Effect of Grain Size Distribution on Frictional Wear and Corrosion Properties of (FeCoNi)86Al7Ti7 High-Entropy Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
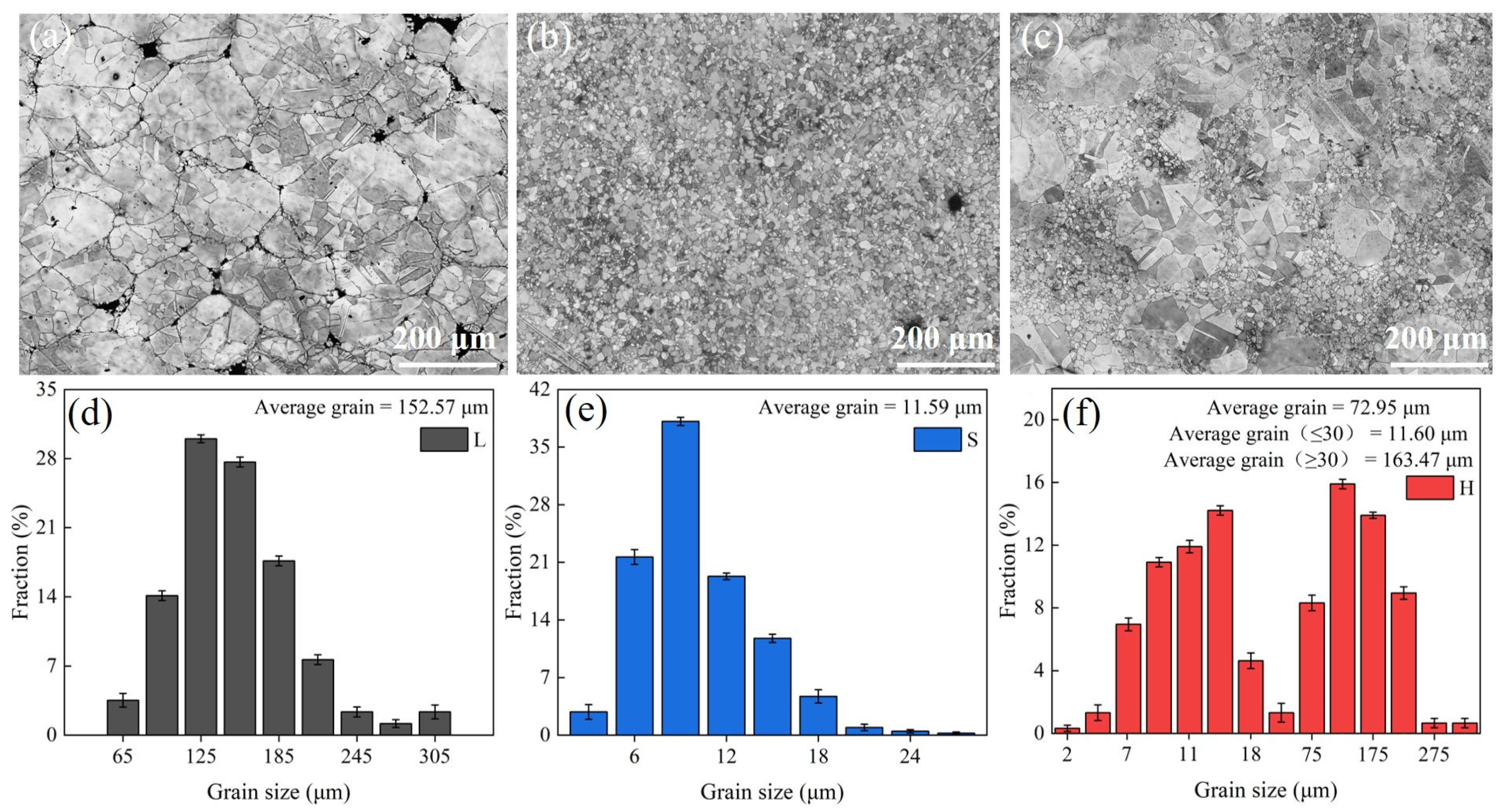
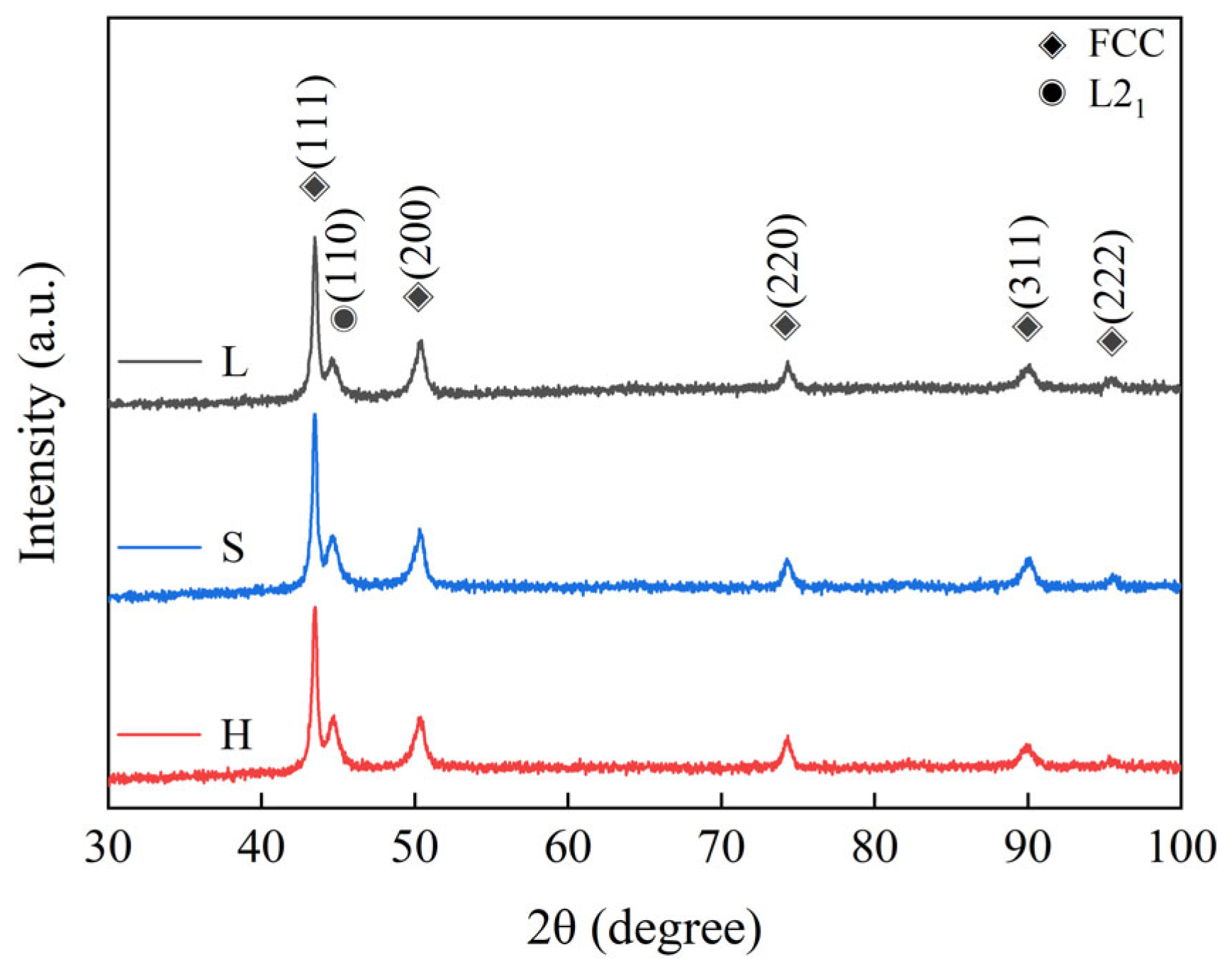
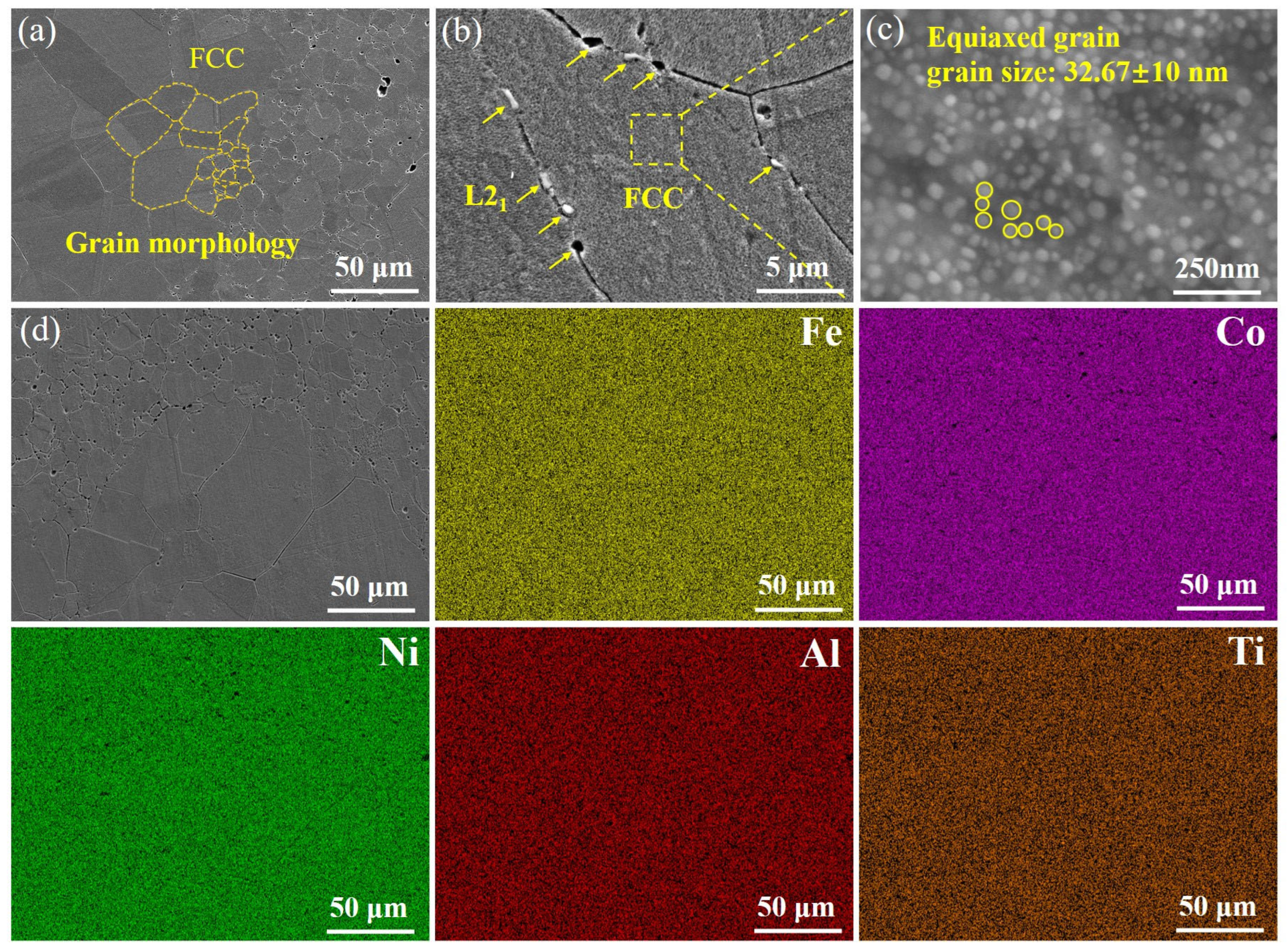
3.1. Microstructural Analysis of SPS Sample
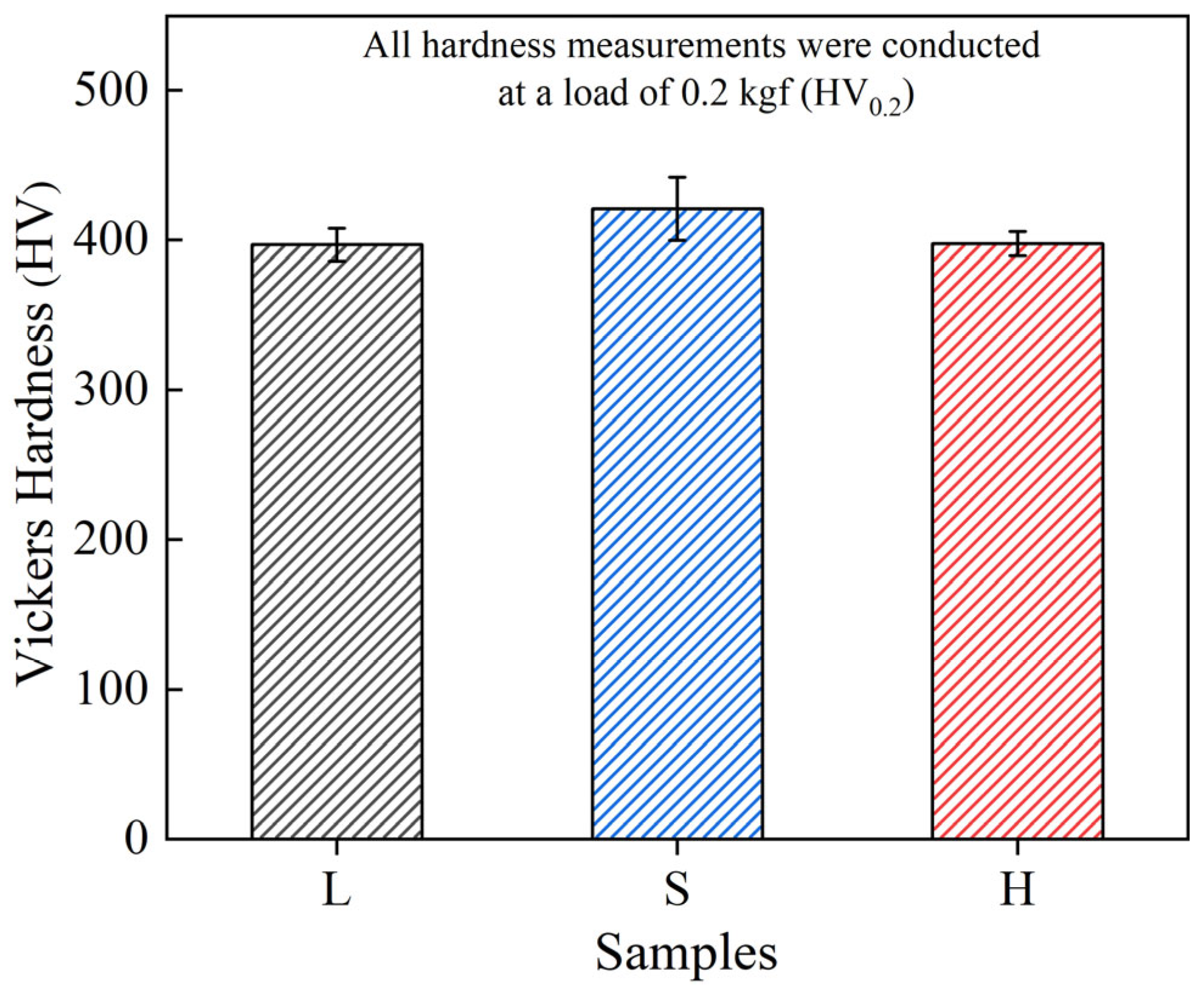
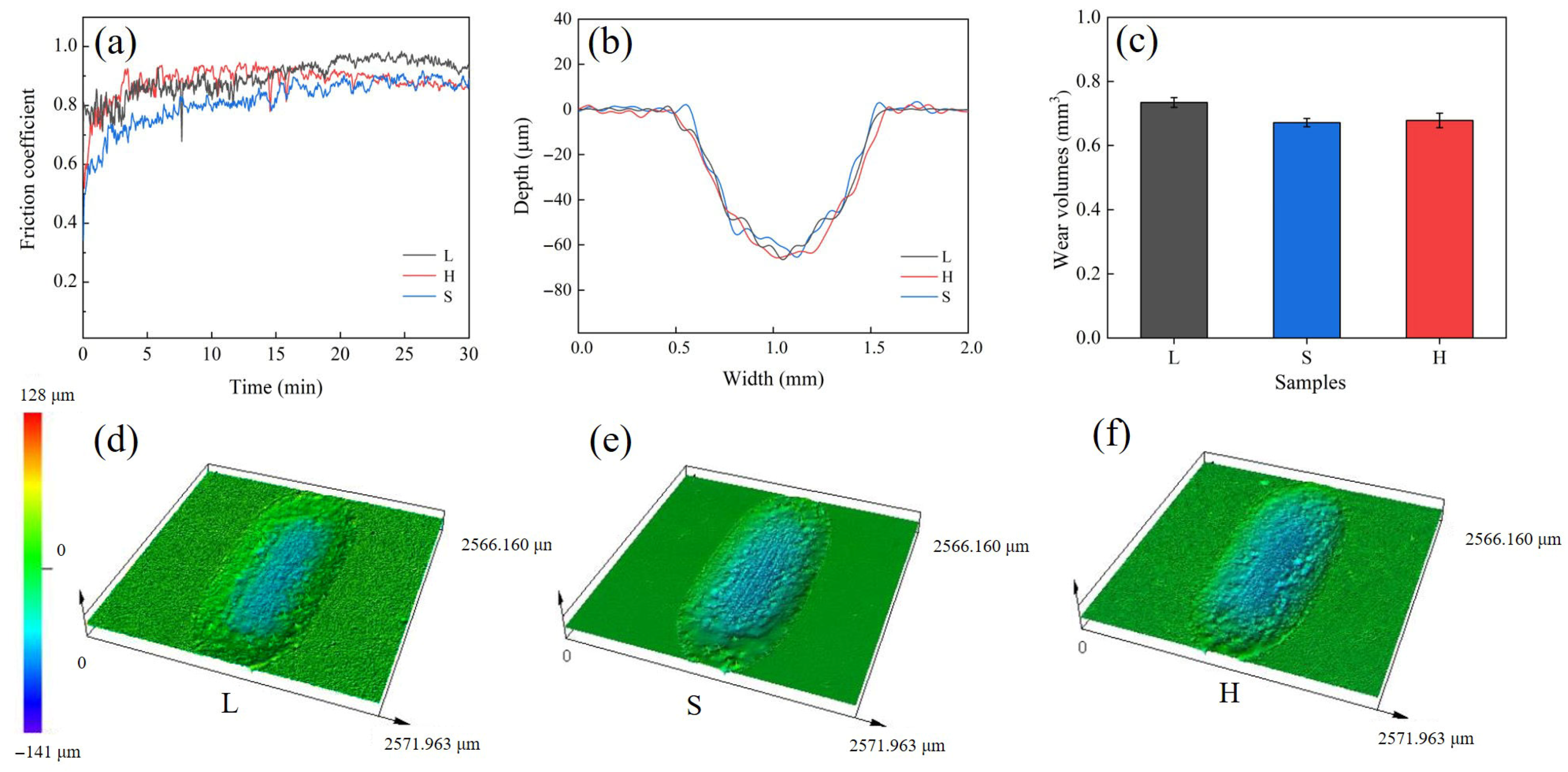
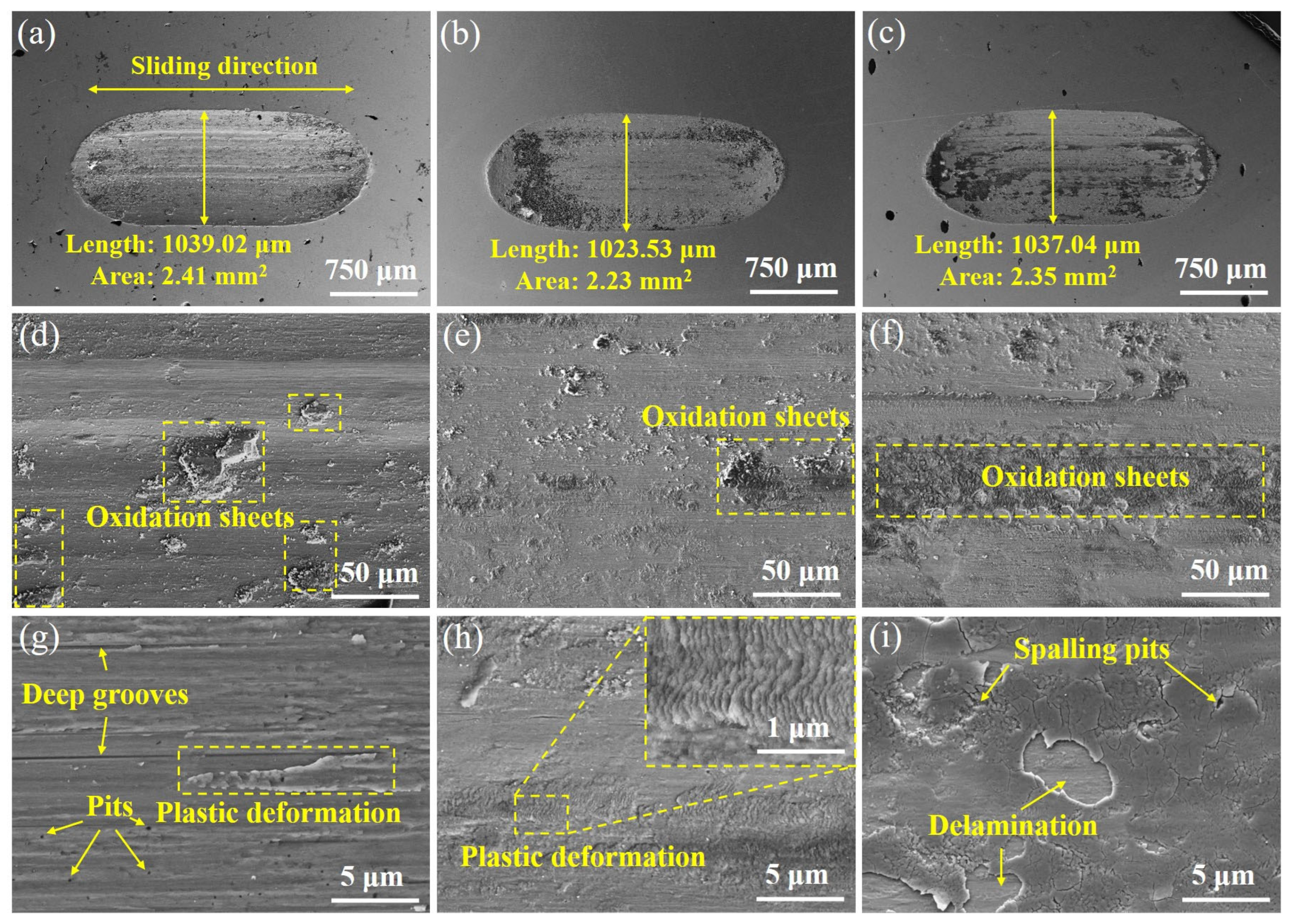
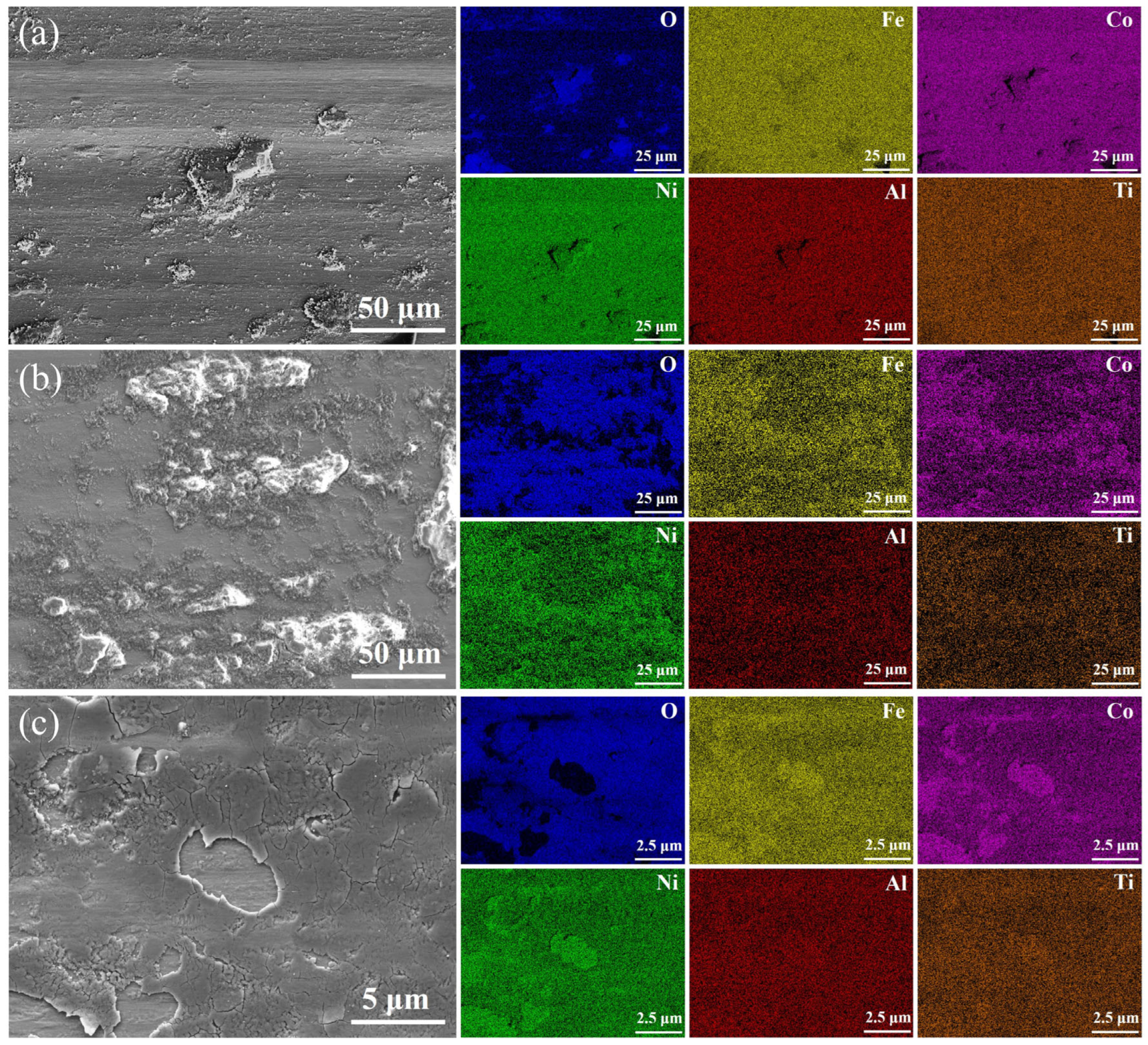
3.2. Hardness and Wear Performance
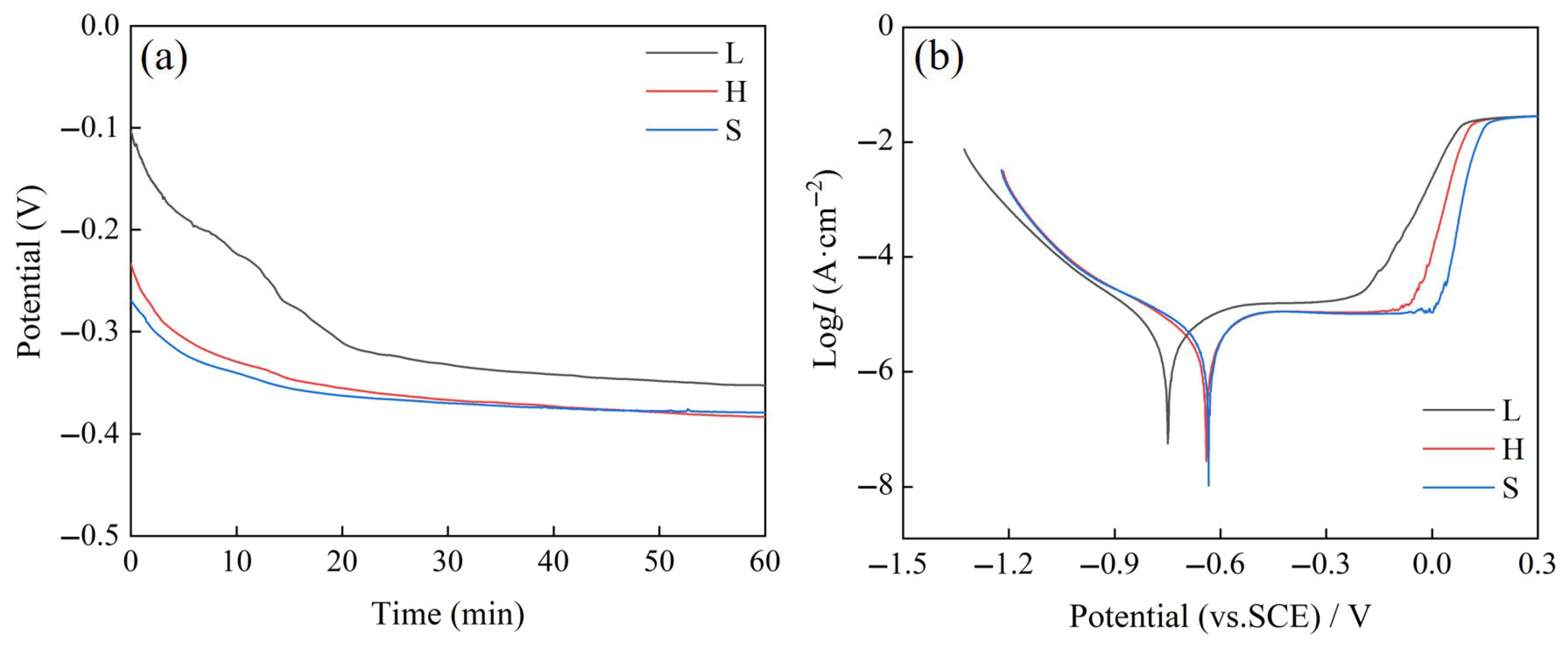
3.3. Electrochemical Corrosion Properties
4. Summary
- (1)
- (FeCoNi)86Al7Ti7 HEAs are mainly composed of a face-centered cubic (FCC) solid solution phase and a body-centered cubic (BCC) structure L21 phase. The surface morphology of L and S samples show equiaxial grains, and the average grain size decreases from 152.57 μm to 11.59 μm due to the difference in the initial powder grain size, while the microstructure of H exhibits a heterogeneous structure consisting of specific spatial distributions of ultrafine and coarse grains, i.e., the coarse-grained regions are surrounded by continuously connected ultra-fine-grained regions.
- (2)
