Tetracyclic Thioxanthene Derivatives: Studies on Fluorescence and Antitumor Activity
Abstract
1. Introduction
2. Results and Discussion
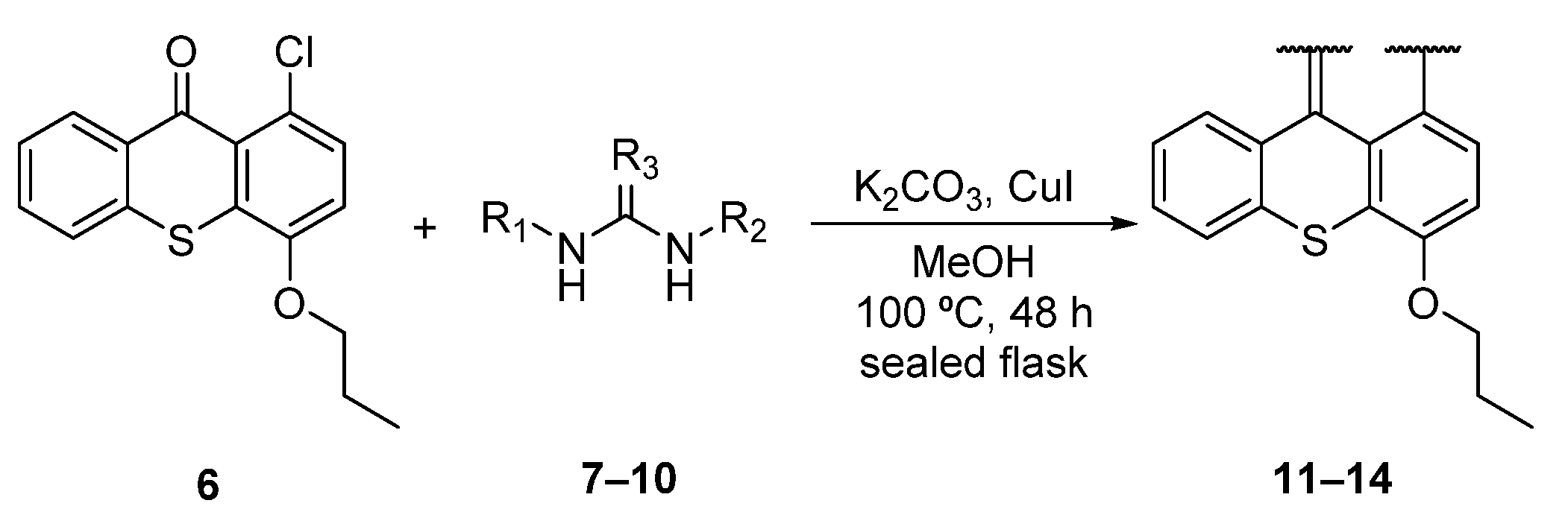
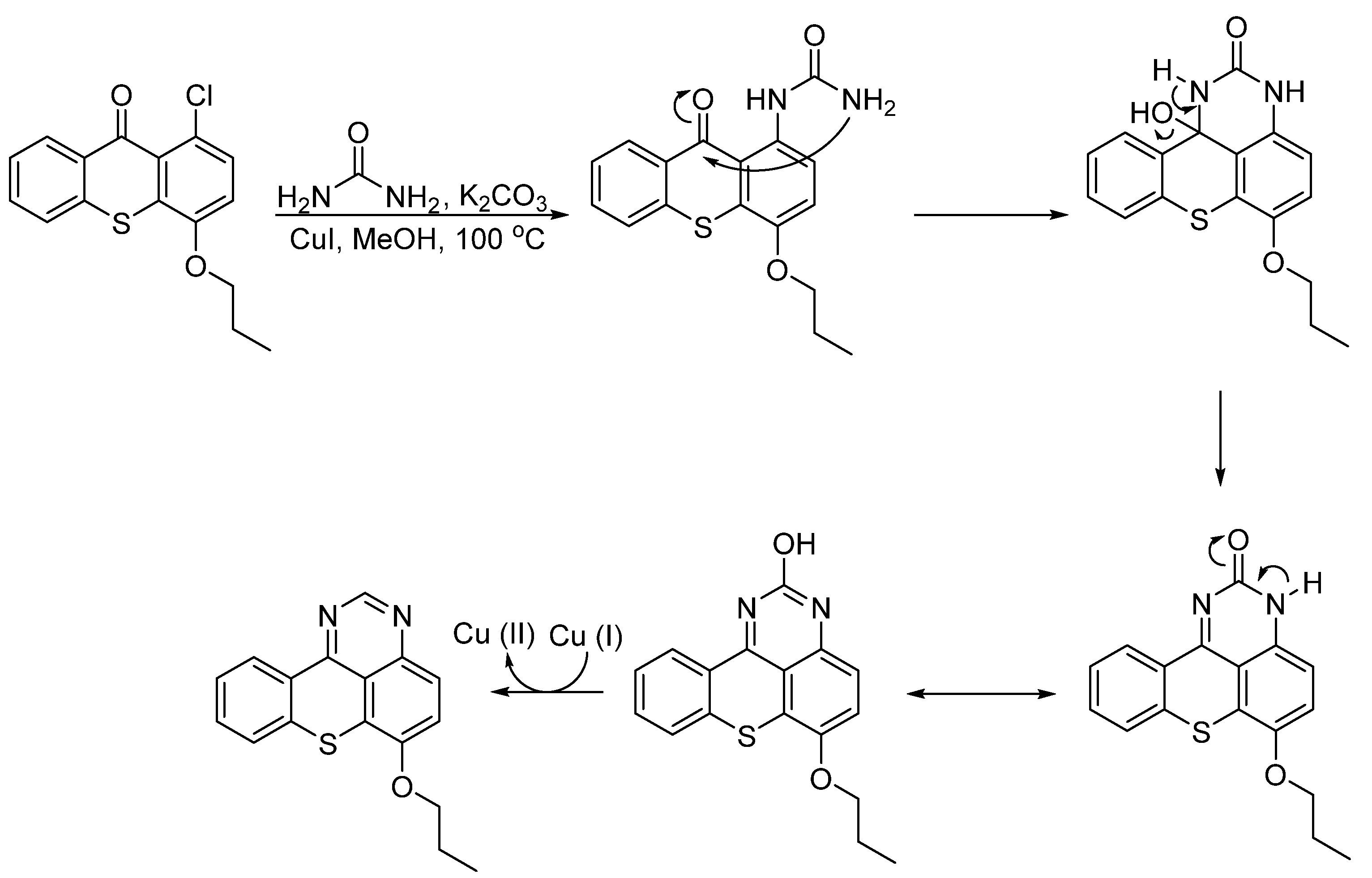
2.1. Chemistry
Synthesis of Aminated Tetracyclic Thioxanthenes
2.2. Biological Activity
Antitumor Activity
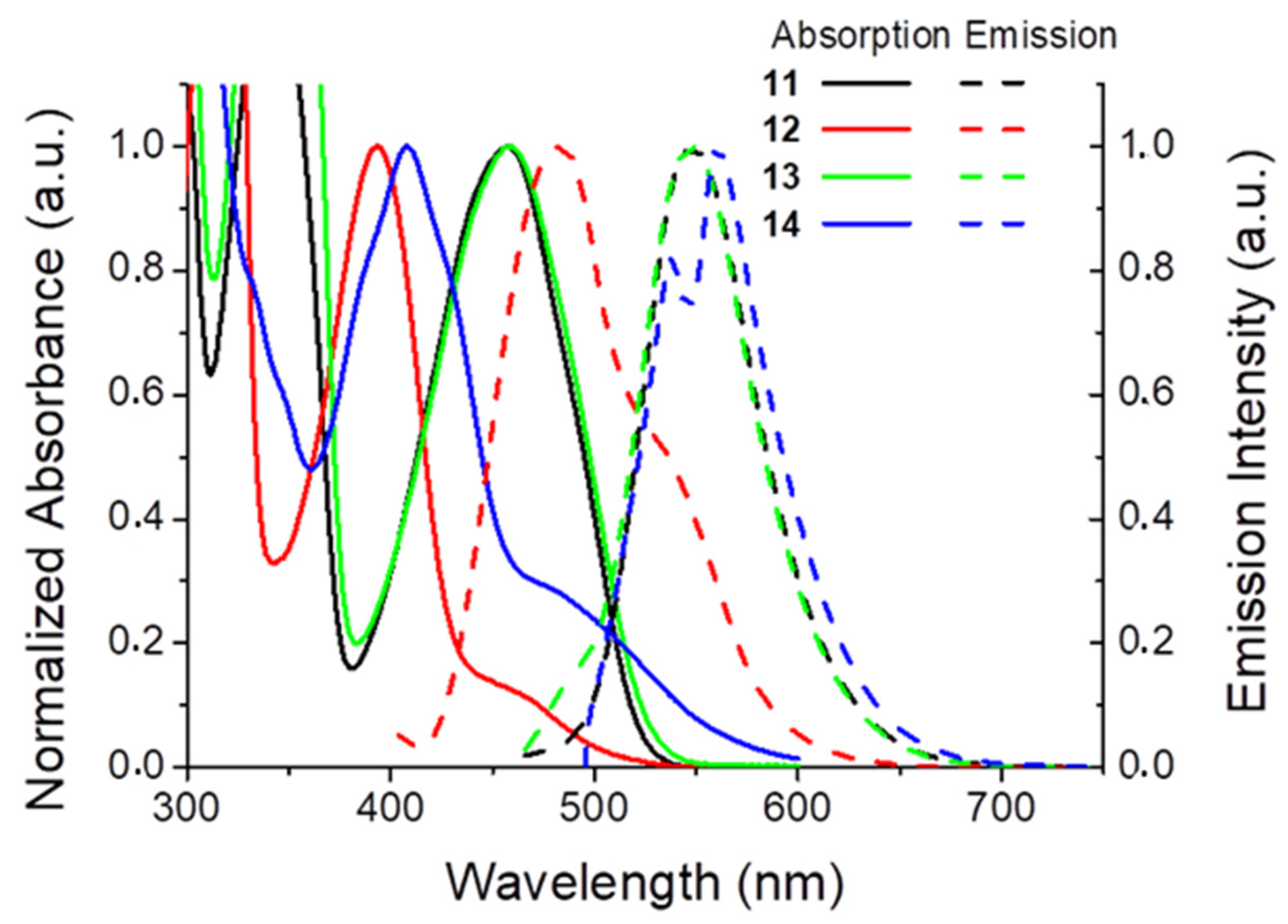
2.3. Fluorescence
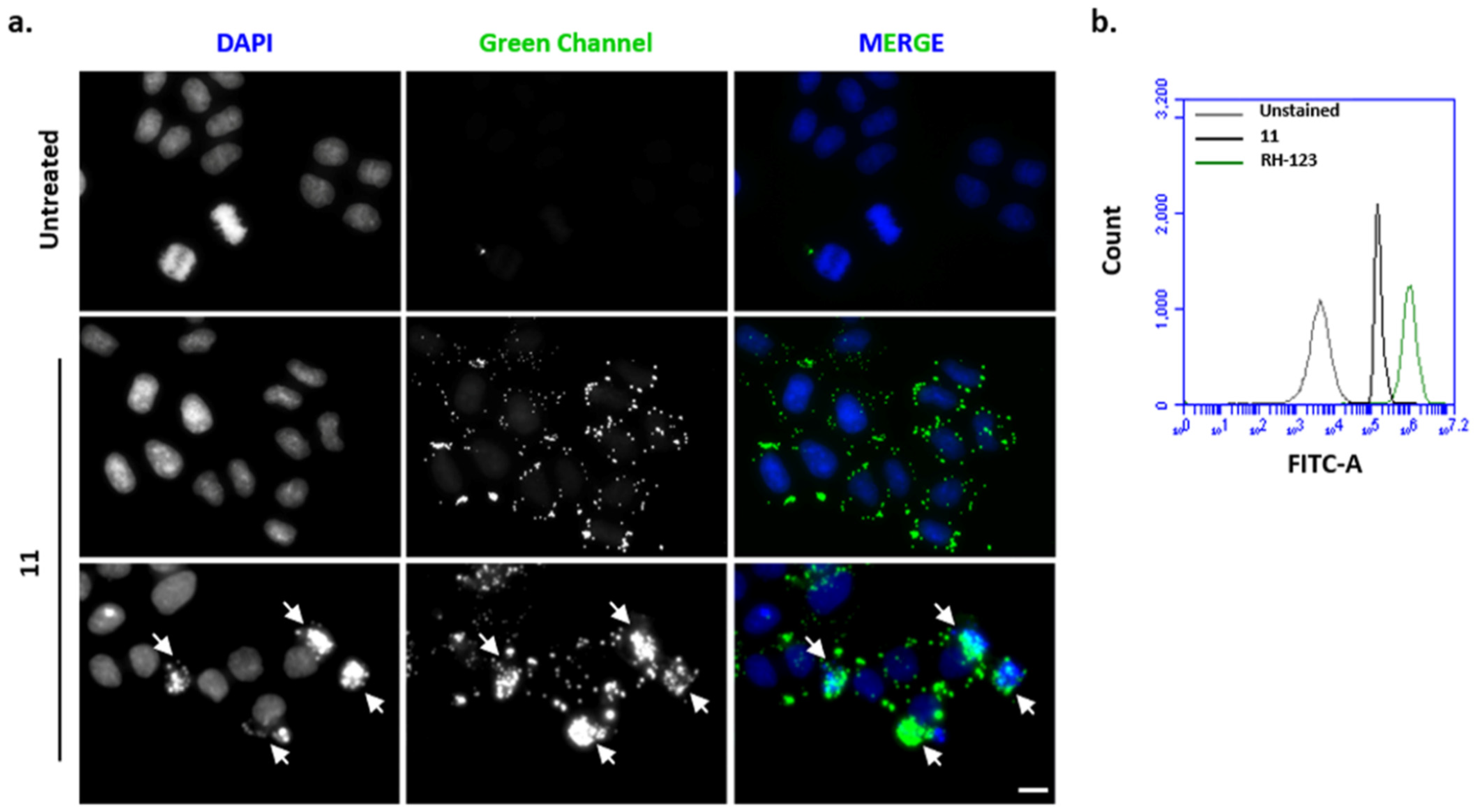
2.4. Fluorescence Microscopy and Flow Cytometry Analysis
2.5. X-ray Crystallography
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Aminated Tetracyclic Thioxanthenes
Synthesis of 6-Propoxythiochromeno[4,3,2-de]quinazolin-2-amine (11)
Synthesis of 6-Propoxythiochromeno[4,3,2-de]quinazoline (12)
Synthesis of N-(2-Chlorobenzyl)-6-propoxythiochromeno[4,3,2-de]quinazolin-2-amine (13)
Synthesis of (E)-6-Propoxy-N,3-di-o-tolylthiochromeno[4,3,2-de]quinazolin-2(3H)-imine (14)
3.2. Biological Activity
Antitumor Activity
3.3. Photophysical Studies
3.4. Fluorescence Microscopy and Flow Cytometry Analysis
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paiva, A.M.; Pinto, M.M.; Sousa, E. A century of thioxanthones: Through synthesis and biological applications. Curr. Med. Chem. 2013, 20, 2438–2457. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Bessa, L.J.; Palmeira, A.; Gomes, A.S.; Vasconcelos, V.; Sousa, E.; Pinto, M.; Martins da Costa, P. Synergistic Effects Between Thioxanthones and Oxacillin Against