Carnot and the Archetype of Waterfalls
Abstract
1. Introduction and Overview
1.1. The Waterfall Analogy
“According to the notions established up to now, we can compare with some accuracy the motive power of heat to that of a waterfall: both have a maximum that cannot be exceeded, whatever the machine used to receive the action of the water, and whatever the substance used to receive the action of the heat. The motive power of a waterfall depends on its height and the quantity of liquid; the motive power of the heat also depends on the quantity of caloric employed and on what could be called, in effect, the height of its fall [here comes Carnot’s Footnote 1, see Section 3.2], that is to say, on the difference in temperature of the bodies between which the transfer of caloric takes place. In the waterfall, the motive power is strictly proportional to the difference in level between the upper and lower reservoirs.”
1.2. Our Starting Point
1.3. Outline of the Paper
2. Waterfalls and Gravitational–Hydraulic Processes
2.1. Schematic Elements of Waterfalls
2.2. Power of and Energy Made Available by a Waterfall
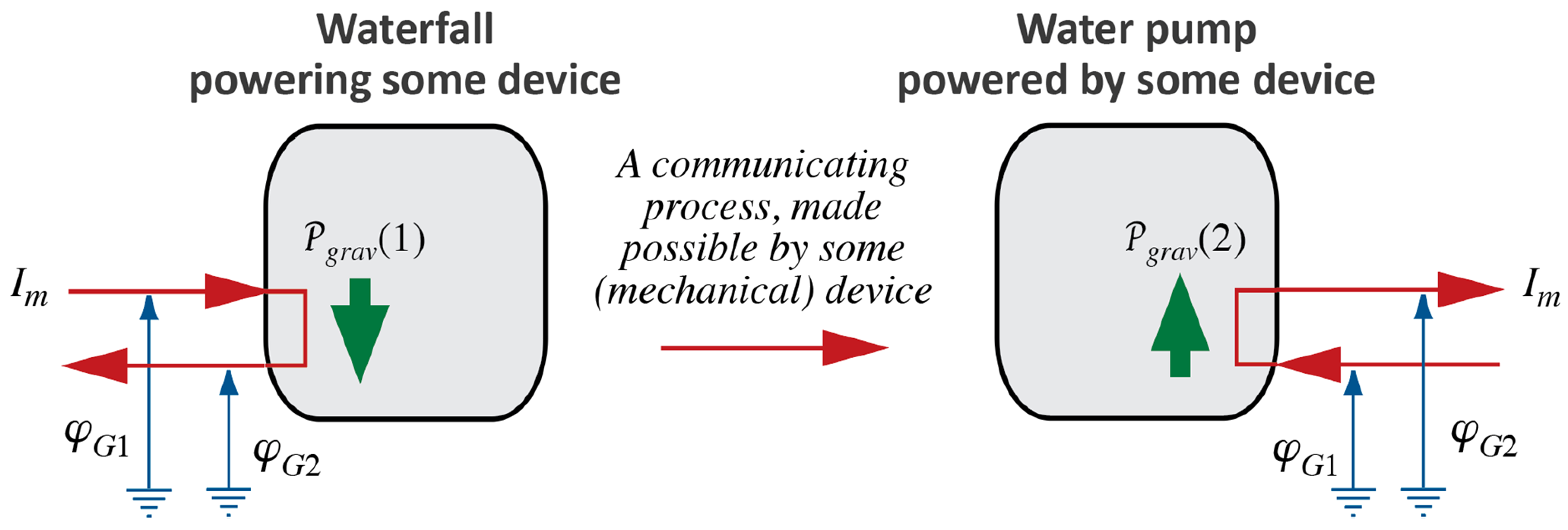
2.3. Lifting or Pumping Water
2.4. The Role of Fluid Medium, Ideal Processes, and Loss of Power
3. Waterfalls and Heat as Forces of Nature
3.1. Heat as a Force of Nature in Carnot’s Thermodynamics
“We are all aware that heat can be the cause of movement, that it even possesses great motive power: the steam engines, now so ubiquitous, are a proof that speaks to anyone who can see.”
“It is to heat that we must attribute the great movements which attract our attention here on Earth; it is to heat that we owe the agitations of the atmosphere, the rise of clouds, the fall of rain and other meteors, the currents of water which channel the surface of the globe and of which man has succeeded in using but a small part for his own purposes; finally, earthquakes and volcanic eruptions also recognize heat as their cause.”
“It is from this immense reservoir that we can draw the moving force necessary for our needs; nature, by offering us fuel everywhere, has given us the faculty, at all times and in all places, of giving birth to heat and to the power which results from it. To develop this power, to appropriate it to our use, such is the object of heat engines.”
3.2. What Carnot’s Waterfall Analogy Tells Us About Heat as a Force
“The subject matter here treated being quite new, we are forced to use expressions which are rather unusual, and which perhaps do not have all the desired clarity.”
3.2.1. Applying the Waterfall Analogy to the Power of Heat
“In the fall of caloric, the motive power undoubtedly increases with the difference in temperature between the hot and cold bodies; but we do not know if it is proportional to this difference.”
3.2.2. The Loss of Power in a Fall of Caloric, and the Production of Caloric
“Wherever there is a difference in temperature, wherever there can be a restoration of equilibrium in the caloric, there can also be production of motive power.”
“Since any restoration of equilibrium in the caloric can be the cause of the production of motive power, any restoration of equilibrium without the production of this power must be considered as a real loss.”
“When ‘thermal agency’ is thus spent in conducting heat through a solid, what becomes of the mechanical effect which it might produce? Nothing can be lost in the operations of nature—no energy can be destroyed. What effect then is produced in place of the mechanical effect which is lost?”
3.2.3. Perpetual Motion and the Medium Used in Heat Engines
“Now, if there were means of employing heat that were preferable to those we have used, i.e., if it were possible, by any method, to make caloric produce a greater quantity of motive power than we have done by our first series of operations, it would suffice to distract a portion of this power to make caloric rise, by the method just indicated, from body B to body A, from the cooler to the furnace, to restore things to their primitive state, and thus be in a position, and thus be in a position to recommence an operation entirely similar to the first, and so on. This would be not only perpetual motion, but an indefinite creation of motive force without the consumption of caloric or any other agent. Such a creation is completely contrary to the ideas received up to now, to the laws of Mechanics and sound Physics; it is inadmissible (footnote). We must therefore conclude that the maximum motive power resulting from the use of steam is also the maximum motive power achievable by any means whatsoever.”
