Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review
Abstract
:1. Introduction

2. Experimental Investigations and 1D Calculations of Exergy and Energy
2.1. Modeling of Exergy and Energy Balance
2.2. Applications in Internal Combustion (IC) Engines
2.3. Applications in Gas Turbine and Power Plants
2.4. Intermediate Conclusions
3. Entropy Generation Analysis Using DNS
3.1. Governing Equations and DNS
3.2. Survey of Applications Related to DNS
4. Numerical-Modeling-Based 3D Investigations: RANS-Based Modeling Approaches
4.1. Governing Averaged Equations and Models
4.1.1. Reynolds Averaging-Based Approach
4.1.2. Assumed Probability Density-Based Approach
4.1.3. DNS Data-Based Approach
4.2. Survey of Applications Using the RANS Approach
5. Numerical-Modeling-Based 3D Investigations: LES-Based Modeling Approaches
5.1. Governing Filtered Equations and Subgrid Scale Models
5.1.1. Thermodynamics-Based Approach
5.1.2. Turbulence-Based Approach
5.1.3. Look-Up-Table-Based Approach
5.2. Survey of Applications Relying on LES
5.3. Near-Wall Challenges in EGA Using LES
6. EGA as an Optimization Tool
7. Conclusions
- Entropy generation modeling using DNS is still computationally expensive and difficult for detailed reaction mechanisms and complex reacting flow configurations.
- In the RANS context, the dissipative nature of the turbulence models as well as the inability to accurately predict the turbulence–chemistry interactions despite of the use of PDF methods, make the approaches used for entropy generation prediction not very accurate, and they can only be applied as a tool to quickly gain a general impression about the second law analysis of the investigated system.
- Investigations within the LES context emerge as promising since new approaches are introduced that are not computationally expensive while being accurate, particularly in representing turbulence–chemistry interactions.
- Different approaches suggested to quantify the entropy generations source terms in the different turbulence framework are still dealing with single phase turbulent flows. Entropy generated in turbulent multiphase flows or sprays, including phase change as well as other processes, such as breakup, collision, coalescence and turbulent dispersion, still need to be considered.
- Soret and Dufour effects are rarely included in entropy generation modeling approaches since, in most of hydrocarbon-air and hydrogen-air flames, these effects have an insignificant role.
- Most experimental investigations are performed in IC engines; however, the tendency is increasing in gas turbines and power plants. All these investigations are focused on simply calculating the exergy efficiency through an exergy balance of the system and observing the effects of certain operating parameters on the gained exergy.
- Experiments found in the literature demonstrated the limitations of experiment-based investigations, since the different entropy generation source terms during combustion and the contribution of each term cannot yet be retrieved by the existing measurement techniques.
- Numerical applications of entropy generation analysis using DNS are rarely found in the literature. Despite the fact that the existing simulations are performed using a single step reaction mechanism, which surely affected the accuracy of the quantitative prediction of the entropy generation analysis, the provided findings remain qualitatively valid, such as the great influence of the wall boundary conditions on entropy generation.
- RANS-based simulations as performed in the literature to quantify the entropy generation source terms allow the report of the major contributions of heat transfer and chemical reactions and to outline the effects of certain operating conditions on the exergy efficiency in IC engines. The presented applications were limited to simply use the exergy balance for computing the exergy efficiency without giving access to the different entropy source terms especially during combustion. Nevertheless, they made it possible to gain some insights into the relationship between the soot formation and entropy production. Such RANS-based investigations were performed under several assumptions for the sake of simplicity, which can affect the accuracy of the results.
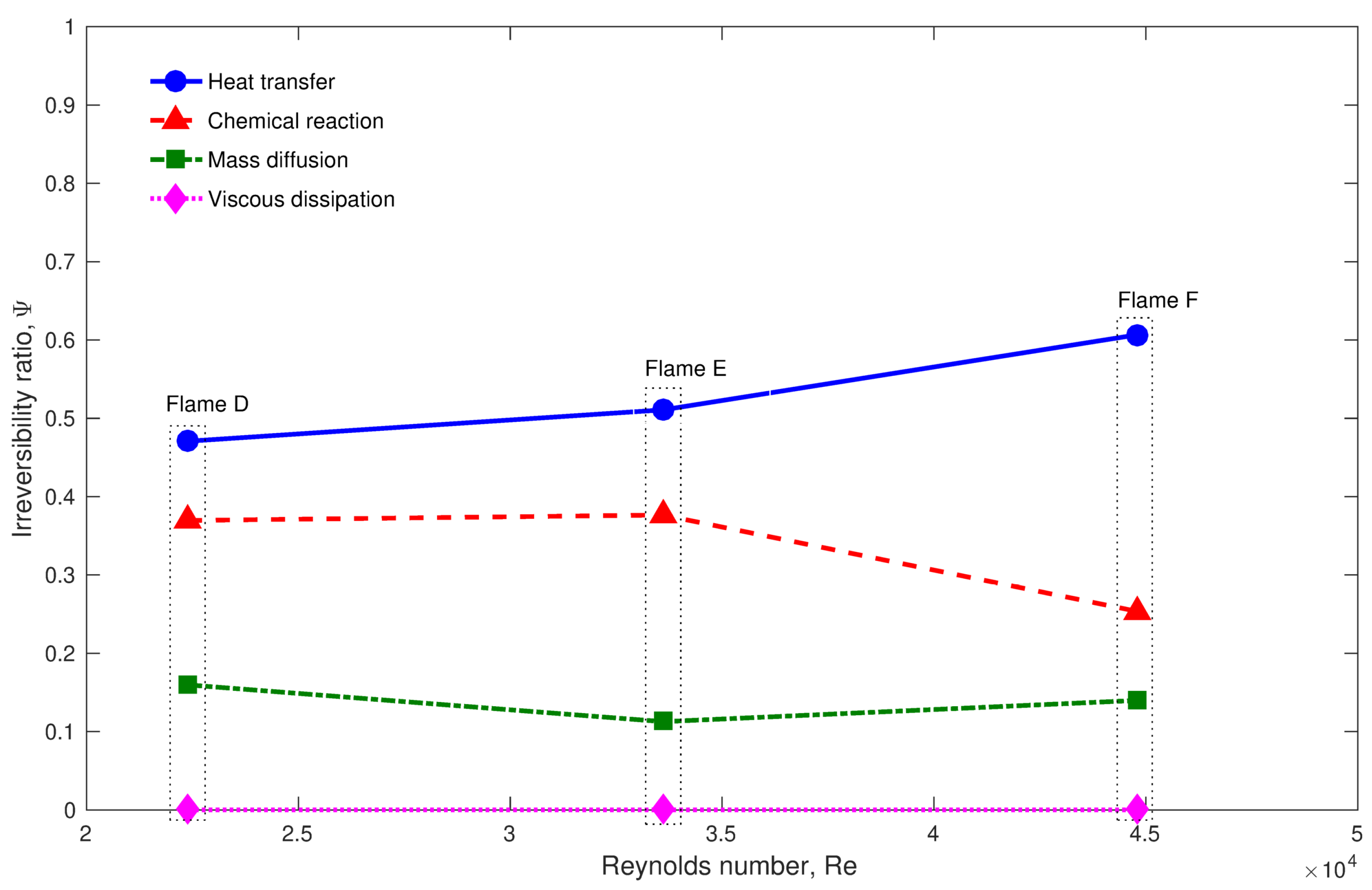
- Different approaches for entropy generation analysis as suggested in the LES context were tested only in Sandia and stratified flames. In both cases, the heat transfer is primarily responsible for entropy generation followed by the chemical reaction.