Methicillin-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 404–415. [Google Scholar] [CrossRef]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid reducing activity and toxicity profiles of a library of polyphenol derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef]
- Palmeira, A.; Vasconcelos, M.H.; Paiva, A.; Fernandes, M.X.; Pinto, M.; Sousa, E. Dual inhibitors of P-glycoprotein and tumor cell growth: (re)discovering thioxanthones. BioChem. Pharmacol. 2012, 83, 57–68. [Google Scholar] [CrossRef]
- Mokhtari Brikci-Nigassa, N.; Nauton, L.; Moreau, P.; Mongin, O.; Duval, R.E.; Picot, L.; Thiéry, V.; Souab, M.; Baratte, B.; Ruchaud, S.; et al. Functionalization of 9-thioxanthone at the 1-position: From arylamino derivatives to [1]benzo(thio)pyrano[4,3,2-de]benzothieno[2,3-b]quinolines of biological interest. Bioorg. Chem. 2020, 94, 103347. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, T.-C.; Lee, C.-C.; Shih, L.-C.; Lin, C.-Y.; Hsieh, Y.-Y.; Ali, A.A.A.; Huang, H.-S. Synthesis and evaluation of new 3-substituted-4-chloro-thioxanthone derivatives as potent anti-breast cancer agents. Arab J. Chem. 2019, 12, 3503–3516. [Google Scholar] [CrossRef]
- Lima, R.T.; Sousa, D.; Gomes, A.S.; Mendes, N.; Matthiesen, R.; Pedro, M.; Marques, F.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. The Antitumor Activity of a Lead Thioxanthone is Associated with Alterations in Cholesterol Localization. Molecules 2018, 23, 3301. [Google Scholar] [CrossRef] [PubMed]