“This leads us to the following general proposition:”
“The motive power of heat is independent of the agents used to produce it; its quantity is determined solely by the temperatures of the bodies between which the heat is ultimately transported.”
“The implication here is that each method of developing motive power achieves the perfection to which it is susceptible.”
3.2.4. A Note on Motive Power and on Power in General
“We use the term motive power here to designate the useful effect that a motor is capable of producing. This effect can always be likened to the lifting of a weight to a certain height; as we know, its measure is the product of the weight multiplied by the height to which it is supposed to be lifted.”
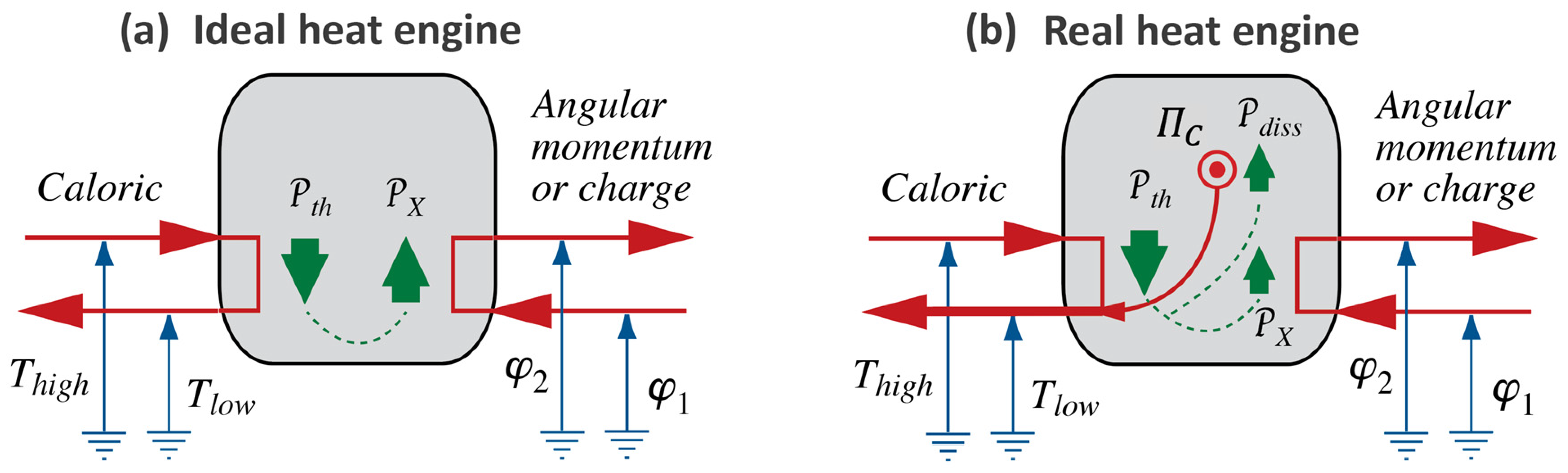
3.2.5. Process Diagrams of Ideal and Real (Dissipative) Heat Engines
3.2.6. A Note Concerning Different Measures of Efficiency
3.2.7. How the Waterfall Analogy and the Caloric Theory Form a Unit
“The production of motive power is thus due, in steam engines, not to an actual consumption of caloric, but to its transport from a hot body to a cold body, …”([1], pp. 10–11; emphasis in the original).
“[…] the motive power of the heat also depends […] on what could be called, in effect, the height of its fall […]”([1], p. 28)
“In other words, the motive power produced would be exactly proportional to the drop in caloric.”([1], p. 79)
3.3. Waterfalls and Ideal Carnot Heat Engines—The Carnot Cycle
3.3.1. The Carnot Cycle
“1. Contact of body A with the air contained in the cylinder, or with the wall of this cylinder, a wall which we will assume transmits caloric easily. This contact brings the air to the same temperature as body A […].”
“2. The piston gradually rises to [a new position]. Contact is always maintained between body A and the air, which is kept at a constant temperature during rarefaction. Body A provides the caloric needed to maintain constant temperature.”
“This condition will be fulfilled if, as we noted above, there is no change of temperature in the bodies that is not due to a change of volume, or, which is the same thing expressed differently, if there is never any contact between bodies of significantly different temperatures.”([1], p. 38)
3.3.2. Ideal Carnot Heat Engines
3.4. Carnot’s Quest for Creating a General Theory of Heat
“To consider the principle of the production of motion by heat in all its generality, it must be conceived independently of any particular mechanism or agent; reasoning must be established that applies not only to steam engines [footnote], but to every conceivable heat engine, whatever the substance used and however it is acted upon.
“Machines that do not receive their motion from heat, those powered by human or animal forces, waterfalls, air currents, etc., can be studied in great detail by the mechanical theory. All cases are foreseen, and all conceivable movements are subject to general principles that are solidly established and applicable in all circumstances. This is the hallmark of a complete theory. Such a theory is obviously lacking for heat engines. It will only be available when the laws of physics are sufficiently extended and generalized to make known in advance all the effects of heat acting in a given way on any body.”
3.5. History of the Waterfall Analogy
3.5.1. On the Origin of the Waterfall Imagery in Carnot’s Work
3.5.2. The Waterfall Analogy in Thermodynamics Shortly After Carnot
“In the use of water-wheels for motive power, the economy of the engine depends not only upon the excellence of its adaptation for actually transmitting any given quantity of water through it, and producing the equivalent of work, but upon turning to account the entire available fall; so, as we are taught by Carnot, the object of a thermodynamic engine is to economize in the best possible way the transference of all the heat evolved, from bodies at the temperature of the source, to bodies at the lowest temperature at which the heat can be discharged. With reference then to any engine of the kind, there will be two points to be considered. (1) The extent of the fall utilised. (2) The economy of the engine, with the fall which it actually uses.”