- The assessment of the new developed approaches shall be further performed in other combustion configurations and extended to complex or/and practical applications, such as multiphase reactive flows and IC engines. Moreover, effects, including non-adiabaticity, differential diffusion, phase change, particle turbulent dispersion, collision, coalescence and breakup, shall be integrated in order to result in an efficient, accurate and inexpensive tool for second law analysis.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| List of symbols | List of greek letter | ||
| Ex | Exergy | Angular speed | |
| T | Temperature | Equivalence ratio | |
| Cp | Specific heat capacity | Density | |
| LHV | Lower heat value | Thermal conductivity | |
| Tr | Engine torque | Viscous stress | |
| P | Pressure | Dynamic viscosity | |
| V | Volume | Stoichiometric coefficients | |
| Q | Heat flux rate | Dimensionless number (=1) | |
| m | Mass flow | Specie | |
| R | Gas constant | Entropy source term | |
| Y | Mass fraction | Reaction source term | |
| u | Velocity | flow variable | |
| t | time | Dissipation rate | |
| F | Body forces | Mass fraction variance | |
| e | Energy | Temperature variance | |
| h | Specific enthalpy | Scalar dissipation | |
| J | Diffusive mass flux | Ratio of specific heats | |
| Ns | Number of species | Quantity of unresolvedness | |
| M | Molecular mass | Irreversibility ratio | |
| r | Molar net rate production | Kinematic viscosity | |
| I | Identity tensor | Kronecker delta | |
| S | Source term | Grid filter width | |
| c | Molar concentration | Crank angle | |
| K | Rate constant of chemical reaction | List of subscripts | |
| Ea | Activation energy | in | Input |
| A | Arrhenius pre-exponential | dest | Destruction |
| s | entropy | 0 | Ambiant |
| D | Diffusion coefficient | ch | Chemical |
| Le | Lewis number | ph | Physical |
| X | Mole fraction | gen | Generation |
| x | spatial coordinate | r | Reaction |
| k | Turbulent kinetic energy | m | Mixture |
| Z | Mixture fraction | i, j | Coordinate directions |
| Ma | Mach number | q | Heat |
| Laminar burning velocity | vs. | Viscous | |
| f | Probability density function | d | Diffusion |
| Sc | Schmidt number | L | Local |
| g | free Gibbs energy | c | Progress variable |
| Pr | Prandtl number | t | Turbulent |
| List of superscripts | sgs | Sub-grid scale | |
| res | Resolved | ||
Appendix A
| Application | Components with Reacting Flow | 1D | RANS | LES | DNS | Entropy Source Terms Calculation Method | Exp. |
|---|---|---|---|---|---|---|---|
| Power plant | Combustion Chamber | [57,58,59,60,61,63,65,67,68] | - | - | - | Skeletal | [57,58,68] |
| Stationary gas turbine | Combustion Chamber | [71,72,73] | - | - | - | Skeletal | - |
| Aircraft gas turbine | Combustion Chamber | [74,75,76,77,78,79] | - | - | - | Skeletal | - |
| IC engine | Combustion Chamber | - | [118,119,120,121,122] | - | - | Skeletal | [37,38,39,40,41,42,43,44,45,46] |
| Jet flame | non premixed | - | [21,22,111,113,115] | [32,33,138] | - | PDF/tabulated/turbulence/DNS data/thermodynamic | - |
| premixed | [109] | - | [139] | [20,27,108] | tabulated/turbulence/DNS data | - | |
| Dilution H2 | - | [21] | - | - | turbulence | - |
References
- Bejan, A. Fundamentals of exergy analysis, entropy generation minimization, and the generation of flow architecture. Int. J. Energy Res. 2002, 26, 545–565. [Google Scholar] [CrossRef]
- Nishida, K.; Takagi, T.; Kinoshita, S. Analysis of entropy generation and exergy loss during combustion. Proc. Comb. Inst 2002, 29, 869–874. [Google Scholar] [CrossRef]
- Keenan, J. Availability and irreversibility in thermodynamics. Brit. J. Appl. Phys. 1951, 2, 183–192. [Google Scholar] [CrossRef]
- Som, S.K.; Datta, A. Thermodynamic irreversibilities and exergy balance in combustion processes. Prog. Energy Combust. 2008, 34, 351–376. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V.; Sciubba, E. Entropy generation analysis as a design tool—A review. Renew. Sustain. Energy Rev. 2015, 43, 1167–1181. [Google Scholar] [CrossRef]
- Bejan, A. Second law analysis in heat transfer. Energy 1980, 5, 720–732. [Google Scholar] [CrossRef]
- Bejan, A. Method of entropy generation minimization, or modeling and optimization based on combined heat transfer and thermodynamics. Rev. Gen. Therm. 1996, 35, 637–646. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Generation Minimization:The Method of Thermodynamic Optimization of Finite-Size Systems and Finite-Time Processes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Sahin, A.Z.; Ben-Mansour, R. Entropy generation in laminar fluid flow through circular pipe. Entropy 2003, 5, 404–416. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, J.; Li, B.; Sunden, B. Entropy generation analysis of fully developed turbulent heat transfer flow in inward helically corrugated tubes. Numer. Heat Transf. A-Appl. 2018, 37, 788–805. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, J.A.; Shahabi, P.B. Effect of non-uniform heating on entropy generation for laminar developing pipe flow of a high prandtl number fluid. Energy Convers. Manag. 2010, 42, 2087–2097. [Google Scholar] [CrossRef]
