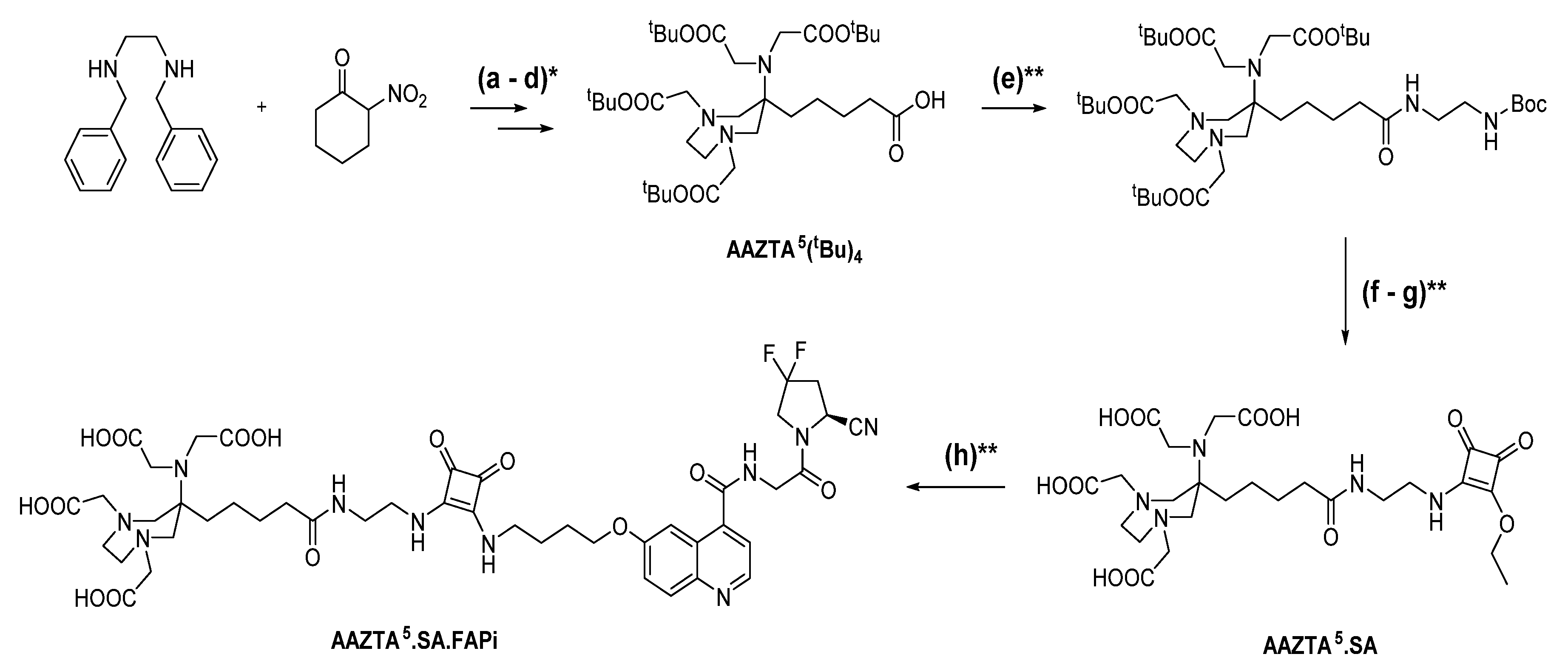
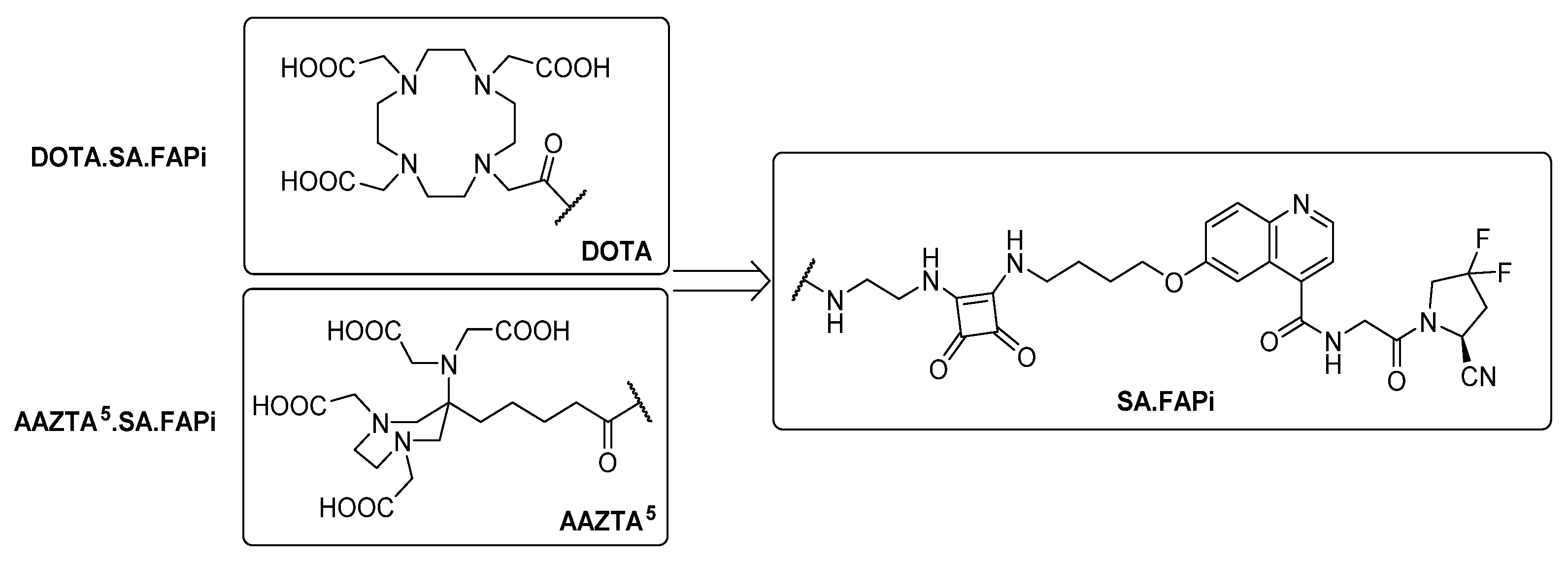
2.1. Synthesis of Chelator Conjugates
2.2. In Vitro Inhibition Measurements
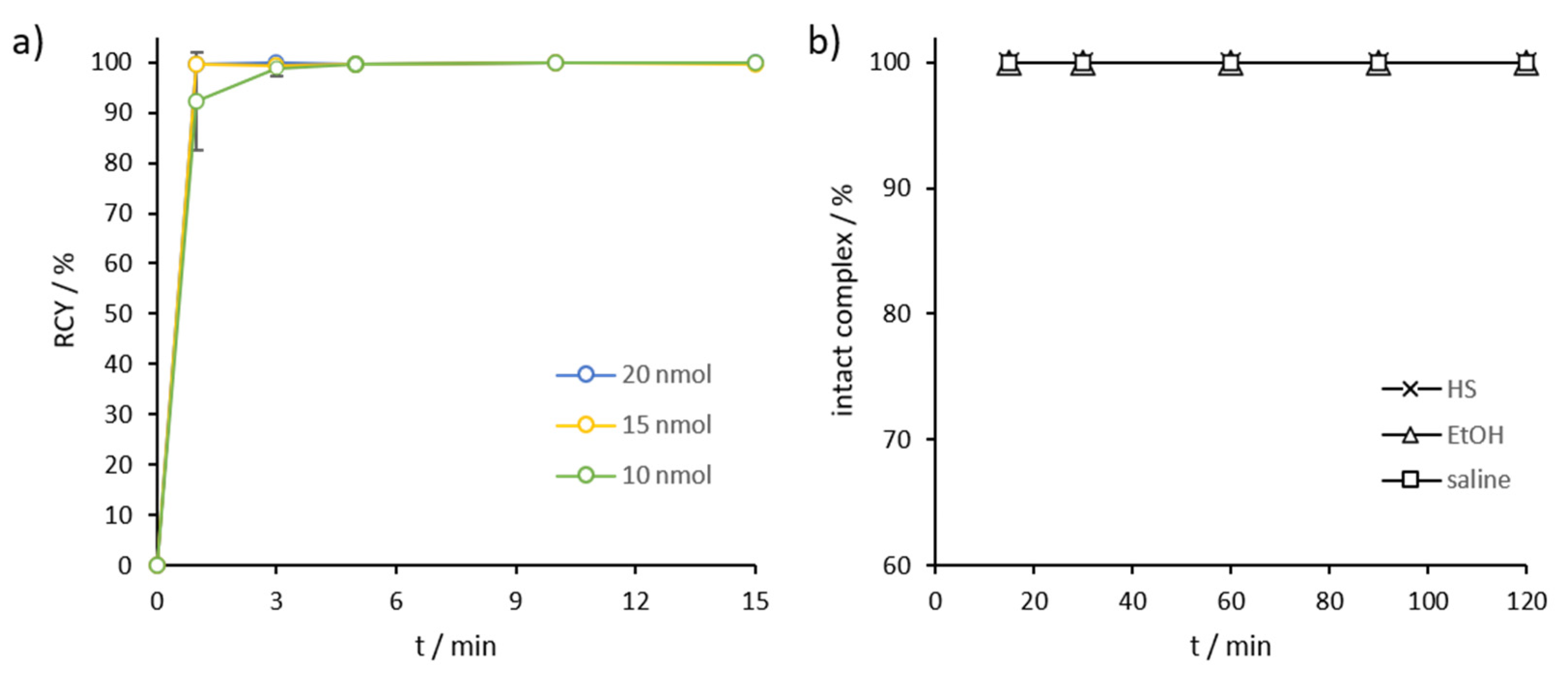
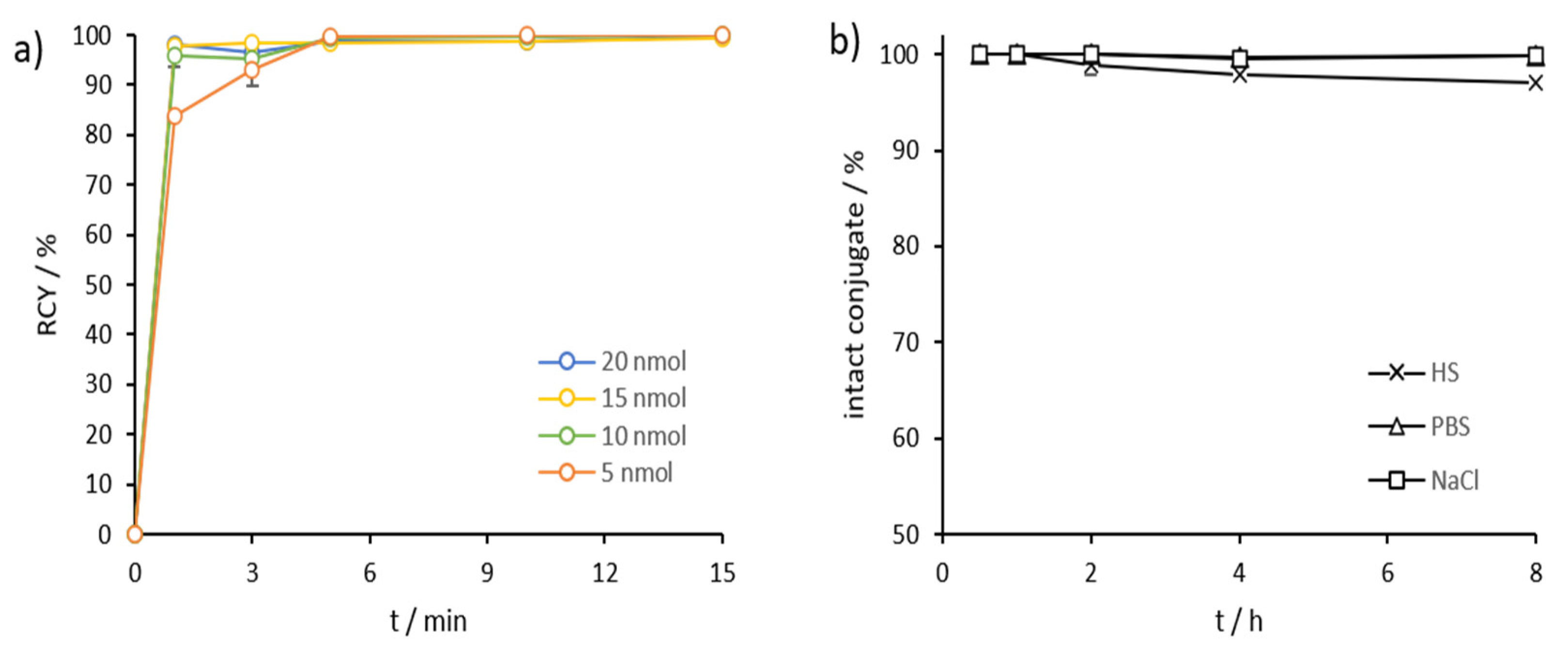
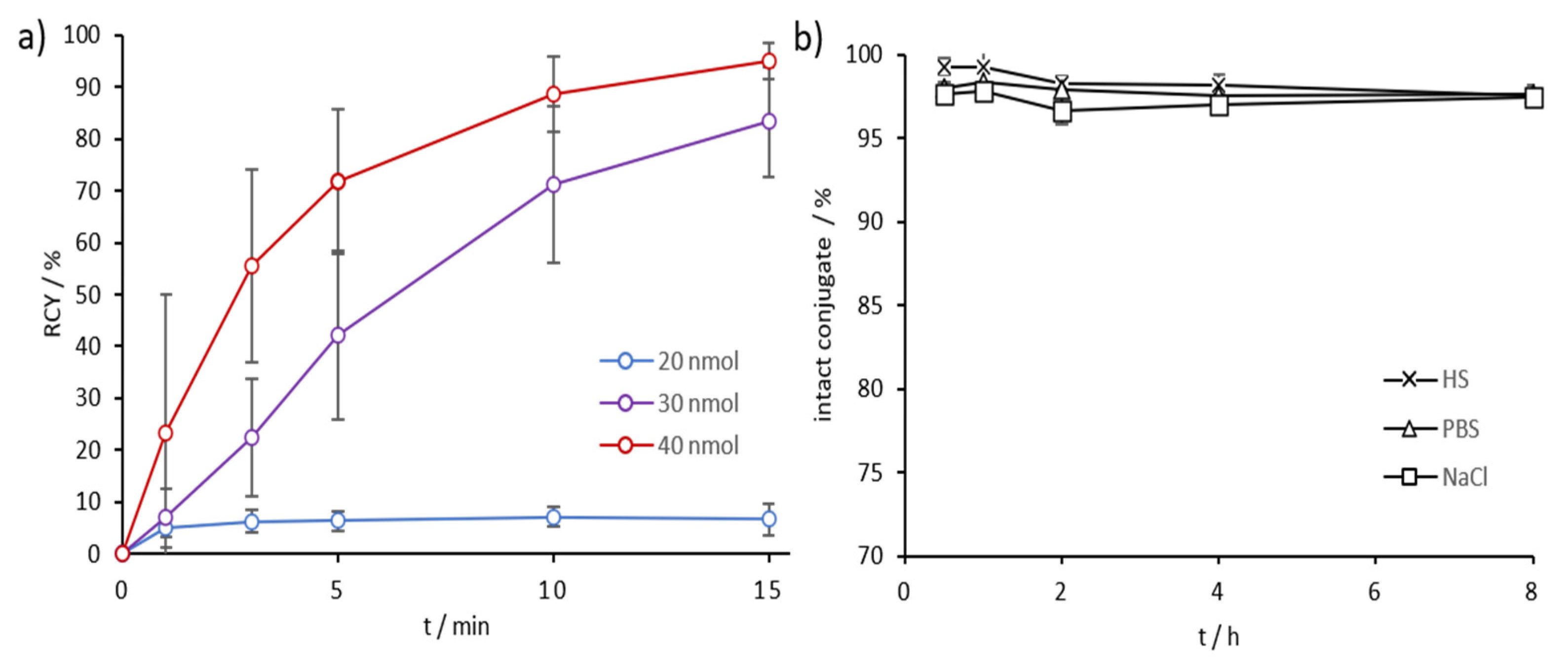
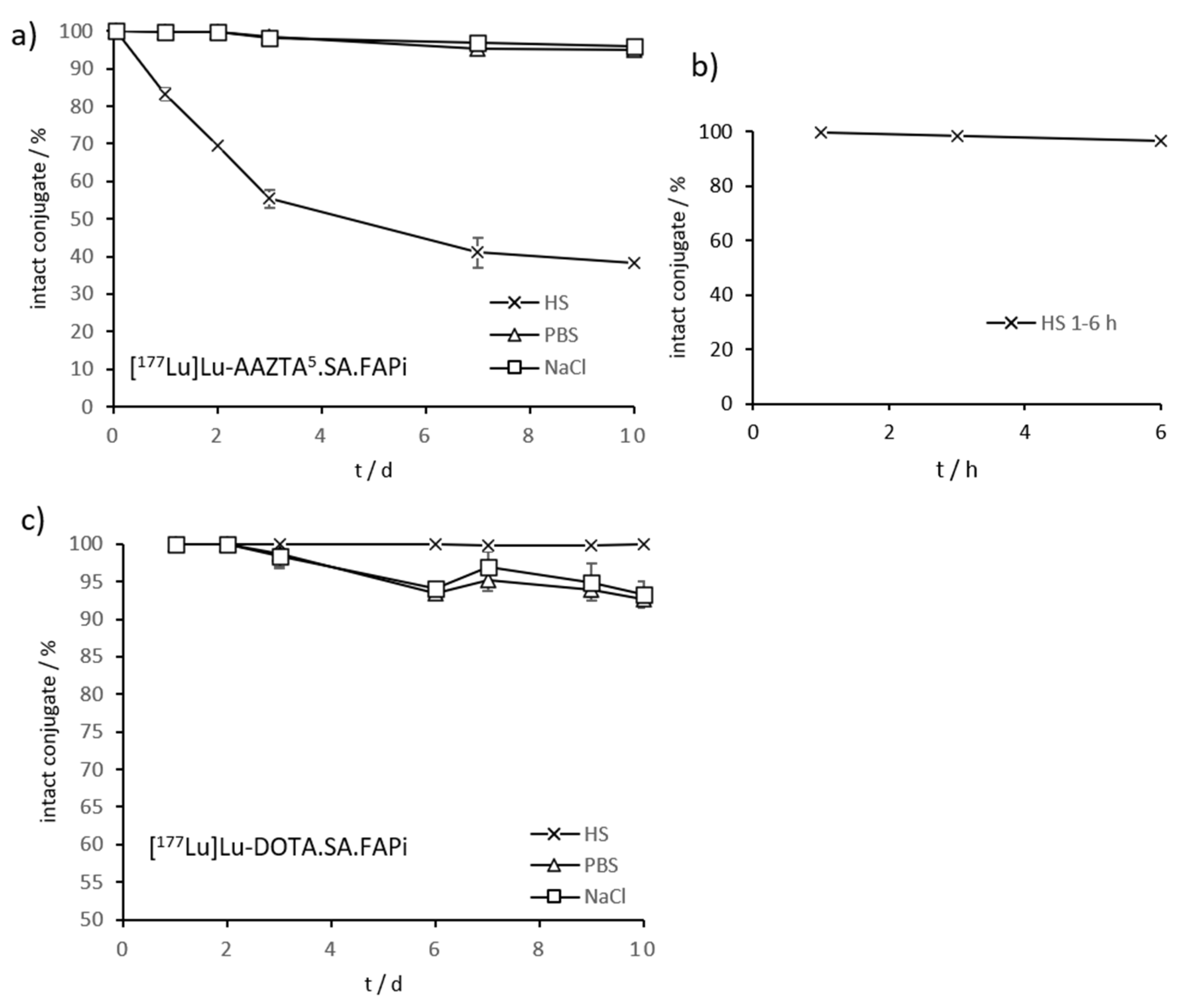
2.3. Radiolabeling and In Vitro Stability in Complexwith Gallium-68, Scandium-44, and Lutetium-177
2.4. Lipophilicity Measurements
3. Materials and Methods
3.1. General
3.2. Organic Synthesis
3.3. Non-Radioactive Compounds and In Vitro Inhibition Studies
3.4. Inhibition Assays
3.5. Radiolabeling and Stability Measurements
3.6. Lipophilicity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Simms, A.E.; Mazur, A.; Wang, S.; León, N.R.; Jones, B.; Aziz, N.; Kelly, T. Fibroblast activation protein-α promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin. Exp. Metastasis 2011, 28, 567–579. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Liu, L.; Yu, J.; Ren, X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol. Ther. 2012, 13, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- De Vlieghere, E.; Verset, L.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-associated fibroblasts as target and tool in cancer therapeutics and diagnostics. Virchows Arch. 2015, 467, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment (review). Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Busek, P.; Mateu, R.; Zubal, M.; Kotackova, L.; Sedo, A. Targeting Fibroblast activation protein in cancer—Prospects and caveats. Front. Biosci. Landmark 2018, 23, 1933–1968. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jaeger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.M.; De Meester, I.; Augustyns, K.; et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 2014, 57, 3053–3074. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Zhang, H.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: A pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Zhang, W.; Li, F. Intense FAPI Uptake in Inflammation May Mask the Tumor Activity of Pancreatic Cancer in 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2020, 45, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Rosar, F.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U.; Ezziddin, S. Positive FAPI-PET/CT in a metastatic castration-resistant prostate cancer patient with PSMA-negative/FDG-positive disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Koerber, S.A.; Staudinger, F.; Kratochwil, C.; Adeberg, S.; Haefner, M.F.; Ungerechts, G.; Rathke, H.; Winter, E.; Lindner, T.; Syed, M.; et al. The role of FAPI-PET/CT for patients with malignancies of the lower gastrointestinal tract—First clinical experience. J. Nucl. Med. 2020, 61, 1331–1336. [Google Scholar] [CrossRef]
- Toms, J.; Kogler, J.; Maschauer, S.; Daniel, C.; Schmidkonz, C.; Kuwert, T.; Prante, O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18F-labeled FAP Inhibitor. J. Nucl. Med. 2020, 61, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Altmann, A.; Kraemer, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and development of 99mTc labeled FAPI-tracers for SPECT-imaging and 188Re therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Gwendolyn, V.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, B.; Gärtner, F.; Marinova, M.; Attenberger, U.; Meisenheimer, M.; Toma, M.; Kristiansen, G.; Feldmann, G.; Moon, E.; Roesch, F.; et al. [68Ga]Ga-DATA5m.SA.FAPi PET/CT: Specific Tracer-uptake in Focal Nodular Hyperplasia and potential Role in Liver Tumor Imaging. Nuklearmedizin 2020, 59, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F. Scandium-44: Benefits of a Long-Lived PET Radionuclide Available from the 44Ti/44Sc Generator System. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Filosofov, D.V.; Loktionova, N.S.; Rösch, F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. Radiochim. Acta 2010, 98, 149–156. [Google Scholar] [CrossRef]