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312. [Google Scholar] [CrossRef]
- Metin, E.; Arsu, N.; Catak, S.; Aviyente, V. Photophysical, kinetic and thermodynamic study of one-component Type II thioxanthone acetic acid photoinitiators. Eur. Polym. J. 2020, 136, 109909. [Google Scholar] [CrossRef]
- Batibay, G.S.; Gunkara, O.T.; Ocal, N.; Arsu, N. Thioxanthone attached polyhedral oligomeric silsesquioxane (POSS) nano-photoinitiator for preparation of PMMA hybrid networks in air atmosphere. Prog. Org. Coat. 2020, 149, 105939. [Google Scholar] [CrossRef]
- Hassan, S.I.; Haque, A.; Jeilani, Y.A.; Ilmi, R.; Faizi, M.S.H.; Khan, I.; Mushtaque, M. Thioxanthone-based organic probe with aggregation enhanced emission and exceptional mineral acids sensing abilities. J. Mol. Struct. 2021, 1224, 129004. [Google Scholar] [CrossRef]
- Archer, S.; Suter, C.M. The Preparation of Some 1-Alkylamino- and Dialkylaminoalkylaminothiaxanthones1. J. Am. Chem. Soc. 1952, 74, 4296–4309. [Google Scholar] [CrossRef]
- Rosi, D.; Peruzzotti, G.; Dennis, E.W.; Berberian, D.A.; Freele, H.; Tullar, B.F.; Archer, S. Hycanthone, a New Active Metabolite of Lucanthone. J. Med. Chem. 1967, 10, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Cioli, D.; Pica-Mattoccia, L.; Archer, S. Antischistosomal drugs: Past, present ... and future? Pharmacol. Ther. 1995, 68, 35–85. [Google Scholar] [CrossRef]