- Saqr, K.M.; Shehata, A.I.; Taha, A.A.; El-Azm, M.A. Cfd modelling of entropy generation in turbulent pipe flow: Effects of temperature difference and swirl intensity. Appl. Therm. Eng. 2016, 100, 999–1006. [Google Scholar] [CrossRef]
- Kock, F.; Herwig, H. Local entropy production in turbulent shear flows: A high-Reynolds number model with wall functions. Int. J. Heat Mass Transf. 2004, 47, 2205–2215. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.C.; Yang, X.; Shi, L. Entropy generation analysis and performance evaluation of turbulent forced convective heat transfer to nanofluids. Entropy 2017, 19, 108. [Google Scholar] [CrossRef] [Green Version]
- Ries, F.; Janicka, J.; Sadiki, A. Thermal transport and entropy production mechanisms in a turbulent round jet at supercritical thermodynamic conditions. Entropy 2017, 19, 404. [Google Scholar] [CrossRef] [Green Version]
- Okong’o, N.A.; Bellan, J. Direct numerical simulations of transitional supercritical binary mixing layers: Heptane and nitrogen. Int. J. Fluid Mech. 2002, 464, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Mohensi, M.; Bazargan, M. Entropy generation in turbulent mixed convection heat transfer to highly variable property pipe flow of supercritical fluids. Energy Convers. Manag. 2014, 87, 552–558. [Google Scholar]
- Datta, A. Effects of gravity on structure and entropy generation of confined laminar diffusion flames. Int. J. Therm. Sci. 2005, 44, 429–440. [Google Scholar] [CrossRef]
- Yapici, H.; KayataŞ, N.; Albayrak, B.; BaŞtrük, G. Numerical calculation of local entropy generation in a methane-air burner. Energy Convers. Manag. 2005, 46, 1885–1919. [Google Scholar] [CrossRef]
- Farran, R.; Chakraborty, N. A direct numerical simulation-based analysis of entropy generation in turbulent premixed flames. Entropy 2013, 15, 1540–1566. [Google Scholar] [CrossRef]
- Emadi, A.; Emami, M.D. Analysis of entropy generation in a hydrogen-enriched turbulent non-premixed flame. Int. J. Hydrogen Energy 2013, 38, 5961–5973. [Google Scholar] [CrossRef]
- Khadidja, S.; Ahmed, O.; Fouzi, T. Entropy generation in turbulent syngas counter-flow diffusion flames. Int. J. Hydrogen Energy 2017, 42, 29532–29544. [Google Scholar]
- Ries, F.; Li, Y.; Klingenberg, D.; Nishad, K.; Janicka, J.; Sadiki, A. Near-wall thermal processes in an inclined impinging jet: Analysis of heat transport and entropy generation mechanisms. Energies 2018, 11, 1354. [Google Scholar] [CrossRef] [Green Version]
- Shuja, S.Z.; Yibas, B.S.; Budair, M.O. Local entropy generation in an impinging jet: Minimum entropy concept evaluating various turbulence models. Comput. Method. Appl. M 2001, 190, 3623–3644. [Google Scholar] [CrossRef]
- Drost, M.K.; White, M.D. Numerical predictions of local entropy generation in an impinging jet. J. Heat Transf. 1991, 113, 823–829. [Google Scholar] [CrossRef]
- Oztop, H.F.; Al-Salem, K. A review on entropy generation in natural and mixed convection heat transfer for energy systems. Renew. Sustain. Energy Rev. 2011, 16, 911–920. [Google Scholar] [CrossRef]
- Chakraborty, N. Modeling of Entropy Generation in Turbulent Premixed Flames for Reynolds Averaged Navier–Stokes Simulations: A Direct Numerical Simulation Analysis. ASME J. Energy Resour. Technol. 2015, 137, 32201. [Google Scholar] [CrossRef]
- Herwig, H.; Kock, F. Local entropy production in turbulent shear flows: A tool for evaluating heat transfer performance. J. Therm. Sci. 2006, 15, 2205–2215. [Google Scholar] [CrossRef]
- Schmandt, B.; Herwig, H. Diffusor and nozzle design optimization by entropy generation minimization. Entropy 2011, 13, 1380–1402. [Google Scholar] [CrossRef]
- Ghisu, T.; Cambuli, F.; Puddu, P.; Mandas, N.; Seshadri, P.; Parks, G.T. Numerical evaluation of entropy generation in isolated airfoils and wells turbines. Meccanica 2018, 56, 3437–3456. [Google Scholar] [CrossRef] [Green Version]
- Ries, F.; Li, Y.; Nishad, K.; Janicka, J.; Sadiki, A. Entropy generation analysis and thermodynamic optimization of jet impingement cooling using large eddy simulation. Entropy 2019, 19, 129. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.; Hadi, F.; Reza, M.; Sheikhi, H. Progress in the prediction of entropy generation in turbulent reacting flows using large eddy simulation. Entropy 2014, 16, 5159–5177. [Google Scholar] [CrossRef] [Green Version]
- Agrebi, S.; Dreßler, L.; Nicolai, H.; Ries, F.; Nishad, K.; Sadiki, A. Analysis of Local Exergy Losses in Combustion Systems Using a Hybrid Filtered Eulerian Stochastic Field Coupled with Detailed Chemistry Tabulation: Cases of Flames D and E. Energies 2021, 14, 6315. [Google Scholar] [CrossRef]
- Ream, A.E.; Slattery, J.C.; Cizmas, P.G.A. The second law of thermodynamics and the numerical simulation of transport phenomena with chemical reactions. In Proceedings of the 10th International Conference on Computational Fluid Dynamics (ICCFD10), Barcelona, Spain, 9–13 July 2018. [Google Scholar]
- Web of Science. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 5 August 2022).