- Due to the fine-grain reinforcement, S exhibits the highest hardness (421.3 HV0.2), the lowest average coefficient of friction (0.87) and the lowest wear rate (7.46 ± 0.14 × 10−3 mm3/(N·m)), and the wear mechanisms operating in both L and S are delamination and abrasive wear. The H sample with heterogeneous structure, on the other hand, achieves similar wear resistance to the S sample through a mixed wear mechanism of adhesive and oxidative wear.
- (3)
- The results of electrochemical corrosion experiments show that all three HEA samples exhibit obvious phenomenon of passivation, and H exhibits strong corrosion resistance similar to that of S. The corrosion potential of H increases to −0.6404 V, the corrosion current density is 8.39 μA/cm2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Samal, S.; Chandra, M.; Li, W.; Habr, S.; Kopeček, J.; Stachiv, I.; Šittner, P. Effect of sintering temperature on microstructure, phase evolution, thermo-mechanical properties of spark plasma sintering of NiTi alloy. Surf. Coat. Tech. 2025, 507, 132152. [Google Scholar] [CrossRef]
- Marinca, T.F.; Popa, F.; Mesaroș, A.Z.; Neamțu, B.V.; Prică, V.C.; Chicinaș, H.F.; Chicinaș, I. Soft magnetic composite obtained by interface reaction upon spark plasma sintering using double-coated Al-permalloy (Ni71.25Fe23.75Al5) composite particles. Appl. Surf. Sci. Adv. 2025, 28, 100793. [Google Scholar] [CrossRef]
- Baliarsingh, S.S.; Das, D.; Behera, R.R.; Roy, S.; Chakrabarty, S.; Mohanta, K. Mechanical and Tribological Properties of Aluminium Based Metal Matrix Composites: An Overview. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yang, L.; Wei, C.; Jiang, F.; Liang, D.; Yan, K.; Cheng, Z.; Li, Z.; Ren, F. Significant Reduction in Friction and Wear of an Ultrafine-Grained Single-Phase FeCoNi Alloy through the Formation of Nanolaminated Structure. Acta Mater. 2024, 263, 119526. [Google Scholar] [CrossRef]
- Li, Y.S.; Li, J.; Mo, J.X.; Tang, J.G.; Lee, S.W. Investigated into High-Temperature Oxidation Behaviors of the Coatings Fabricated on Ti6Al4V by Laser Cladding MoNbTiZr with Varying Contents of TaC. J. Alloys Compd. 2025, 1022, 179944. [Google Scholar] [CrossRef]
- Chen, D.; Guo, J.; Guo, L.; Tan, X.; Ba, Y.; Zhang, L.; Zhang, Q. A phase prediction strategy of high entropy alloys manufactured by selective laser melting based on feature selection and machine learning. J. Mater. Res. Technol. 2025, 37, 79–88. [Google Scholar] [CrossRef]