- Barbosa, J.; Lima, R.T.; Sousa, D.; Gomes, A.S.; Palmeira, A.; Seca, H.; Choosang, K.; Pakkong, P.; Bousbaa, H.; Pinto, M.M.; et al. Screening a Small Library of Xanthones for Antitumor Activity and Identification of a Hit Compound which Induces Apoptosis. Molecules 2016, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.T.; Sousa, D.; Paiva, A.M.; Palmeira, A.; Barbosa, J.; Pedro, M.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. Modulation of Autophagy by a Thioxanthone Decreases the Viability of Melanoma Cells. Molecules 2016, 21, 1343. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.-F.; Chen, Z.; Kakkad, S.; Pomper, M.G.; Bhujwalla, Z.M. Theranostic imaging of cancer. Eur. J. Radiol. 2012, 81 (Suppl. S1), S124–S126. [Google Scholar] [CrossRef]
- Hedidi, M.; Maillard, J.; Erb, W.; Lassagne, F.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; Roisnel, T.; Dorcet, V.; Hamzé, M.; et al. Fused Systems Based on 2-Aminopyrimidines: Synthesis Combining Deprotolithiation-in situ Zincation with N-Arylation Reactions and Biological Properties. Eur. J. Org. Chem. 2017, 2017, 5903–5915. [Google Scholar] [CrossRef]
- Ataci, N.; Arsu, N. Studies of the binding mode of TXNHCH2COOH with calf thymus DNA by spectroscopic methods. Spectrochim. Acta A Mol. BioMol. Spectrosc. 2016, 169, 128–133. [Google Scholar] [CrossRef]