- el Hossaini, M.K. Review of the New Combustion Technologies in Modern Gas Turbines. In Progress in Gas Turbine Performance; Benini, E., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Canakci, M.; Hosoz, M. Energy and exergy analyses of a diesel engine fuelled with various biodiesels. Energy Sources Part B Econ. Plan. Policy 2006, 1, 379–394. [Google Scholar] [CrossRef]
- Ghazikhani, M.; Feyz, M.E.; Joharchi, A. Experimental investigation of the exhaust gas recirculation effects on irreversibility and brake specific fuel consumption of indirect injection diesel engines. Appl. Therm. Eng. 2010, 30, 1711–1718. [Google Scholar] [CrossRef]
- Sekmen, P.; Yılbaşı, Z. Application of energy and exergy analyses to a CI engine using biodiesel fuel. Math. Comput. Appl. 2011, 16, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Mohammadkhani, F.; Khalilarya, S.; Mirzaee, I. Energetic and exergetic analysis of internal combustion engine cogeneration system. J. Energy Eng. Manag. 2012, 2, 24–31. [Google Scholar]
- Panigrahi, N.; KumarMohanty, M.; RanjanMishra, S.; Mohanty, R.C. Performance, emission, energy, and exergy analysis of a C.I. engine using Mahua Biodiesel Blends with Diesel. Int. Sch. Res. Not. 2014, 2014, 207465. [Google Scholar] [CrossRef]
- Özkan, M. A comparative study on energy and exergy analyses of a CI engine performed with different multiple injection strategies at part load: Effect of injection pressure. Entropy 2015, 17, 244–263. [Google Scholar] [CrossRef]
- Meisami, F.; Ajam, H. Energy, exergy and economic analysis of a Diesel engine fueled with castor oil biodiesel. Int. J. Engine Res. 2015, 16, 691–702. [Google Scholar] [CrossRef]
- Sayin Kul, B.; Kahraman, A. Energy and Exergy Analyses of a Diesel Engine Fuelled with Biodiesel-Diesel Blends Containing 5% Bioethanol. Entropy 2016, 18, 387. [Google Scholar] [CrossRef]
- Kamboj, S.K.; Karimi, M.N. Exergy analysis of internal combustion engine based cooling cycle. Int. Therm. Environ. Eng. 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Jannatkhah, J.; Najafi, B.; Ghaebi, H. Energy-exergy analysis of compression ignition engine running with biodiesel fuel extracted from four different oil-basis materials. Int. J. Green Energy 2019, 16, 749–762. [Google Scholar] [CrossRef]
- Teng, H.; Kinoshita, C.M.; Masutani, S.M.; Zhou, J. Entropy generation in multicomponent reacting flows. J. Energy Resour.-ASME 1998, 120, 226–232. [Google Scholar] [CrossRef]
- Datta, A.; Som, S.K. Energy and exergy balance in a gas turbine combustor. Proc. Inst. Mech. Eng. 1999, 213, 23–32. [Google Scholar] [CrossRef]
- Sanli, B.G.; Özcanli, M.; Serin, H. Assessment of thermodynamic performance of an IC engine using microalgae biodiesel at various ambient temperatures. Fuel 2020, 277, 118108. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Dabi, M.; Saha, U.K. A Compendium of Methods for Determining the Exergy Balance Terms Applied to Reciprocating Internal Combustion Engines. J. Energy Resour. Technol. 2021, 143, 120801. [Google Scholar] [CrossRef]
- Dimitrova, Z.; Marechal, F. Energy Integration on Multi-periods for Vehicle Thermal Powertrains. Can. J. Chem. Eng. 2017, 95, 235–264. [Google Scholar] [CrossRef]
- Khaliq, A.; Trivedi, S.K. Second Law Assessment of a Wet Ethanol Fuelled HCCI Engine Combined with Organic Rankine Cycle. ASME J. Energy Resour. Technol. 2012, 134, 22201. [Google Scholar] [CrossRef]
- Salek, F.; Babaie, M.; Ghodsi, A.; Hosseini, S.V.; Zare, A. Energy and Exergy Analysis of a Novel Turbo-compounding System for Supercharging and Mild Hybridization of a Gasoline Engine. J. Therm. Anal. Calorim. 2020, 145, 817–828. [Google Scholar] [CrossRef]
- Ntziachristos, L.; Samaras, Z.; Zervas, E.; Dorlhene, P. Effects of a Catalysed and an Additized Partied Filter on the Emissions of a Diesel Passenger Car Operating on Low Sulphur Fuels. Atmos. Environ. 2005, 39, 4925–4936. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Giakoumis, E.G. Second-law analyses applied to internal combustion engines operation. Prog. Energy Combust. Sci. 2006, 32, 2–47. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gakkhar, R.P. Application of Exergy Analysis to Internal Combustion Engine: A Review. J. Aeronaut. Automot. Eng. (JAAE) 2015, 2, 101–106. [Google Scholar]
- Ibrahim, T.K.; Basrawi, F.; Awad, O.I.; Abdullah, A.N.; Najafi, G.; Mamat, R.; Hagos, F.Y. Thermal performance of gas turbine power plant based on exergy analysis. Appl. Therm. Eng. 2017, 115, 977–985. [Google Scholar] [CrossRef]
- Haouam, A.; Derbal, C.; Mzad, H. Thermal performance of a gas turbine based on an exergy analysis. In Proceedings of the 12th International Conference on Computational Heat, Mass and Momentum Transfer (ICCHMT), Rome, Italy, 3–6 September 2019. [Google Scholar]
- Omar, J.K.; Thamir, K.I.; Firas, B.I.; Ahmed, T.A.; Saiful, H.B.A.H. Modeling and analysis of optimal performance of a coal-fired power plant based on exergy evaluation. Energy Rep. 2022, 8, 2179–2199. [Google Scholar]