3.5.3. Waterfall Analogy and the Caloric Theory in More Recent Work
4. Archetypes, Schematism, Metaphor, and Analogy
4.1. A Waterfall as an Archetypal Causative Entity in Nature
4.2. Experiencing, Image Schemas, and Primary Metaphors
4.3. Blending as Compression over Phenomena: Origins of Analogy
4.4. Carnot’s Waterfall Analogy as a Spontaneous Mental Activity
5. The Waterfall Analogy and Modern Macroscopic Physics
5.1. Forces of Nature and the Waterfall Analogy in Macroscopic Physics
5.2. Constructing a Generalized Energy Principle: Extending the Waterfall Image
5.3. Examples of Models
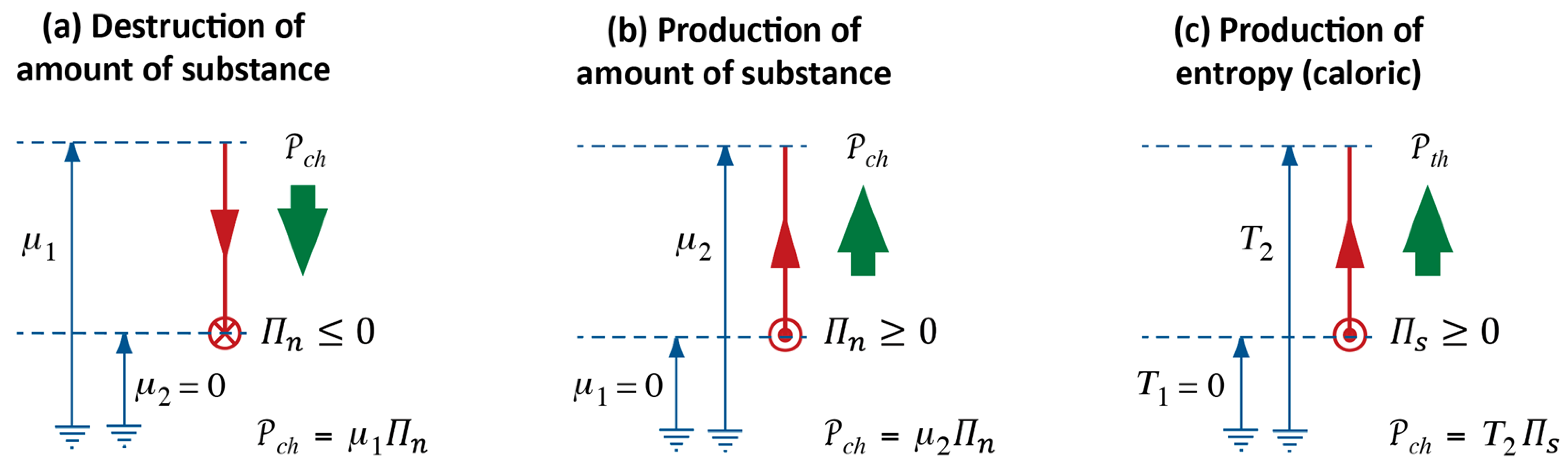
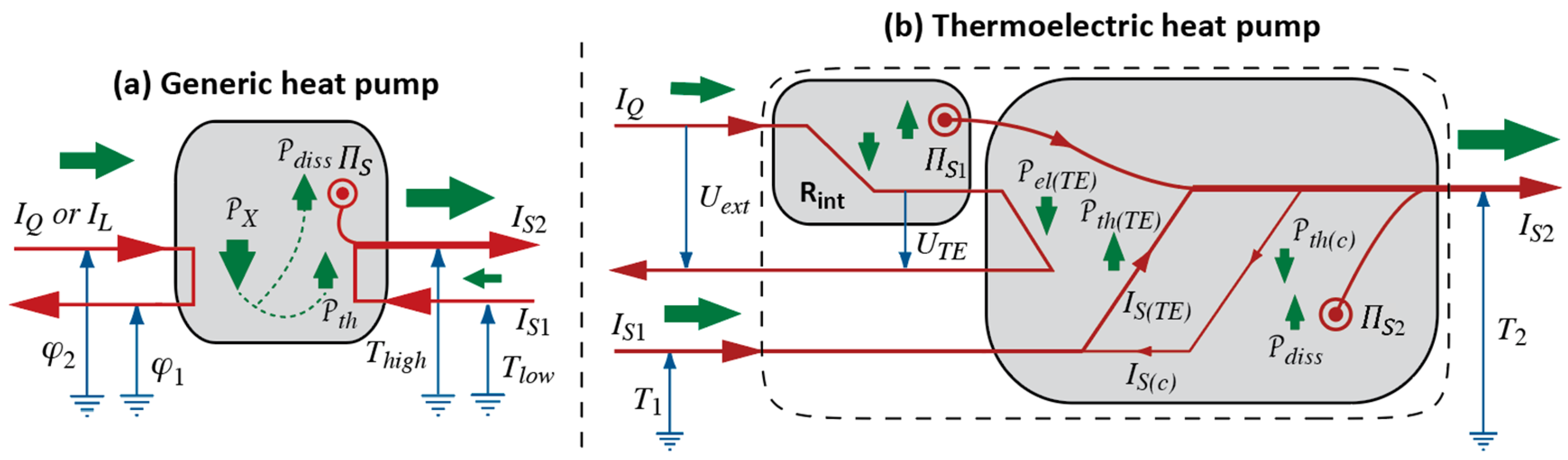
5.3.1. Generic and Thermoelectric Heat Pumps
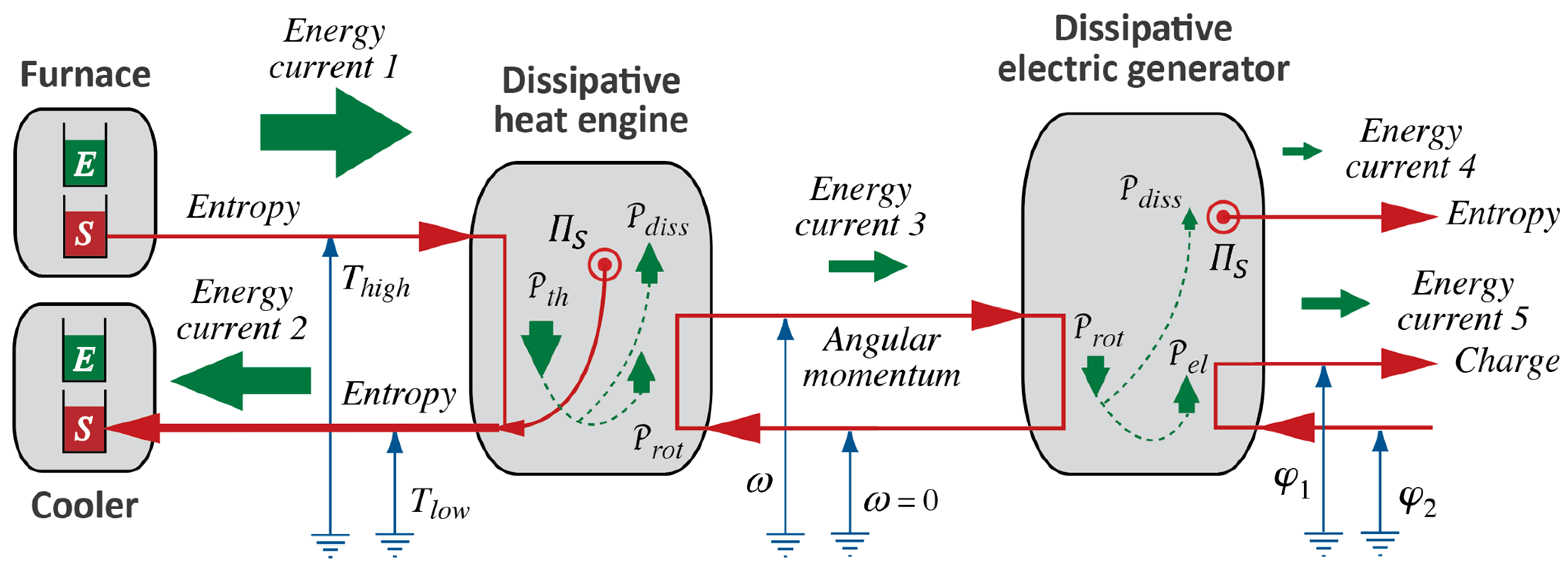
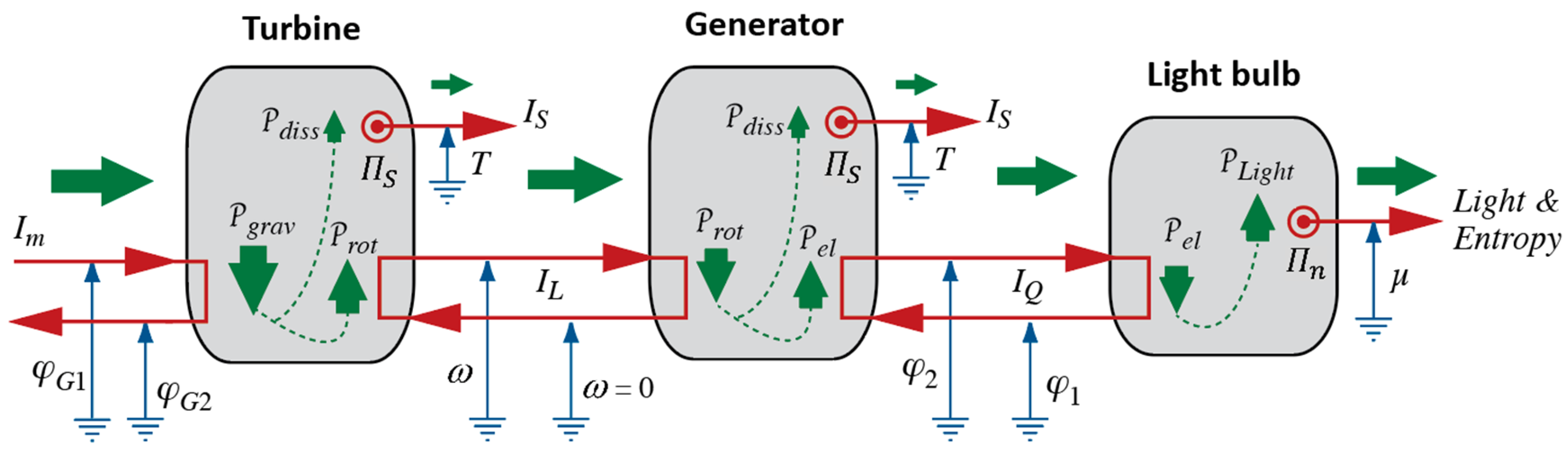
5.3.2. Hydraulic Power Plant
5.3.3. Breaking of a Flywheel
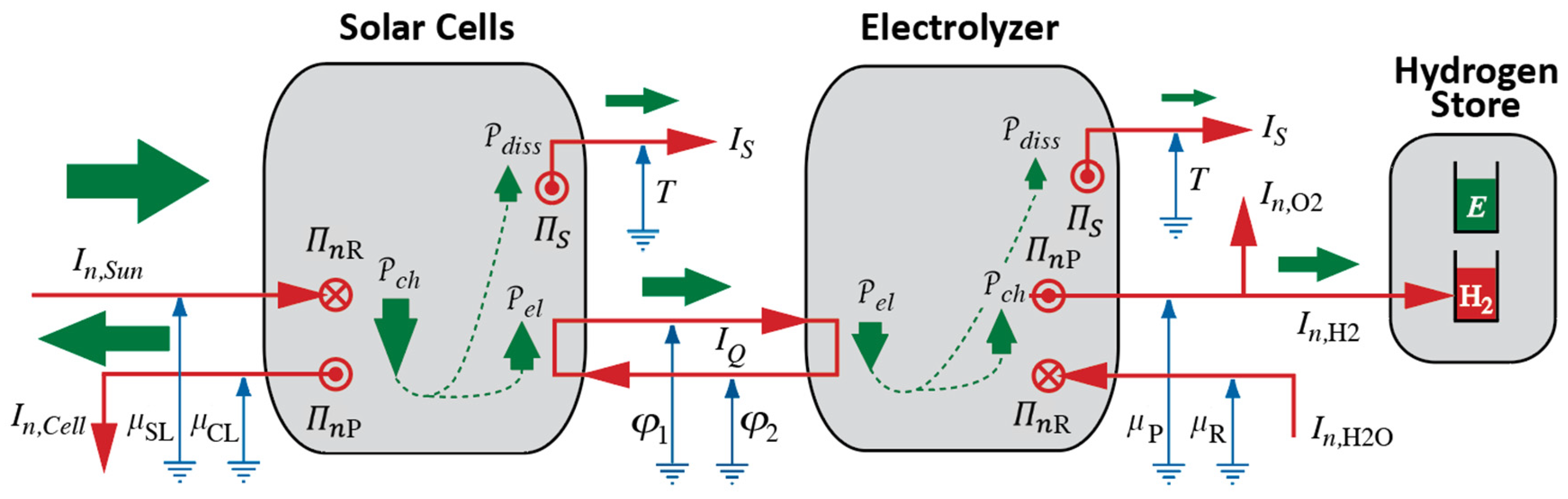
5.3.4. Sun Electrolyzer, and Hydrogen Storage
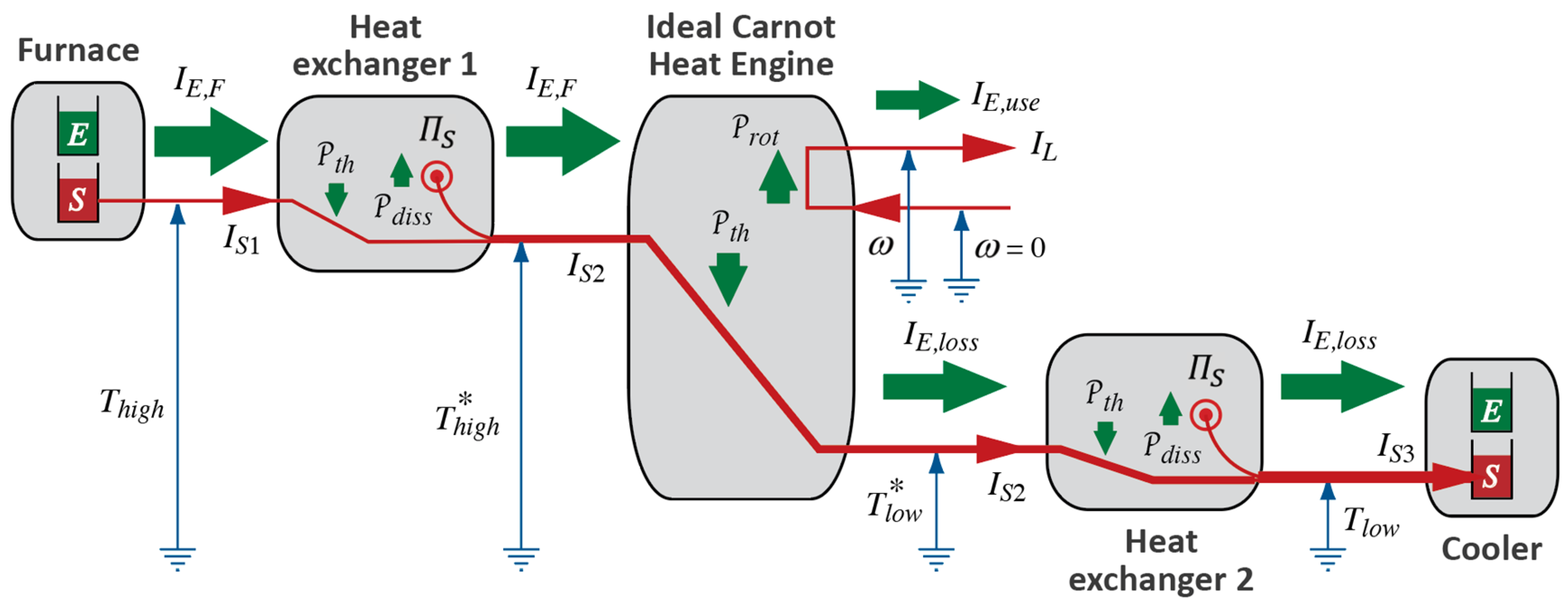
5.3.5. Models of Endoreversible Systems and Processes
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carnot, S. Réflexions sur la Puissance Motrice du feu et sur les Machines Propres à Développer Cette Puissance; Bachelier: Paris, France, 1824. [Google Scholar]
- Fox, R. The Caloric Theory of Gases. From Lavoisier to Regnault; Clarendon Press: Oxford, UK, 1971. [Google Scholar]
- Truesdell, C.A. The Tragicomical History of Thermodynamics 1822–1854; Springer: New York, NY, USA, 1980. [Google Scholar]
- Fuchs, H.U.; D’Anna, M.; Corni, F. Entropy and the Experience of Heat. Entropy 2022, 24, 646. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.U. The Dynamics of Heat. A Unified Approach to Thermodynamics and Heat Transfer, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Truesdell, C.A.; Toupin, R.A. The Classical Field Theories. In Encyclopedia of Physics; Flügge, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1960; Volume III/1. [Google Scholar]
- Truesdell, C.A.; Noll, W. The Non-Linear Field Theories of Mechanics. In Encyclopedia of Physics; Flügge, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1965; Volume III/3. [Google Scholar]