- Ataci, N.; Kazancioglu, E.O.; Kalındemirtas, F.D.; Kuruca, S.E.; Arsu, N. The interaction of light-activaTable 2-thioxanthone thioacetic acid with ct-DNA and its cytotoxic activity: Novel theranostic agent. Spectrochim. Acta A Mol. BioMol. Spectrosc. 2020, 239, 118491. [Google Scholar] [CrossRef] [PubMed]
- Ataci, N.; Ozcelik, E.; Arsu, N. Spectrophotometric study on binding of 2-thioxanthone acetic acid with ct-DNA. Spectrochim. Acta A Mol. BioMol. Spectrosc. 2018, 204, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Esen, D.S.; Temel, G.; Balta, D.K.; Allonas, X.; Arsu, N. One-Component Thioxanthone Acetic Acid Derivative Photoinitiator for Free Radical Polymerization. PhotoChem. Photobiol. 2014, 90, 463–469. [Google Scholar] [CrossRef]
- Wang, D.Z.; Yan, L.; Ma, L. Facile Preparation of 4-Substituted Quinazoline Derivatives. J. Vis. Exp. 2016, 53662. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Ma, D. Synthesis of 3-Substituted and 2,3-Disubstituted Quinazolinones via Cu-Catalyzed Aryl Amidation. Org. Lett. 2012, 14, 1150–1153. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Rurack, K. Determination of the Photoluminescence Quantum Yield of Dilute Dye Solutions (IUPAC Technical Report). Chem. Int. Newsmag. IUPAC 2014, 36. [Google Scholar] [CrossRef][Green Version]
- Gales, L.; Damas, A.M. Xanthones-A Structural Perspective. Curr. Med. Chem. 2005, 12, 2499–2515. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Gales, L.; Damas, A.M.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Studies on the Structural and Thermodynamic Properties of Two Sulfur Heterocyclic Keto Compounds. J. Chem. Eng. Data 2010, 55, 5009–5017. [Google Scholar] [CrossRef]