- Haseli, Y.; Hornbostel, K. Demonstration of an inverse relationship between thermal efficiency and specific entropy generation for combustion systems. J. Energy Resour. Technol. 2018, 141, 14501. [Google Scholar] [CrossRef]
- Alberto, F.; Samiran, S.; Rosaria, V. Exergetic analysis of a natural gas combined-cycle power plant with a molten carbonate fuel cell for carbon capture. Sustainability 2022, 14, 533. [Google Scholar] [CrossRef]
- Ismail, N.C.; Abdullah, M.Z.; Mustafa, K.F.; Mazlan, N.M.; Gunnasegaran, P.; Irawan, A.P. Double-Layer Micro Porous Media Burner from Lean to Rich Fuel Mixture: Analysis of Entropy Generation and Exergy Efficiency. Entropy 2021, 23, 1663. [Google Scholar] [CrossRef]
- Temraz, A.; Rashad, A.; Elweteedy, A.; Alobaid, F.; Epple, B. Energy and Exergy Analyses of an Existing Solar-Assisted Combined Cycle Power Plant. Appl. Sci. 2020, 10, 4980. [Google Scholar] [CrossRef]
- Alobaid, F.; Ströhle, J. Thermochemical Conversion Processes for Solid Fuels and Renewable Energies. Appl. Sci. 2021, 11, 1907. [Google Scholar] [CrossRef]
- Temraz, A.; Alobaid, F.; Link, J.; Elweteedy, A.; Epple, B. Development and Validation of a Dynamic Simulation Model for an Integrated Solar Combined Cycle Power Plant. Energies 2021, 14, 3304. [Google Scholar] [CrossRef]
- Aljundi, I.H. Energy and exergy analysis of a steam power plant in Jordan. Appl. Therm. Eng. 2009, 29, 324–328. [Google Scholar] [CrossRef]
- Vuckovic, G.D.; Stojiljkovic, M.M.; Vukic, M.V.; Stefanovic, G.M.; Dedeic, E.M. Advanced Exergy Analysis Furthermore, Exergoeconomic Performance Evaluation Of Thermal Processes In An Existing Industrial Plant. Energy Convers. Manag. 2014, 85, 655–662. [Google Scholar] [CrossRef]
- Dang, R.; Mangal, S.K.; Suman, G. Energy and Exergy Analysis of Thermal Power Plant at Design and Off Design Load. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 29. [Google Scholar]
- Kumar, U.; Pal, M.K.; Bahirat, S. A review of exergy and energy analysis of coal based combined thermal power plant. Int. J. Adv. Manag. Technol. Eng. Sci. 2018, 8, 1525–1534. [Google Scholar]
- Hamayun, M.H.; Hussain, M.; Shafiq, I.; Ahmed, A.; Park, Y.K. Investigation of the thermodynamic performance of an existing steam power plant via energy and exergy analyses to restrain the environmental repercussions: A simulation study. Environ. Eng. Res. 2021, 27. [Google Scholar] [CrossRef]
- Peng, P.; Kirtipal, B.; Andres, J.G.; Junior, N. Waste heat recovery in CO2 compression. Int. J. Greenh. Gas Control. 2014, 30, 86–96. [Google Scholar]
- Guillermo, V.O.; Carlos, A.P.; Jhan, P.R. Thermoeconomic modelling and parametric study of a simple ORC for the recovery of waste heat in a 2 MW gas engine under different working fluids. Appl. Sci. 2019, 9, 4526. [Google Scholar] [CrossRef] [Green Version]
- Samira, M.M.; Rahim, K.S.; Mohammad, N. Comparison of different optimized heat driven power-cycle configurations of a gas engine. Appl. Therm. Eng. 2020, 179, 115768. [Google Scholar]
- Ramazan, A.; Önder, T.; Önder, A.; Hakan, A.; Kateryna, S. Environmental impact assessment of a turboprop engine with the aid of exergy. Energy 2013, 58, 664–671. [Google Scholar]
- Onder, T.; Hakan, A. Exergetic and exergo-economic analyses of an aero-derivative gas turbine engine. Energy 2014, 74, 638–650. [Google Scholar]
- Yasin, S.; Emin, A.; Arif, H.; Hikmet, K.T. Advanced exergy analysis of an aircraft gas turbine engine: Splitting exergy destructions into parts. Energy 2015, 90, 1219–1228. [Google Scholar]
- Fernandes, A.; Woudstra, T.; Aravind, P.V. System simulation and exergy analysis on the use of biomass-derived liquid-hydrogen for SOFC/GT powered aircraft. Int. J. Hydrogen Energy 2015, 40, 4683–4697. [Google Scholar] [CrossRef]
- Ramazan, A.; Onder, T.; Hakan, A. Dynamic exergo-environmental analysis of a turboprop aircraft engine at various torques. Energy 2019, 186, 115894. [Google Scholar]
- Ramazan, A.; Onder, T. Economy and exergy of aircraft turboprop engine at dynamic loads. Energy 2020, 213. [Google Scholar]
- Mahmud, S.; Fraser, R.A. The second law analysis in fundamental convective heat transfer problems. Int. J. Therm. Sci. 2003, 42, 177–186. [Google Scholar] [CrossRef]
- Mukherjee, P.; Biswas, G.; Nag, P.K. Second-law analysis of heat transfer in swirling flow through a cylindrical duct. J. Heat Transf. 1987, 109, 308–313. [Google Scholar] [CrossRef]
- Sahin, A.Z. Second law analysis of laminar viscous flow through a duct subjected to constant wall temperature. J. Heat Transf. 1998, 120, 76–83. [Google Scholar] [CrossRef]
- Sahin, A.Z. A second law comparison for optimum shape of duct subjected to constant wall temperature and laminar flow. J. Heat Transf. 1998, 33, 425–430. [Google Scholar]
- Zimmermann, S.; Tiwari, M.K.; Meijer, I.; Paredes, S.; Michel, B.; Poulikakos, D. Hot water cooled electronics: Exergy analysis and waste heat reuse feasibility. Int. J. Heat Mass Transf. 2012, 55, 6391–6399. [Google Scholar] [CrossRef]
- Poulikakos, D.; Johnson, J.M. Second law analysis of combined heat and mass transfer phenomena in external flow. Energy 1989, 14, 67–73. [Google Scholar] [CrossRef]
