1. Introduction
The combustion-induced driven flow is the phenomenon that occurs in traditional cooking stoves widely used in the developing world, and mainly in rural areas, where the major portion of the population uses biomass fuel as the primary source of energy [
1,
2,
3]. Inefficient stoves are important sources of emissions of pollutants hazardous to human health, such as carbon monoxide (CO), particulate matters (PM) and polycyclic aromatic hydrocarbons (PAHs) [
4,
5,
6,
7]. The Clean Cooking Alliance reports that every year four million people die from illnesses associated with smoke from cooking activities and, at the same time, burning woodfuels contributes to about 2% of global CO
emissions [
8]. Thus, the challenge for clean cooking designers is to create user-friendly appliances that can maintain high overall efficiency and reduce harmful emissions to levels low enough to ensure health, environment and climate co-benefits [
7].
Given its importance, the conception of clean cooking stoves is now increasingly deserving of the attention of researchers [
9,
10,
11]. Starting in the 1980s, early modelling efforts have been initiated to design more efficient cooking stoves. Since then, two types of models have emerged from researchers. The primary type is a zonal model in which conservation of mass, momentum and energy are applied to different zones within the stove [
9]. In the second type of model, Computational Fluid Dynamics (CFD) is used to represent detailed informations inside the computational domain, such as regions of high soot and CO concentrations [
12,
13,
14]. While remarkable progress has been made in the modelling of cooking stoves, many questions remain unanswered.
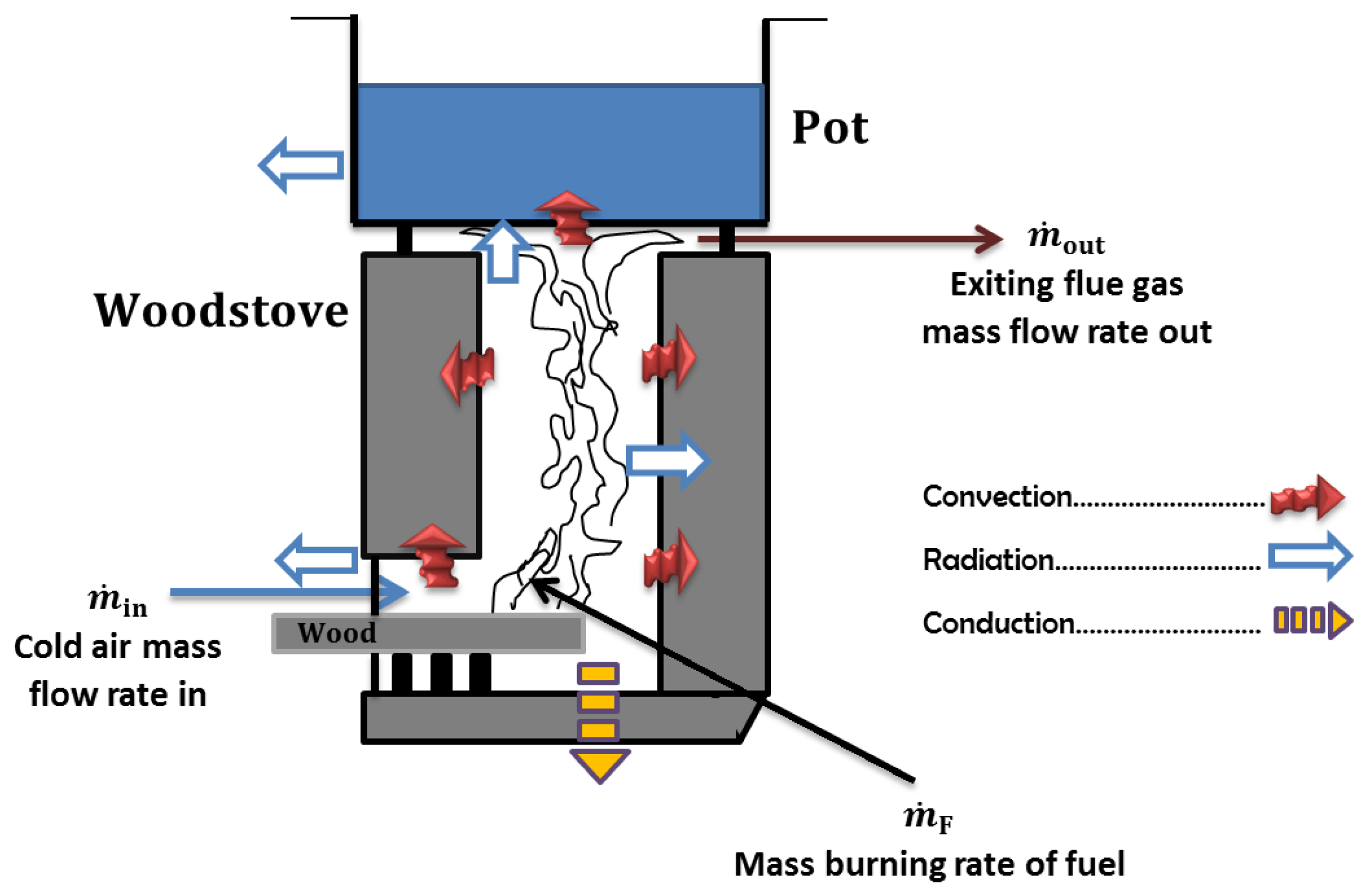
Figure 1 presents significant features of a natural draft stove burning wood fuel including a horizontal combustion chamber, and under the cooking pot there is an insulated short chimney, inside which takes place a buoyant flow of hot gases [
11]. Buoyancy occurring in the stove results from a conversion process between two forms of energy (internal and mechanical) conserving the overall energy according to the first law of thermodynamics. The efficiency of this conversion is globally assessed by the stove loss coefficient. The derivation of this quantity in cooking stove modelling is still uncertain. How this stove flow loss coefficient varies when fire takes place under different woodburning rates remains a challenging concern.
To better understand the importance of the issue, it is worth mentioning in this study that in woodstove modelling, great attention has always been focused on the influence of design parameters such as geometry or insulation materials [