- Badisa, R.B.; Ayuk-Takem, L.T.; Ikediobi, C.O.; Walker, E.H. Selective Anticancer Activity of Pure Licamichauxiioic-B Acid in Cultured Cell Lines. Pharm. Biol. 2006, 44, 141–145. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]


| Amine | Thioxanthene | Yield |
|---|---|---|
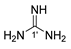 7 |  11 | 25% |
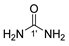 8 | 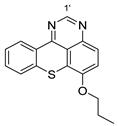 12 | 11% |
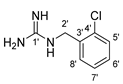 9 |  13 | 45% |
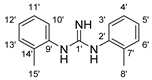 10 | 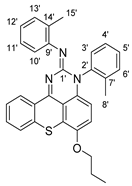 14 | 13% |
| Compound | Antitumor Activity (µM) | ||
|---|---|---|---|
| A375-C5 | MCF-7 | NCI-H460 | |
| 11 | 6.48 ± 0.88 | 6.39 ± 0.49 | 5.66 ± 0.89 |
| 12 | 38.77 ± 5.93 | 33.82 ± 1.61 | 31.19 ± 1.53 |
| 13 | 22.88 ± 3.75 | 18.85 ± 1.42 | 16.97 ± 3.12 |
| 14 | 8.02 ± 3.59 | 9.04 ± 0.89 | 10.64 ± 0.31 |
| Doxorubicin | 0.016 ± 0.005 | 0.018 ± 0.003 | 0.024 ± 0.006 |
| Compound | HPAEpiC | Selectivity Index | ||
|---|---|---|---|---|
| (GI50, µM) | A375-C5 | MCF-7 | NCI-H460 | |
| 11 | 26.75 ± 1.20 | 4.13 | 4.19 | 4.73 |
| 12 | 39.28 ± 0.25 | 1.01 | 1.16 | 1.26 |
| 13 | 127.90 ± 1.56 | 5.59 | 6.79 | 7.54 |
| 14 | >150 | - | - | - |
| Doxorubicin | 0.054 ± 0.005 | 3.38 | 3.00 | 2.25 |
| Compound | λmax (nm) | log ε | λem (nm) | Stokes’ Shift (nm) | ΦF % 1 |