- Van Duong, L.; Thinh, N.Q.; Linh, N.N.; Khanh, D.Q.; Kim, H.; Song, J.W.; Phuong, D.D. Microstructure, Mechanical Properties, and Corrosion Behavior of TiVNbZrHf High Entropy Alloys Fabricated by Multi-Step Spark Plasma Sintering. Int. J. Refract. Met. Hard Mater. 2024, 119, 106529. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Chu, C.; Fu, Z. Enhancing Mechanical and Anti-Corrosion Properties in a L12 Nanoprecipitates Reinforced Fe35.4Ni24Cr21Co9Mo2Cu1.6Al3Ti4 Multi-Principal Element Alloy via Tailoring Grain Size. J. Alloys Compd. 2025, 1010, 177235. [Google Scholar] [CrossRef]
- Abolkassem, S.; Elsayed, A.; Kariya, S.; Umeda, J.; Kondoh, K. Microstructure, Mechanical, and Magnetic Properties of Powder Metallurgy FeCoNiSi–Cu, FeCoNiSi–Mn, and FeCoNiSi-Ti Equiatomic HEAs Manufactured by Spark Plasma Sintering. J. Mater. Res. Technol. 2024, 33, 9426–9438. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Yu, Y.; Liu, S.; Tian, H.; Jiang, L.; Chen, F.; Gao, H. Combining Spark Plasma Sintering with Laser Cladding: A New Strategy for Fabricating Microstructure of Defect-Free and Highly Wear-Resistant AlCoCrFeNi High-Entropy Alloy. J. Mater. Res. Technol. 2024, 32, 2101–2114. [Google Scholar] [CrossRef]