16,
17,
18,
19], whereas little prior consideration has been devoted to the operating firepower level impacting aerodynamics and chemistry in the stove. The firepower–performance dependency becomes a key issue whose interest has been growing only in the last 10 years [
20]. The works of Agenbroad et al. [
21,
22,
23] represent an important benchmark in identifying the influence of operating firepower on woodburning stove behaviour. Moving beyond empirical/observational approaches, these authors developed and validated experimentally on steady state assumption an analytical and simplified stove flow theory that predicts mass-flow rate and exhaust-gas temperature from stove design and operating firepower.
Despite the Agenbroad simplified stove flow model, and other previous investigations, there is still a limited understanding of variations in the stove flow loss coefficient. Published contributions explicitly addressing access to the stove loss coefficient (also named the discharge coefficient) are very scarce.
Table 1 summarizes some of the few papers dealing with the loss coefficient issue in stove modelling.
Following a common fluid mechanics approach, this loss coefficient is associated with an overall pressure drop through stove geometry. Thus, as a conclusion of the literature review in
Table 1, losses in the flow field are supposed to be systematically characterized by empirical friction factors and single-valued head loss coefficients of different conduit components such as sudden contraction at inlet, friction loss in elbow length, loss due to friction in the pot gap zone, etc. This way of proceeding continues to appear in the eyes of many as the single rule to predict stove flow behaviour [
11,
26].
However, from a thermodynamics point of view, in addition to fluid friction, a real process can present other kinds of irreversibilities associated with heat-transfer mechanisms, mixing, chemical reactions, etc., and all resulting in the loss of process efficiency [
27,
28,
29] related to the entropy-generation rate. Therefore, an energy devaluation (energy quality loss) manifests in a destruction of available work commonly known as exergy. Losses in the internal flow field of a technical application like energy conversion in a woodburning stove can from now be assumed to be losses of flow exergy. The notion on the quality of energy and its change during thermodynamic processes is today well addressed in many contributions, to name just a few, [
30,
31,
32,
33,
34,
35,
36].