- San, J.Y.; Worek, W.M.; Lavan, Z. Entropy generation in combined heat and mass transfer. Int. J. Heat Mass Transf. 1987, 30, 1359–1369. [Google Scholar] [CrossRef]
- Ben Nejma, F.; Mazgar, A.; Abdallah, N.; Charrada, K. Entropy generation through combined non-grey gas radiation and forced convection between two parallel plates. Energy 2008, 33, 1169–1178. [Google Scholar] [CrossRef]
- Mahmud, S.; Fraser, R.A. Mixed convection-radiation interaction in a vertical porous channel: Entropy generation. Energy 2003, 28, 1557–1577. [Google Scholar] [CrossRef]
- Hotz, N.; Lee, M.T.; Grigoropoulos, C.P.; Senn, S.M.; Poulikakos, D. Exergetic analysis of fuel cell micropowerplants fed by methanol. Int. J. Heat Mass Transf. 2006, 49, 2397–2411. [Google Scholar] [CrossRef]
- Hotz, N.; Senn, S.M.; Poulikakos, D. Exergy analysis of a solid oxide fuel cell micropowerplant. J. Power Sources 2006, 158, 333–347. [Google Scholar] [CrossRef]
- Haworth, D.C.; Poinsot, T.J. Numerical simulations of Lewis number effects in turbulent premixed flames. J. Fluid. Mech. 1992, 244, 405–436. [Google Scholar] [CrossRef]
- Rutland, C.J.; Trouvé, A. Direct simulations of premixed turbulent flames with non-unity Lewis numbers. Combust. Flame 1993, 94, 41–57. [Google Scholar] [CrossRef]
- Chakraborty, N.; Cant, R.S. Influence of Lewis Number on curvature effects in turbulent premixed flame propagation in the thin reaction zones regime. Phys. Fluids 2005, 17, 105105. [Google Scholar] [CrossRef]
- Chakraborty, N.; Cant, R.S. Influence of Lewis number on strain rate effects in turbulent premixed flame propagation in the thin reaction zones regime. Int. J. Heat Mass Trans. 2006, 49, 2158–2172. [Google Scholar] [CrossRef]
- Yuan, J.; Ju, Y.; Law, C.K. Coupled hydrodynamic and diffusional-thermal instabilities in flame propagation at small Lewis numbers. Phys. Fluids 2005, 17, 74106. [Google Scholar] [CrossRef]
- Chakraborty, N.; Klein, M. Influence of Lewis number on the surface density function transport in the thin reaction zones regime for turbulent premixed flames. Phys. Fluids 2008, 20, 65102. [Google Scholar] [CrossRef]
- Chakraborty, N.; Klein, M.; Swaminathan, N. Effects of Lewis number on reactive scalar gradient alignment with local strain rate in turbulent premixed flames. Proc. Combust. Inst. 2009, 32, 1409–1416. [Google Scholar] [CrossRef]
- Chakraborty, N.; Katragadda, M.; Cant, R.S. Effects of Lewis number on turbulent kinetic energy transport in turbulent premixed combustion. Phys. Fluids 2011, 23, 75109. [Google Scholar] [CrossRef]
- Veynante, D.; Trouvé, A.; Bray, K.N.C.; Mantel, T. Gradient and counter-gradient turbulent scalar transport in turbulent premixed flames. J. Fluid Mech. 1997, 332, 263–293. [Google Scholar] [CrossRef]
- Boger, M.; Veynante, D.; Boughanem, H.; Trouvé, A. Direct Numerical Simulation analysis of flame surface density concept for Large Eddy Simulation of turbulent premixed combustion. Proc. Combust. Inst. 1998, 27, 917–925. [Google Scholar] [CrossRef]
- Charlette, F.; Meneveau, C.; Veynante, D. A power-law flame wrinkling model for LES of premixed turbulent combustion. Part I: Nondynamic formulation and initial tests. Combust. Flame 2002, 131, 159–180. [Google Scholar] [CrossRef]
- Swaminathan, N.; Bray, K.N.C. Effect of dilatation on scalar dissipation in turbulent premixed flames. Combust. Flame 2005, 143, 549–565. [Google Scholar] [CrossRef]
- Swaminathan, N.; Grout, R. Interaction of turbulence and scalar fields in premixed flames. Phys. Fluids 2006, 18, 45102. [Google Scholar] [CrossRef]
- Grout, R. An age extended progress variable for conditioned reaction rates. Phys. Fluids 2007, 19, 105107. [Google Scholar] [CrossRef]
- Han, I.; Huh, K.H. Roles of displacement speed on evolution of flame surface density for different turbulent intensities and Lewis numbers for turbulent premixed combustion. Combust. Flame 2008, 152, 194–205. [Google Scholar] [CrossRef]
- Han, I.; Huh, K.H. Effects of Karlovitz number on the evolution of the flame surface density in turbulent premixed flames. Proc. Combust. Inst. 2009, 32, 1419–1425. [Google Scholar] [CrossRef]
- Im, H.G.; Chen, J.H. Preferential diffusion effects on the burning rate of interacting turbulent premixed Hydrogen-Air flames. Combust. Flame 2002, 131, 246–258. [Google Scholar] [CrossRef]
- Ghai, S.K.; Ahmed, U.; Chakraborty, N.; Klein, M. Entropy Generation during Head-On Interaction of Premixed Flames with Inert Walls within Turbulent Boundary Layers. Entropy 2022, 24, 463. [Google Scholar] [CrossRef] [PubMed]
- Salimath, P.S.; Ertesvåg, I.S. Local entropy generation and entropy fluxes of a transient flame during head-on quenching towards solid and hydrogen-permeable porous walls. Int. J. Hydrogen Energy 2021, 46, 26616–26630. [Google Scholar] [CrossRef]
- Sierra-Pallares, J.; del Valle, J.G.; García-Carrascal, P.; Ruiz, F.C. Numerical study of supercritical and transcritical injection using different turbulent Prandtl numbers: A second law analysis. J. Supercrit. Fluid 2016, 115, 86–98. [Google Scholar] [CrossRef]