- Truesdell, C.A. Rational Thermodynamics, 2nd ed.; Springer: New York, NY, USA, 1984. [Google Scholar]
- Müller, I. Thermodynamics; Pitman: Boston, MA, USA, 1985. [Google Scholar]
- Jou, D.; Casas-Vázquez, J.; Lebon, G. Extended Irreversible Thermodynamics, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Callendar, H.L. The caloric theory of heat and Carnot’s principle. Proc. Phys. Soc. 1911, 23, 153–189. [Google Scholar] [CrossRef]
- Job, G. Neudarstellung der Wärmelehre–Die Entropie als Wärme; Akad. Verlagsgesellschaft: Frankfurt, Germany, 1972. [Google Scholar]
- Falk, G. Entropy, a resurrection of caloric-a look at the history of thermodynamics. Eur. J. Phys. 1985, 6, 108–115. [Google Scholar] [CrossRef]
- Mareš, J.J.; Hubík, P.; Šesták, J.; Špička, V.; Krištofik, J.; Stávek, J. Phenomenological approach to the caloric theory of heat. Thermochim. Acta 2008, 474, 16–24. [Google Scholar] [CrossRef]
- Herrmann, F.; Pohlig, M. Which Physical Quantity Deserves the Name “Quantity of Heat”? Entropy 2021, 23, 1078. [Google Scholar] [CrossRef]
- Johnson, M. The Body in the Mind. The Bodily Basis of Meaning, Imagination, and Reason; The University of Chicago Press: Chicago, IL, USA, 1987. [Google Scholar]
- Hampe, B. (Ed.) From Perception to Meaning: Image Schemas in Cognitive Linguistics; De Gruyter: Berlin, Germany, 2005. [Google Scholar]
- Cienki, A. Frames, Idealized Cognitive Models, and Domains. In The Oxford Handbook of Cognitive Linguistics; Geeraerts, D., Cuyckens, H., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 170–187. [Google Scholar]
- Fauconnier, G. Mental Spaces: Aspects of Meaning Construction in Natural Language, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Lakoff, G.; Johnson, M. Metaphors We Live by; University of Chicago Press: Chicago, IL, USA, 1980. [Google Scholar]
- Lakoff, G.; Johnson, M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought; Basic Books: New York, NY, USA, 1999. [Google Scholar]