In recent years, the second law of thermodynamics in analyzing energy conversion in power-generating units permits a fresh look to evaluate some key features of the flow dynamics, heat transfer and chemical reactions through various systems. Many works dealing with entropy-generation analysis in flows involving heat transfer in natural convection processes can be found in the literature, e.g., [
27,
37]. These works concluded that the second law analysis plays a vital role in determining the frictional and heat-transfer losses [
38]. Recently, refs. [
39,
40,
41,
42] and Schmandt [
43,
44] also analyzed the basic principles of entropy and its role in the momentum and heat transfer. However, these authors made an attempt to understand the physics beyond convective heat-transfer processes in an original way by introducing some alternative non-dimensional parameters that allow to also assess qualitative aspects during the energy-transfer processes.
To the best of our knowledge, no scientific paper has addressed woodburning stove engineering from the angle of the second law analysis. In researching this, not a single paper contains reference to words like entropy or exergy. Even when Agenbroad et al. [
21,
22] mentioned reduction of chimney effect due to a non-ideal heat addition profile assumption, the entropy or exergy concept was not dealt with in their model (see
Table 1). Thus, it is not surprising to see that researchers in the cooking stove community focused exclusively on viscous and frictional losses when addressing the issue of stove flow resistance. The evaluation is being performed as if losses were occurring in an “isothermal cold flow field”, whereas heat-transfer processes are identified to be in turn important sources of irreversibilities [
45].
The present contribution presents a new outlook on the stove flow loss coefficient assessment based on an exergetic analysis of the flowing fluid and making particular use of qualitative assessment numbers in energy-transfer processes. The study derives a simplified analytical model that permits to evaluate the entropy-generation rate due to steady-flow combustion and viscous dissipation in a natural draft shielded fire stove burning wood fuel. The effect of varying woodburning rate (or firepower) on the entropy-generation rate is assessed in a steady-state assumption. To validate this model, experiments have been conducted first using a G3300 cooking stove model without a cooking pot in place to better isolate the physical processes governing the intrinsic behaviour of the stove. Then, we referred to published literature [
15,
23,
46] to confirm the practical case of a stove operating with a cooking piece in place.
The idea in carrying out this study is motivated by the observation, in the literature, of often erroneous results or simply the lack of information concerning the loss coefficient of cooking stoves. The loss coefficient is likely a determinant parameter in stove engineering. Without a comprehensive assessment method of this parameter, it will remain challenging to evaluate proper stove-operating behaviour. This work attempts to reconsider flow and heat transfer issues through a holistic approach.
This paper is organised as follows. The theoretical background along with the derivation of the loss coefficient is provided in
Section 2. Since validation data shall be generated, the experimental setup together with the materials and methods utilized is introduced in
Section 3. The obtained results and related discussions are presented in
Section 4. The last
Section 5 is devoted to conclusions.
5. Conclusions
The second law of thermodynamics analysis was performed to assess the loss coefficient in buoyantly-driven biomass cooking stoves. Accordingly, a simplified mathematical model of the entropy-generation rate in the flow field was developed. To validate the model, experiments were conducted first on a G3300 woodburning cookstove operating without pot to better isolate physical processes governing the basic behaviour of the stove. For the practical case of a stove operating with the cooking pot in place, data from published literature have served for validation. In particular, mass-flow rate and flue gas temperature at different firepower levels have been monitored.