- Stanciu, D.; Isvoranu, D.; Marinescu, M. Second Law Analysis of Diffusion Flames. Int. J. Appl. Thermodyn. 2001, 4, 1–18. [Google Scholar]
- Som, S.K.; Agrawal, G.K.; Chakraborty, S. Thermodynamics of flame impingement heat transfer. J. Appl. Phys. 2007, 102, 43506. [Google Scholar] [CrossRef]
- Tehrani, F.B.; Abedinejad, M.S.; Mohammadi, M. Analysis of relationship between entropy generation and soot formation in turbulent Kerosene/Air jet diffusion flames. Energy Fuels 2019, 33, 9184–9195. [Google Scholar] [CrossRef]
- Burke, S.P.; Schumann, T.E.W. Diffusion flames. Ind. Eng. Chem. 1928, 20, 998–1004. [Google Scholar] [CrossRef]
- Nadim, P. Irreversibility of Combustion, Heat and Mass Transfer, Master of Science in Energy and Environment. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, June 2011. [Google Scholar]
- Magnusen, B.F.; Hjertager, B.H. On the Mathematical Model of Turbulent Combustion, with Special Emphasis on Soot Formation and Combustion. In Proceedings of the 16th Symposium On Combustion (The Combustion Institute), Cambridge, MA, USA, 15–20 August 1976. [Google Scholar]
- Hall, R.; Smooke, M.; Colket, M. Physical and Chemical Aspects of Combustion: A Tribute to Irvine Glassman; Dryer, F.L., Sawyer, R.F., Eds.; Gordon and Breach: Philadelphia, PA, USA, 1997; p. 189. [Google Scholar]
- Jafarmadar, S. Three-dimensional modeling and exergy analysis in Combustion Chambers of an indirect injection diesel engine. Fuel 2013, 107, 439–447. [Google Scholar] [CrossRef]
- Jafarmadar, S. Exergy analysis of hydrogen/diesel combustion in a dual fuel engine using three-dimensional model. Int. J. Hydrogen Energy 2014, 39, 9505–9514. [Google Scholar] [CrossRef]
- Abbasi, A.; Emamverdi, O. Influence of nozzle hole diameter on the first and second law balance in direct injection diesel engine. Journel Power Technol. 2014, 94, 20–33. [Google Scholar]
- Hamdi, F.; Agrebi, S.; Idrissi, M.S.; Mondo, K.; Labiadh, Z.; Sadiki, A.; Chrigui, M. Impact of Spray Cone Angle on the Performances of Methane/Diesel RCCI Engine Combustion under Low Load Operating Conditions. Entropy 2022, 24, 650. [Google Scholar] [CrossRef] [PubMed]
- Mondo, K.; Agrebi, S.; Hamdi, F.; Lakhal, F.; Sadiki, A.; Chrigui, M. Impact of Multi-Component Surrogates on the Performances, Pollutants, and Exergy of IC Engines. Entropy 2022, 24, 671. [Google Scholar] [CrossRef]
- Slimi, K.; Saati, A.A. Entropy generation rate due to radiative transfer within a vertical channel filled with a semi-transparent porous medium. Arab. J. Sci. Eng. 2012, 37, 803–820. [Google Scholar] [CrossRef]
- Caldas, M.; Semiao, V. Entropy generation through radiative transfer in participating media: Analysis and numerical computation. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 423–437. [Google Scholar] [CrossRef]
- Mahmud, S.; Fraser, R.A. Analysis of mixed convection—Radiation interaction in a vertical channel: Entropy generation. Exergy Int. J. 2002, 2, 330–339. [Google Scholar] [CrossRef]
- Pope, S. PDF methods for turbulent reactive flows. Prog. Energy Combust. Sci. 1985, 11, 119–192. [Google Scholar] [CrossRef]
- Pope, S.B. A monte carlo method for the PDF equations of turbulent reactive flow. Combust. Sci. Technol. 1981, 25, 159–174. [Google Scholar] [CrossRef]
- Safari, M.; Sheikhi, M.R.H.; Janbozorgi, M.; Metghalchi, H.; Sheikhi, R.H. Entropy transport equation in large eddy simulation for exergy analysis of turbulent combustion systems. Entropy 2010, 12, 434. [Google Scholar] [CrossRef]
- Safari, M.; Sheikhi, M.R.H. Large eddy simulation for prediction of entropy generation in a non-premixed turbulent jet flame. J. Energy Resour. Technol. 2014, 136, 22002. [Google Scholar] [CrossRef]
- Sheikhi, M.R.H.; Safari, M.; Metghalchi, H. Large Eddy simulation for local entropy generation analysis of turbulent flows. J. Energy Resour. Technol. 2012, 134, 41603. [Google Scholar] [CrossRef]
- Sheikhi, M.R.H.; Givi, P.; Pope, S.B. Frequency-velocity-scalar filtered mass density function for large eddy simulation of turbulent flows. Phys. Fluids 2009, 21, 75102. [Google Scholar] [CrossRef] [Green Version]
- Sheikhi, M.R.H.; Givi, P.; Pope, S.B. Velocity-scalar filtered mass density function for large eddy simulation of turbulent reacting flows. Phys. Fluids 2007, 19, 95106. [Google Scholar] [CrossRef]
- Sheikhi, M.R.H.; Drozda, T.G.; Givi, P.; Pope, S.B. Velocity-scalar filtered density function for large eddy simulation of turbulent flows. Phys. Fluids 2003, 15, 2321. [Google Scholar] [CrossRef] [Green Version]
- Lilly, D. The representation of small-scale turbulence in numerical simulation experiments. IBM Form 1967, 281, 95–210. [Google Scholar]
- Schmidt, H.; Schumann, U. Coherent structure of the convective boundary layer derived from large-eddy simulations. J. Fluid Mech. 1989, 200, 511–562. [Google Scholar] [CrossRef] [Green Version]
- Corrsin, S. On the spectrum of isotropic temperature fluctuations in an isotropic turbulence. J. Appl. Phys. 1951, 22, 469–473. [Google Scholar] [CrossRef]
- TNF Workshop. Available online: http://www.ca.sandia.gov/TNF (accessed on 28 June 2021).