- Gibbs, R. The Poetics of Mind. Figurative Thought, Language, and Understanding; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Amin, T.G.; Jeppsson, F.; Haglund, J. Conceptual metaphor and embodied cognition in science learning. Introduction to the special issue. Int. J. Sci. Educ. 2015, 37, 745–758. [Google Scholar] [CrossRef]
- Gentner, D.; Jeziorski, M. The shift from metaphor to analogy in western science. In Metaphor and Thought, 2nd ed.; Ortony, A., Ed.; Cambridge University Press: Cambridge, UK, 1993; pp. 447–480. [Google Scholar]
- Gentner, D. Structure Mapping: A Theoretical Framework for Analogy. Cogn. Sci. 1983, 7, 155–170. [Google Scholar]
- Caracciolo, M. The Experientiality of Narrative. An Enactivist Approach; De Gruyter: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Fuchs, H.U. From Stories to Scientific Models and Back: Narrative framing in modern macroscopic physics. Int. J. Sci. Educ. 2015, 37, 934–957. [Google Scholar] [CrossRef]
- Fuchs, H.U.; Dumont, E.; Corni, F. Narrative minds in the construction and use of theories of forces of nature—A model of experience at different scales. In Narrative and Cognition in Literature and Science; Sinding, M., Heydenreich, A., Mecke, K., Eds.; De Gruyter: Berlin, Germany, 2025. [Google Scholar]
- Carnot, S.; Fox, R. Reflexions on the Motive Power of Fire: A Critical Edition with the Surviving Scientific Manuscripts; Manchester University Press: Manchester, UK, 1986. [Google Scholar]
- Gillispie, C.C.; Pisano, R. Lazare and Sadi Carnot: A Scientific and Filial Relationship; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Norton, J.D. How analogy helped create the new science of thermodynamics. Synthese 2022, 200, 269. [Google Scholar] [CrossRef]
- Keenan, J.H. Availability and Irreversibility in Thermodynamics. Br. J. Appl. Phys. 1951, 2, 183–192. [Google Scholar] [CrossRef]
- Bejan, A. Advanced Engineering Thermodynamics; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Corning, P.A.; Kline, S.J. Thermodynamics, Information and Life Revisited, Part I: ‘To Be or Entropy’. Syst. Res. 1998, 15, 273–295. [Google Scholar] [CrossRef]