- Pruszyński, M.; Loktionova, N.S.; Filosofov, D.V.; Rösch, F. Post-elution processing of 44Ti/44Sc generator-derived 44Sc for clinical application. Appl. Radiat. Isot. 2010, 68, 1636–1641. [Google Scholar] [CrossRef]
- Hernandez, R.; Valdovinos, H.; Yang, Y.; Chakravarty, R.; Hong, H.; Barnhart, T.; Cai, W. Sc: An Attractive Isotope for Peptide-Based PET Imaging. Mol. Pharm. 2014, 11, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, E.; Loktionova, N.S.; Fellner, M.; Roesch, F.; Thews, O.; Pawlak, D.; Archimandritis, S.C.; Mikolajczak, R. 44Sc-DOTA-BN[2–14]NH2 in comparison to 68Ga-DOTA-BN[2–14]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl. Radiat. Isot. 2012, 70, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI Radiopharm. Chem. 2017, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thorp-Greenwood, F.L.; Coogan, M.P. Multimodal radio- (PET/SPECT) and fluorescence imaging agents based on metallo-radioisotopes: Current applications and prospects for development of new agents. J. Chem. Soc. Dalton Trans. 2011, 40, 6129–6143. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical translation and first in-human use of [44Sc]Sc-PSMA-617 for pet imaging of metastasized castrate-resistant prostate cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef]