- Agrebi, S.; Dreßler, L.; Nishad, K. The Exergy Losses Analysis in Adiabatic Combustion Systems including the Exhaust Gas Exergy. Entropy 2022, 24, 564. [Google Scholar] [CrossRef]
- Dressler, L.; Nicolai, H.; Agrebi, S.; Ries, F.; Sadiki, A. Computation of Entropy Production in Stratified Flames Based on Chemistry Tabulation and an Eulerian Transported Probability Density Function Approach. Entropy 2022, 24, 615. [Google Scholar] [CrossRef]
- Piomelli, U. Wall-layer models for large-eddy simulations. Prog. Aerosp. Sci. 2008, 44, 437–446. [Google Scholar] [CrossRef]
- Li, Y.; Ries, F.; Leudesdorff, W.; Nishad, K.; Pati, A.; Hasse, C.; Janicka, J.; Jakirlic, S.; Sadiki, A. Non-equilibrium wall functions for large eddy simulations of complex turbulent flows and heat transfer. Int. J. Heat Fluid Flow 2021, 88. [Google Scholar] [CrossRef]
- Li, Y.; Ries, F.; Nishad, K.; Sadiki, A. Predictions of Conjugate Heat Transfer in Turbulent Channel Flow Using Advanced Wall-Modeled Large Eddy Simulation Techniques. Entropy 2021, 23, 725. [Google Scholar] [CrossRef]
- Ordonez, J.C.; Cavalcanti, E.J.C.; Carvalho, M. Energy, exergy, entropy generation minimization, and exergoenvironmental analyses of energy systems—A mini-review. Front. Sustain. 2022, 3, 902071. [Google Scholar] [CrossRef]
- Kleidon, A.; Malhi, Y.; Cox, P.M. Maximum entropy production inenvironmental and ecological systems. Phil. Trans. R. Soc. B 2010, 365, 1297–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmandi, H.R.; Amani, E. A numerical investigation of the entropy generation in and thermodynamic optimization of a combustion chamber. Energy 2015, 81, 706–718. [Google Scholar] [CrossRef]

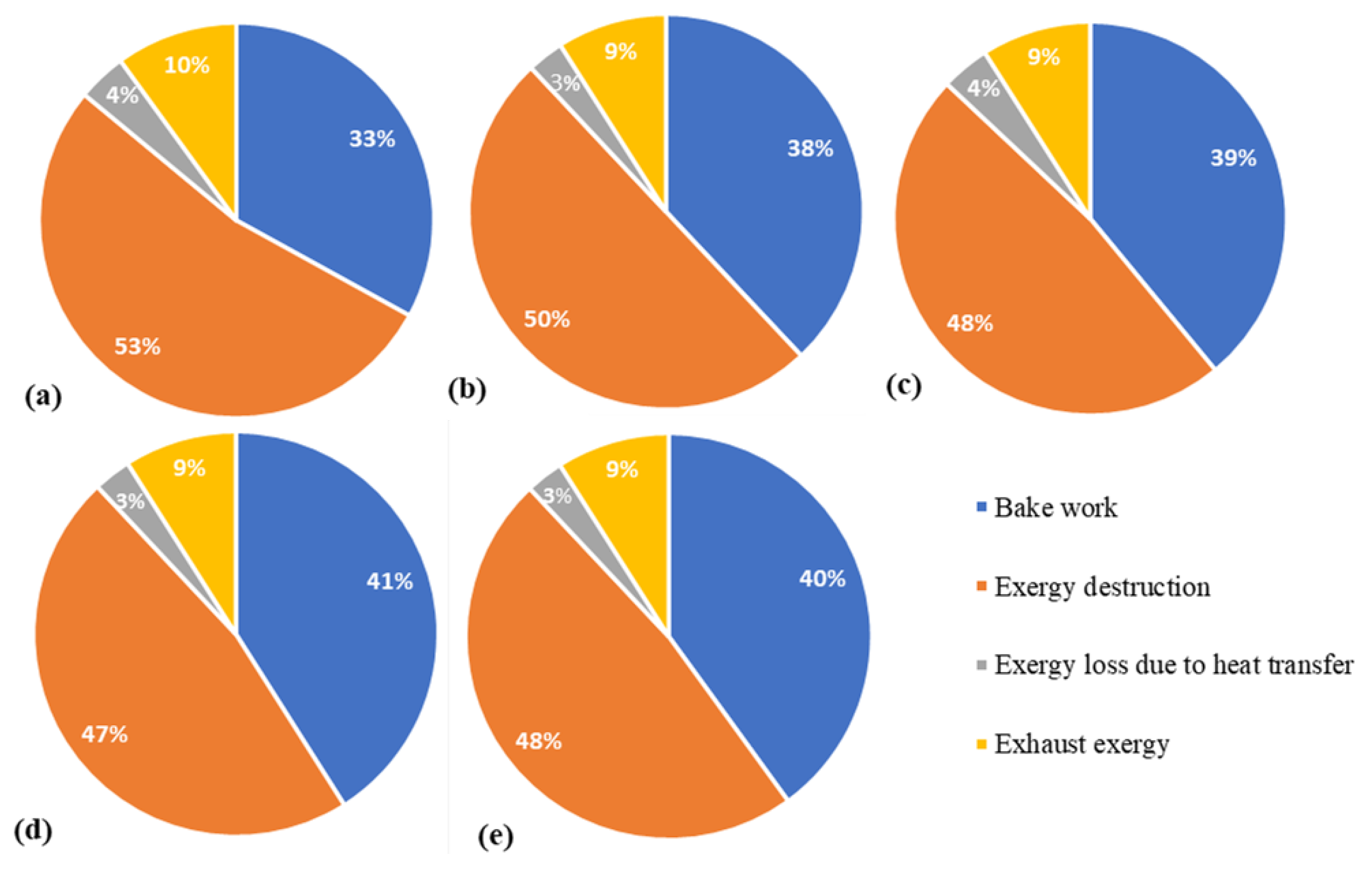


| (W/K) | (W/K) | (W/K) | (W/K) | (W/K) | (W/K) | (W/K) | (W/K) | Entropy Flux (W/K) | Error (%) |
|---|---|---|---|---|---|---|---|---|---|
| 8.1 × | 0.0011 | 0.456 | 28.499 | 0.0283 | 2.896 | 41.967 | 73.847 | 69.397 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiki, A.; Agrebi, S.; Ries, F. Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review. Entropy 2022, 24, 1099. https://doi.org/10.3390/e24081099
Sadiki A, Agrebi S, Ries F. Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review. Entropy. 2022; 24(8):1099. https://doi.org/10.3390/e24081099
Chicago/Turabian StyleSadiki, Amsini, Senda Agrebi, and Florian Ries. 2022. "Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review" Entropy 24, no. 8: 1099. https://doi.org/10.3390/e24081099
APA StyleSadiki, A., Agrebi, S., & Ries, F. (2022). Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review. Entropy, 24(8), 1099. https://doi.org/10.3390/e24081099