- Corning, P.A.; Kline, S.J. Thermodynamics, Information and Life Revisited, Part II: ‘Thermoeconomics’ and ‘Control Information’. Syst. Res. 1998, 15, 453–482. [Google Scholar] [CrossRef]
- Falk, G.; Herrmann, F.; Schmid, G.B. Energy forms or energy carriers? Am. J. Phys. 1983, 51, 1074–1077. [Google Scholar] [CrossRef]
- Fuchs, H.U.; Corni, F. Primary Physical Science Education—A Narrative Approach to Encounters with Nature; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Bejan, A. Entropy Generation Minimization. The Method of Thermodynamic Optimization of Finite-Size Systems and Finite-Time Processes; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Sieniutycz, S.; De Vos, A. (Eds.) Thermodynamics of Energy Conversion and Transport; Springer: New York, NY, USA, 2000. [Google Scholar]
- Thomson, W. An account of Carnot’s theory of the motive power of heat; with numerical results deduced from Regnault’s experiments on steam. Trans. R. Soc. Edinb. 1849, 16, 541–574. [Google Scholar] [CrossRef]
- Bohren, C.F. Joule’s waterfall measurements: A great story, but is it true? Am. J. Phys. 2010, 78, 6. [Google Scholar] [CrossRef]
- Saslow, W.M. A History of Thermodynamics: The Missing Manual. Entropy 2020, 22, 77. [Google Scholar] [CrossRef]
- Truesdell, C.A. Absolute Temperatures as a Consequence of Carnot’s General Axiom. Arch. Hist. Exact Sci. 1979, 20, 357–380. [Google Scholar] [CrossRef]
- Hanlon, R.T. Block by Block: The Historical and Theoretical Foundations of Thermodynamics; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Laplace, P.S. Sur la Vitesse du Son dans l’air at dans l’eau. Ann. Chim. Phys. 1816, 3, 238–241. [Google Scholar]
- Fox, R. Watt’s Expansive Principle in the Work of Sadi Carnot and Nicolas Clément. Notes Rec. R. Soc. Lond. 1970, 24, 233–253. [Google Scholar]
- Salvi, P.R.; Schettino, V. Sadi Carnot’s Réflexions and the foundation of thermodynamics. Substantia 2019, 3, 73–96. [Google Scholar] [CrossRef]
- Carnot, L. Essay upon machines in general. Philos. Mag. 1808, 30. 8–15, 154–158, 207–221, 310–320; 31, 28–36, 136–144, 220–228, 295–305. [Google Scholar] [CrossRef]
- Communication by an anonymous reviewer. 2024.