- Kang, M.; Rizi, M.S.; Jo, S.-J.; Ebrahimian, M.; Nersisyan, H.; Lee, J.H.; Moon, J.; Kim, H.S.; Hong, S.-J. Microstructure Evolution and Mechanical Properties of CoCrFeMnNi HEA-MXene Composites Prepared by Spark Plasma Sintering. J. Alloys Compd. 2025, 1010, 177494. [Google Scholar] [CrossRef]
- Yang, R.; Li, F.; Huang, Z.; Li, Y.; Yang, H.; Qiao, J. Tribological Behavior of Fe40Mn20Cr20Ni20 HEA Sliding against Various Counterface Materials. Tribol. Int. 2024, 200, 110084. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, R.; Mo, J.; Lee, S. Investigation into Tribocorrosion Behaviors of CoCrFeNiNb Laser-Clad Coatings with Y2O3. Mater. Res. Express 2024, 11, 116517. [Google Scholar] [CrossRef]
- Zhicheng, L.; Dejun, K. Microstructure, Corrosive–Wear and Immersion Corrosion Performances of Laser Cladded FeCoNiCr–Mo (Al, Ti) High–Entropy Alloy Coatings. Corros. Sci. 2024, 227, 111766. [Google Scholar] [CrossRef]
- Ke, X.; Li, J.; Ke, Z.; Zhou, Q.H. Al0. 5CoCrFeNi-yNb high-entropy alloys via spark plasma sintering: Microstructural evolution, property enhancement, and wear resistance optimization. Intermetallics 2025, 185, 108862. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Y.; Zhao, Y.; Ma, K.; Cui, J. Corrosion Behaviors and Mechanism of AlxCrFeMnCu High-Entropy Alloys in a 3.5 wt% NaCl Solution. Corros. Sci. 2024, 233, 112087. [Google Scholar] [CrossRef]
- Li, R.L.; Li, J.; Yan, Y.N.; Shao, M.; Li, J. Tribocorrosion Resistance of CoCrFeNiNb Laser-Clad Coatings in the Neutral and Acid Solutions. Opt. Laser Technol. 2023, 158, 108817. [Google Scholar] [CrossRef]
- Huang, G.Q.; Sun, T.; Meng, F.Q.; Jiang, X.W.; Chou, T.H.; Ju, J.; Gan, J.; Yang, T.; Li, M.S.; Shen, Z.K.; et al. Microstructural Evolution and Wear Behavior of Friction Stir Processed L12 Strengthened AlFeCrCuNi-Type High-Entropy Alloy. Mater. Charact. 2024, 216, 114250. [Google Scholar] [CrossRef]
- Ma, P.; Yang, H.; Zhang, Z.; Xie, X.; Yang, P.; Konda-Gokuldoss, P.; Zhang, H.; Jia, Y. Study on the microstructure and Mechanical Properties of Additively Manufactured FeCoCrMnNi High-Entropy Alloy Composite after Aging. J. Cent. South Univ. 2025, 32, 1167–1178. [Google Scholar] [CrossRef]
- An, F.; Zou, S.; Hou, J.; Sun, T.; Liu, J.; Li, Z.; Qian, B.; Zeng, G.; Zhang, X.; Tan, X.; et al. Advanced Interstitial High-Entropy Alloy Engineered for Exceptional Wear Resistance via Dual Nanoprecipitation-Induced Heterogeneous Structure. Mater. Sci. Eng. A 2024, 904, 146714. [Google Scholar] [CrossRef]