|---|---|---|---|---|---|
| 11 | 456 | 3.67 | 548 | 92 | 29.0 |
| 12 | 394 | 3.72 | 483 | 89 | 50.7 |
| 13 | 457 | 3.72 | 548 | 91 | 31.3 |
| 14 | 480 | 4.00 | 560 | 80 | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durães, F.; Silva, P.M.A.; Novais, P.; Amorim, I.; Gales, L.; Esteves, C.I.C.; Guieu, S.; Bousbaa, H.; Pinto, M.; Sousa, E. Tetracyclic Thioxanthene Derivatives: Studies on Fluorescence and Antitumor Activity. Molecules 2021, 26, 3315. https://doi.org/10.3390/molecules26113315
Durães F, Silva PMA, Novais P, Amorim I, Gales L, Esteves CIC, Guieu S, Bousbaa H, Pinto M, Sousa E. Tetracyclic Thioxanthene Derivatives: Studies on Fluorescence and Antitumor Activity. Molecules. 2021; 26(11):3315. https://doi.org/10.3390/molecules26113315
Chicago/Turabian StyleDurães, Fernando, Patrícia M. A. Silva, Pedro Novais, Isabel Amorim, Luís Gales, Cátia I. C. Esteves, Samuel Guieu, Hassan Bousbaa, Madalena Pinto, and Emília Sousa. 2021. "Tetracyclic Thioxanthene Derivatives: Studies on Fluorescence and Antitumor Activity" Molecules 26, no. 11: 3315. https://doi.org/10.3390/molecules26113315
APA StyleDurães, F., Silva, P. M. A., Novais, P., Amorim, I., Gales, L., Esteves, C. I. C., Guieu, S., Bousbaa, H., Pinto, M., & Sousa, E. (2021). Tetracyclic Thioxanthene Derivatives: Studies on Fluorescence and Antitumor Activity. Molecules, 26(11), 3315. https://doi.org/10.3390/molecules26113315