- Pisano, R.; Franckowiak, R.; Anakkar, A. Reading Science, Technology and Education: A Tradition Dating back to Science into the History and Historiography. Int. J. Hist. Sci. 2017, 3, 77–97. [Google Scholar] [CrossRef][Green Version]
- Müller, I. A History of Thermodynamics: The Doctrine of Energy and Entropy; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Lervig, P. Sadi Carnot and the Steam Engine: Nicolas-Clément’s Lectures on Industrial Chemistry 1823–1828. Brit. J. Hist. Sci. 1985, 18, 147–196. [Google Scholar] [CrossRef]
- Grinevald, J. La révolution carnotienne thermodynamique, économie et idéologie. Rev. Eur. Des Sci. Soc. 1976, 14, 39–79. [Google Scholar]
- Kerker, M. Sadi Carnot and the steam engine engineers. Isis 1960, 51, 257–270. [Google Scholar] [CrossRef]
- Clapeyron, E. Mémoire sur la puissance motrice de la chaleur. J. L’ecole Polytech. 1834, 14, 153–190. [Google Scholar]
- Corni, F.; Fuchs, H.U. Primary Physical Science for Student Teachers at Kindergarten and Primary School Levels: Part I—Foundations of an Imaginative Approach to Physical Science. Interchange 2020, 51, 315–343. [Google Scholar] [CrossRef]
- Fuchs, H.U. A Direct Entropic Approach to Uniform and Spatially Continuous Dynamical Models of Thermoelectric Devices. Review Article. Energy Harvest. Syst. 2014, 1, 253–265. [Google Scholar] [CrossRef][Green Version]
- Smith, C.W. William Thomson and the Creation of Thermodynamics: 1840–1855. Arch. Hist. Exact Sci. 1977, 16, 231–288. [Google Scholar] [CrossRef]
- Thomson, W. On the Dynamical Theory of Heat, with numerical remits deduced from Mr Joule’s equivalent of a Thermal Unit, and M. Regnault’s Observations on Steam. Trans. R. Soc. Edinb. 1851, 20, 261–288. [Google Scholar] [CrossRef]
- Clausius, R. Über die bewegende Kraft der Wärme und die Gesetze, welche sich daraus für die Wärmelehre selbst ableiten lassen. Ann. Phys. 1850, 155, 368–397. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatics; Wiley: New York, NY, USA, 1985; p. 318. [Google Scholar]
- Grassmann, P. Physikalische Grundlagen der Verfahrenstechnik; 3. Auflage; Otto Salle Verlag: Frankfurt, Germany, 1983. [Google Scholar]
- Fuchs, H.U. Entropy in the teaching of introductory thermodynamics. Am. J. Phys. 1987, 55, 215–219. [Google Scholar] [CrossRef]
- Barnett, M.K. Sadi Carnot and the Second Law of Thermodynamics. Osiris 1958, 13, 327–357. [Google Scholar] [CrossRef]
- Erlichson, H. Sadi Carnot, ‘Founder of the Second Law of Thermodynamics’. Eur. J. Phys. 1999, 20, 183. [Google Scholar] [CrossRef]
- Lemons, D.S.; Penner, M.K. Sadi Carnot’s contribution to the second law of thermodynamics. Am. J. Phys. 2008, 76, 21–25. [Google Scholar] [CrossRef]
- Wang, L.-S. The Second Law: From Carnot to Thomson-Clausius, to the Theory of Exergy, and to the Entropy-Growth Potential Principle. Entropy 2017, 19, 57. [Google Scholar] [CrossRef]
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures on Physics; Section 44-2 and 44-3; Addison-Wesley Publishing Company: Reading, MA, USA, 1963; Volume I. [Google Scholar]
- D’Anna, M.; Lubini, P.; Fuchs, H.U.; Corni, F. A Direct Entropic Approach to the Thermal Balance of Spontaneous Chemical Reactions. Entropy 2024, 26, 450. [Google Scholar] [CrossRef]
- Lakoff, G.; Nuñez, R. Where Mathematics Comes from: How the Embodied Mind Brings Mathematics into Being; Basic Books: New York, NY, USA, 2001. [Google Scholar]
- Gentner, D.; Gentner, D.R. Flowing waters or teeming crowds: Mental models of electricity. In Mental Models; Gentner, D., Stevens, A., Eds.; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1983; pp. 99–129. [Google Scholar]
- Knox, J. Archetype, Attachment, Analysis; Routledge: London, UK, 2003. [Google Scholar]
- Merchant, J. The image schema and innate archetypes: Theoretical and clinical implications. J. Anal. Psychol. 2016, 61, 63–78. [Google Scholar] [CrossRef]
- Dewey, J. Experience and nature. In The Later Works (1925–1953, Vol.1); Boydston, J.A., Ed.; Southern Illinois University Press: Carbondale, IL, USA, 1925. [Google Scholar]
- Talmy, L. Toward a Cognitive Semantics (Vol. I and II); MIT Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Grady, J.E. Foundations of Meaning: Primary Metaphors and Primary Scenes. Ph.D. Dissertation, University of Berkeley, Berkeley, CA, USA, 1997. [Google Scholar]
- Grady, J.E. Primary metaphors as inputs to conceptual integration. J. Pragmat. 2005, 37, 1595–1614. [Google Scholar] [CrossRef]
- Grady, J.E. Image schemas and perception: Refining a definition. In From Perception to Meaning: Image Schemas in Cognitive Linguistics; Hampe, B., Ed.; De Gruyter: Berlin, Germany, 2005; pp. 35–55. [Google Scholar]
- Sherin, B.L. How Students Understand Physics Equations. Cogn. Instr. 2001, 19, 479–541. [Google Scholar] [CrossRef]
- Fauconnier, G. Mental Spaces. In The Oxford Handbook of Cognitive Linguistics; Geeraerts, D., Cuyckens, H., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 351–376. [Google Scholar]
- Fauconnier, G.; Turner, M. The Way We Think. Conceptual Blending and the Mind’s Hidden Complexities; Basic Books: New York, NY, USA, 2002. [Google Scholar]