- Fröss-Baron, K.; Garske-Román, U.; Welin, S.; Granberg, D.; Eriksson, B.; Khan, T.; Sandström, M.; Sundin, A. 177Lu-DOTATATE therapy of advanced pancreatic neuroendocrine tumors heavily pretreated with chemotherapy; analysis of outcome, safety and their determinants. Neuroendocrinology 2020, 111, 330–334. [Google Scholar] [CrossRef]
- Forrer, F.; Uusijärvi, H.; Storch, D.; Maecke, H.R.; Mueller-Brand, J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J. Nucl. Med. 2005, 46, 1310–1316. [Google Scholar] [PubMed]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; van Echteld, C.J.A. [177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: A Phase-II study. Theranostics 2016, 6, 501–510. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: Safety and efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2020, 23, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Retz, M.; Tauber, R.; Knorr, K.; Kratochwil, C.; Eiber, M. Radionuklidtherapie des Prostatakarzinoms mittels PSMA-Lutetium. Urologe 2017, 56, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef]
- Sinnes, J.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Greifenstein, L.; Grus, T.; Nagel, J.; Sinnes, J.P.; Rösch, F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA5 for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl. Radiat. Isot. 2020, 156, 108867. [Google Scholar] [CrossRef]
- De Decker, A.; Vliegen, G.; Van Rompaey, D.; Peeraer, A.; Bracke, A.; Verckist, L.; Jansen, K.; Geiss-Friedlander, R.; Augustyns, K.; De Winter, H.; et al. Novel Small Molecule-Derived, Highly Selective Substrates for Fibroblast Activation Protein (FAP). ACS Med. Chem. Lett. 2019, 10, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- De Meester, I.; Vanhoof, G.; Lambeir, A.M.; Scharpé, S. Use of immobilized adenosine deaminase (EC 3.5.4.4) for the rapid purification of native human CD26/dipeptidyl peptidase IV (EC 3.4.14.5). J. Immunol. Methods 1996, 189, 99–105. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-based post-processing of generator-derived 68Ga Toward kit-type preparation of 68Ga-radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef]

| Compound | DPPs IC50 (µM) | PREP IC50 (µM) | FAP IC50 (nM) | Selectivitiy Index (FAP/PREP) |
|---|---|---|---|---|
| AAZTA5.SA.FAPi | >1 | 2.4 ± 0.4 | 0.56 ± 0.02 | 4286 |
| [natSc]Sc-AAZTA5.SA.FAPi | >1 | 3.6 ± 0.8 | 0.57 ± 0.04 | 6316 |
| [natLu]Lu-AAZTA5.SA.FAPi | >1 | 3.2 ± 0.6 | 0.55 ± 0.04 | 5818 |
| DOTA.SA.FAPi | n.d. | 5.4 ± 0.3 a | 0.9 ± 0.1 a | 6000 |
| [natGa]Ga-DOTA.SA.FAPi | >1 | 8.7 ± 0.9 a | 1.4 ± 0.2 a | 6214 |
| [natLu]Lu DOTA.SA.FAPi | >1 | 2.5 ± 0.4 a | 0.8 ± 0.2 a | 3125 |
| DATA5m.SA.FAPi | n.d. | 1.7 ± 0.1 a | 0.8 ± 0.2 a | 2113 |