- Yu, J. Tribological Behavior and Wear Mechanism of In-Situ Precipitation Strengthened High-Entropy Alloys with Heterogeneous Design. Vacuum 2025, 234, 114053. [Google Scholar] [CrossRef]
- Wei, L.; Qin, W.-M.; Chen, J.-Y.; Lei, W.-X.; Xi, J.-Y. L21-Strengthened Face-Centered Cubic High-Entropy Alloy with Well Pitting Resistance. Corros. Sci. 2023, 215, 111043. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; He, C.; Chen, S.; Yu, J.; Nie, C.; Zhang, M.; Gong, P.; Hu, Z.; Li, B.; et al. An Investigation on Corrosion Resistance of Laser-Cladded FeCoNiCrCu High-Entropy Alloy Coatings Based on Different Initial Powder Particle Sizes. Surf. Coat. Technol. 2025, 503, 132030. [Google Scholar] [CrossRef]
- Yan, X.; Guo, H.; Yang, W.; Pang, S.; Wang, Q.; Liu, Y.; Liaw, P.K.; Zhang, T. Al0.3CrxFeCoNi High-Entropy Alloys with High Corrosion Resistance and Good Mechanical Properties. J. Alloys Compd. 2021, 860, 158436. [Google Scholar] [CrossRef]
- ASTM G99-95; American Society for Testing and Materials. Standard Test Method for Wear Testing with a Pin—on—Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 1995.