- Turner, M. The Art of Compression. In The Artful Mind; Turner, M., Ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Scherr, R.E.; Close, H.G.; McKagan, S.B.; Vokos, S. Representing energy. I. Representing a substance ontology for energy. Phys. Rev. Spec. Top.-Phys. Educ. Res. 2012, 8, 020114. [Google Scholar] [CrossRef]
- Harrer, B.W. On the origin of energy: Metaphors and manifestations as resources for conceptualizing and measuring the invisible, imponderable. Am. J. Phys. 2017, 85, 454–460. [Google Scholar] [CrossRef]
- Amin, T.G. Coordinating metaphors in science, learning and instruction. The case of energy. In How Metaphors Guide, Teach and Popularize Science; Berger, A., Smith, T.H., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2020; pp. 73–110, Chapter 3; ISBN 9789027261441. [Google Scholar]
- Atkins, L. Why We Eat Calories: A Plurality Metaphor of Energy in Scientific Disciplines. Sci. Educ. 2024. [Google Scholar] [CrossRef]
- Rubin, D.; Krempl, E.; Lai, W.M. Introduction to Continuum Mechanics; 3rd ed.; 4th ed.; Elsevier: Burlington, MA, USA, 2009. [Google Scholar]
- Feldhoff, A. Power Conversion and Its Efficiency in Thermoelectric Materials. Entropy 2020, 22, 803. [Google Scholar] [CrossRef]
- Corni, F.; Dumont, E.; Fuchs, H.U.; Schütz, E. The Energy Principle in a Model of Electric Braking of a Flywheel. In Proceedings of the 2016 GIREP Conference, Krakow, Poland, 30 August–3 September 2016; Available online: https://digitalcollection.zhaw.ch/handle/11475/12026 (accessed on 20 November 2024).
- Fuchs, H.U. Introductory Chemical Dynamics—Using the Chemical Potential from the Start. Rev. Cub. Fis. 2010, 27, 119–124. [Google Scholar]
- Job, G.; Rüffler, R. Physical Chemistry from a Different Angle; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Herrmann, F.; Würfel, P. The semiconductor diode as a rectifier, a light source, and a solar cell: A simple explanation. Am. J. Phys. 2006, 74, 591–594. [Google Scholar] [CrossRef]
- Dumont, E.; Fuchs, H.U.; Corni, F.; Contini, A.; Altiero, T.; Romagnoli, M.; Karwasz, G.P. FCHgo: Fuel Cells HydroGen educatiOnal model for schools, an imaginative approach to hydrogen and fuel cell technology for young students and their teachers. J. Phys. Conf. Ser. 2021, 1229, 012019. [Google Scholar] [CrossRef]
- De Vos, A. Thermodynamics of Solar Energy Conversion; Wiley-VCH Verlag: New York, NY, USA, 2008. [Google Scholar]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Novikov, I.I. Efficiency of atomic energy installation. At. Energiya 1957, 3, 409–412. [Google Scholar]
- Gordon, J.M.; Zarmi, Y. Wind energy as a solar-driven heat engine: A thermodynamic approach. Am. J. Phys. 1989, 57, 995–998. [Google Scholar] [CrossRef]




| Water, Waterfalls, and Water Wheels | Heat, Falling Caloric, and Heat Engines |
|---|---|
| Vertical level, level difference | Temperature, temperature difference |
| Gravitational tension | Thermal tension |
| Amount of water (mass of water) | Amount of heat, i.e., caloric |
| Flow/fall of water | Flow/fall of caloric |
| Amount of water contained in a system | Heat (caloric) contained in materials (assumption of a heat function) |
| Conservation of amount of water | Conservation of quantity of caloric |
| Power of falling water (falling water produces motive power) | Power of falling caloric (falling caloric produces motive power) |
| Power is proportional to both gravitational tension and flow of water | Power is proportional to both thermal tension and flow of caloric |
| Loss of power of falling water (water falling without “producing motive power”) | Loss of power of falling caloric (caloric falling through a temperature difference without “producing motive power”) |
| Water falling and water wheel | Caloric falling and heat engine |
| Water can be lifted/pumped (brought back to a high level) | Heat (caloric) can be pumped (brought back to high temperature) |
| Power is independent of medium falling | Power is independent of working fluid |
| Water pump | Heat pump |
| Impossibility of hydraulic perpetual motion machine | Impossibility of thermal perpetual motion machine |
| Force of Nature | Intensity | Extension | ||
|---|---|---|---|---|
| Fluids | Pressure | Volume | ||
| Electricity | Electric potential | Charge | ||
| Heat | Temperature | Entropy (Caloric) | ||
| Substances | Chemical potential | Amount of substance | ||
| Gravitation | Gravitational potential | Gravitational mass | ||
| Linear Motion | Velocity | Momentum | ||
| Rotation | Angular velocity | Angular momentum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, H.U.; Dumont, E.; Corni, F. Carnot and the Archetype of Waterfalls. Entropy 2024, 26, 1066. https://doi.org/10.3390/e26121066
Fuchs HU, Dumont E, Corni F. Carnot and the Archetype of Waterfalls. Entropy. 2024; 26(12):1066. https://doi.org/10.3390/e26121066
Chicago/Turabian StyleFuchs, Hans U., Elisabeth Dumont, and Federico Corni. 2024. "Carnot and the Archetype of Waterfalls" Entropy 26, no. 12: 1066. https://doi.org/10.3390/e26121066
APA StyleFuchs, H. U., Dumont, E., & Corni, F. (2024). Carnot and the Archetype of Waterfalls. Entropy, 26(12), 1066. https://doi.org/10.3390/e26121066