| [natGa]Ga-DATA5m.SA.FAPi | >1 | 4.7 ± 0.3 a | 0.7 ± 0.1 a | 6714 |
| UAMC1110-FAP inhibitor | >10 | 1.8 ± 0.01 b | 0.43 ± 0.07 a | 4186 |
| Compound | LogD7.4 |
|---|---|
| [68Ga]Ga-AAZTA5.SA.FAPi | −2.53 ± 0.13 |
| [44Sc]Sc-AAZTA5.SA.FAPi | −2.50 ± 0.11 |
| [68Ga]Ga-DOTA.SA.FAPi | −2.68 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, E.S.; Van Rymenant, Y.; Battan, S.; De Loose, J.; Bracke, A.; Van der Veken, P.; De Meester, I.; Rösch, F. In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules 2021, 26, 3482. https://doi.org/10.3390/molecules26123482
Moon ES, Van Rymenant Y, Battan S, De Loose J, Bracke A, Van der Veken P, De Meester I, Rösch F. In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules. 2021; 26(12):3482. https://doi.org/10.3390/molecules26123482
Chicago/Turabian StyleMoon, Euy Sung, Yentl Van Rymenant, Sandeep Battan, Joni De Loose, An Bracke, Pieter Van der Veken, Ingrid De Meester, and Frank Rösch. 2021. "In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi" Molecules 26, no. 12: 3482. https://doi.org/10.3390/molecules26123482
APA StyleMoon, E. S., Van Rymenant, Y., Battan, S., De Loose, J., Bracke, A., Van der Veken, P., De Meester, I., & Rösch, F. (2021). In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules, 26(12), 3482. https://doi.org/10.3390/molecules26123482