- Özbay Kısasöz, B. Influence of Initial Grain Size on the Formation of Sigma and Its Effect on Corrosion and Wear Properties of 2205 Duplex Stainless Steel. Mater. Chem. Phys. 2025, 339, 130693. [Google Scholar] [CrossRef]
- Lai, J.; Dong, H.; Wang, Y.; Mu, Y.; Jiang, H.; Jia, Y.; Yang, P.; Xie, X.; Jia, Y.; Wang, G. Ultra-High Hardness and Wear Resistance Combined with Excellent Corrosion Resistance of Novel Dual-Phase Al-Cr-Fe-V-Ti High-Entropy Alloys. J. Alloys Compd. 2025, 1029, 180855. [Google Scholar] [CrossRef]
- Shahbazkhan, A.; Sabet, H.; Abbasi, M. Investigation of Bonding Strength and Hot Corrosion Behavior of NiCoCrAlSi High Entropy Alloy Applied on IN-738 Superalloy by SPS Method. J. Alloys Compd. 2022, 911, 164997. [Google Scholar] [CrossRef]
- Ouyang, J.; Gao, Y. Effect of Degradable (CaP and ZnO) Nanoparticals Void-Plugging MgFe-LDHs Film on the Pitting-Responsive Behaviors of AZ31 Magnesium Alloy. Corros. Sci. 2023, 222, 111425. [Google Scholar] [CrossRef]

| Sample | Content of Elements (at%) | |||||
|---|---|---|---|---|---|---|
| O | Fe | Co | Ni | Al | Ti | |
| L | 12.17 | 25.42 | 25.43 | 24.65 | 6.13 | 6.21 |
| S | 46.29 | 15.47 | 15.25 | 14.98 | 4.20 | 3.81 |
| H | 45.80 | 15.56 | 15.23 | 14.68 | 3.85 | 3.87 |
| Sample | Ecorr (V) | Icorr (μA/cm2) |
|---|---|---|
| L | −0.7492 | 8.32 |
| S | −0.6333 | 11.62 |
| H | −0.6404 | 8.39 |
| Sample | Rs (Ω·cm2) | CPEdl (10−6 F·cm−2) | n | Rp (103 Ω·cm2) | L (103 Hz·cm2) | RL (103 Ω·cm2) |
|---|---|---|---|---|---|---|
| L | 4.7 | 0.9 | 0.87 | 222.6 | 342.4 | 289.4 |
| S | 2.8 | 0.7 | 0.83 | 373.6 | 3243 | 664 |
| H | 5.6 | 0.7 | 0.82 | 312 | 59.8 | 350.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Ma, P.; Yang, H.; Xie, K.; Wan, S.; Sheng, C.; Chen, Z.; Yang, H.; Jia, Y.; Prashanth, K.G. Effect of Grain Size Distribution on Frictional Wear and Corrosion Properties of (FeCoNi)86Al7Ti7 High-Entropy Alloys. Entropy 2025, 27, 747. https://doi.org/10.3390/e27070747
Sun Q, Ma P, Yang H, Xie K, Wan S, Sheng C, Chen Z, Yang H, Jia Y, Prashanth KG. Effect of Grain Size Distribution on Frictional Wear and Corrosion Properties of (FeCoNi)86Al7Ti7 High-Entropy Alloys. Entropy. 2025; 27(7):747. https://doi.org/10.3390/e27070747
Chicago/Turabian StyleSun, Qinhu, Pan Ma, Hong Yang, Kaiqiang Xie, Shiguang Wan, Chunqi Sheng, Zhibo Chen, Hongji Yang, Yandong Jia, and Konda Gokuldoss Prashanth. 2025. "Effect of Grain Size Distribution on Frictional Wear and Corrosion Properties of (FeCoNi)86Al7Ti7 High-Entropy Alloys" Entropy 27, no. 7: 747. https://doi.org/10.3390/e27070747
APA StyleSun, Q., Ma, P., Yang, H., Xie, K., Wan, S., Sheng, C., Chen, Z., Yang, H., Jia, Y., & Prashanth, K. G. (2025). Effect of Grain Size Distribution on Frictional Wear and Corrosion Properties of (FeCoNi)86Al7Ti7 High-Entropy Alloys. Entropy, 27(7), 747. https://doi.org/10.3390/e27070747