For the parameters under study, it turned out that the entropy generation due to fluid friction is negligible compared to the global dissipation process. Therefore, heat-transfer processes are revealed to be the main source of irreversibilities in the flowing fluid. Energy flux applied as flow work in cooking stove is pure exergy which is lost in consecutive dissipative processes. Furthermore, analysis shows:
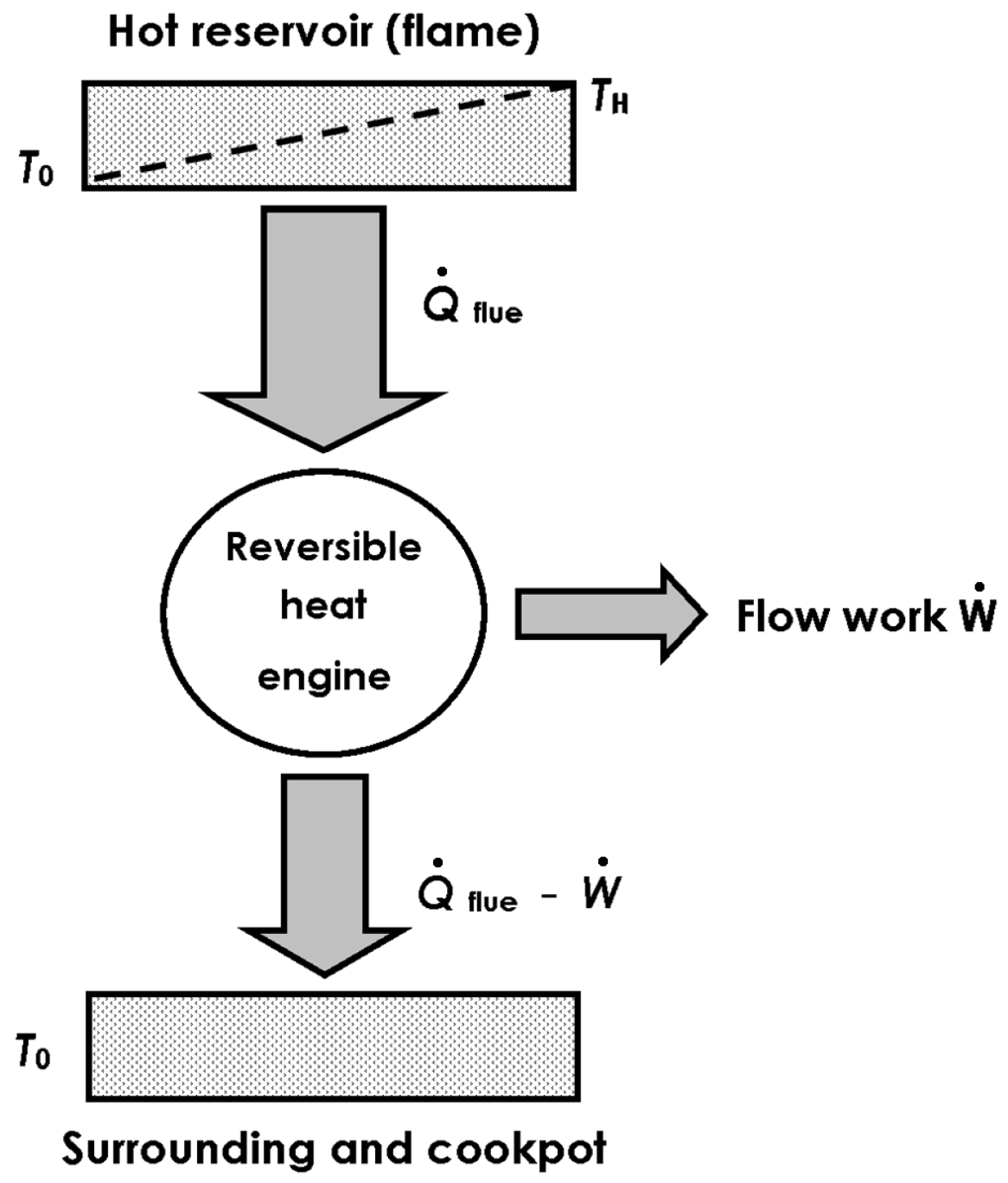
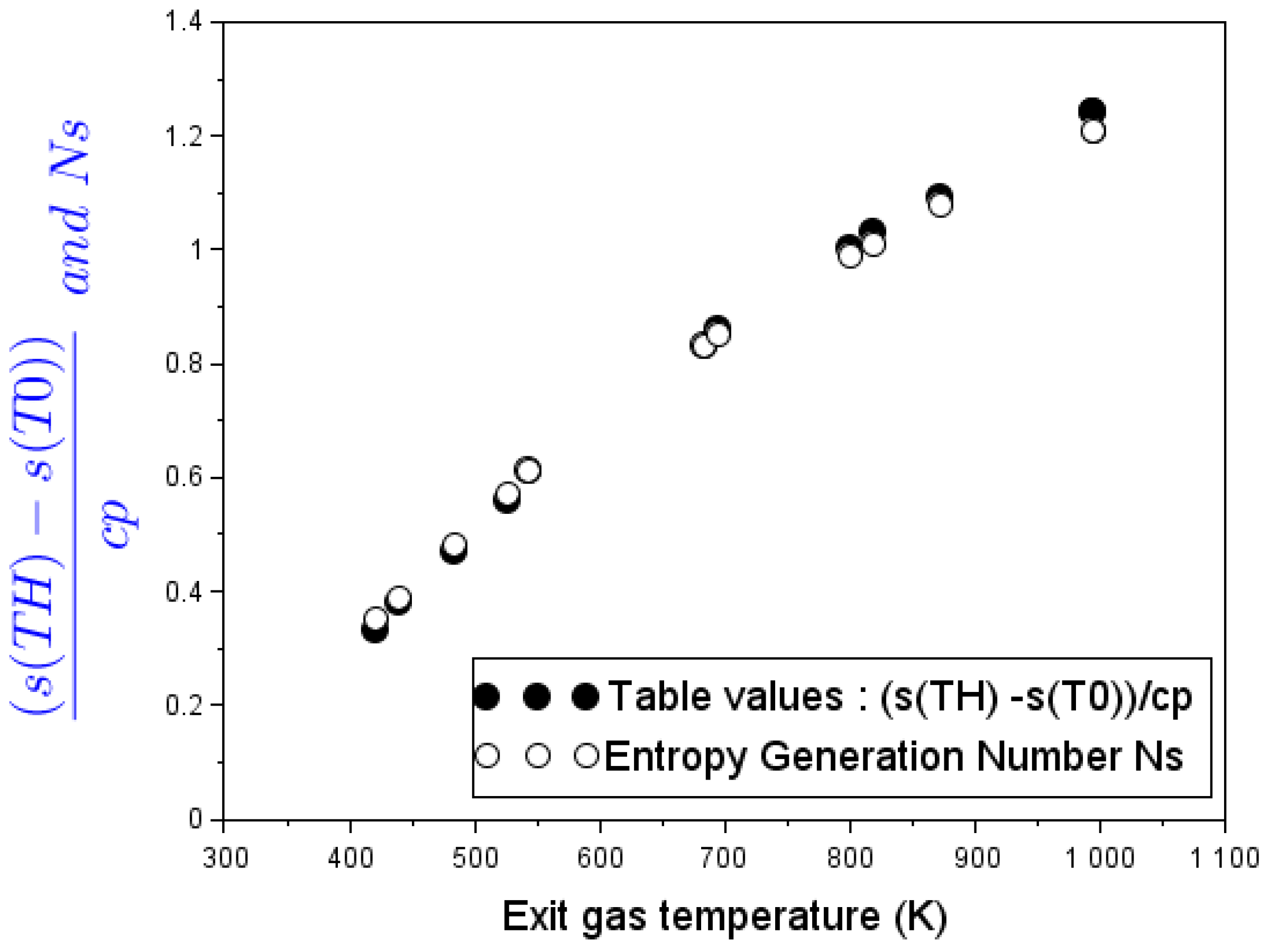
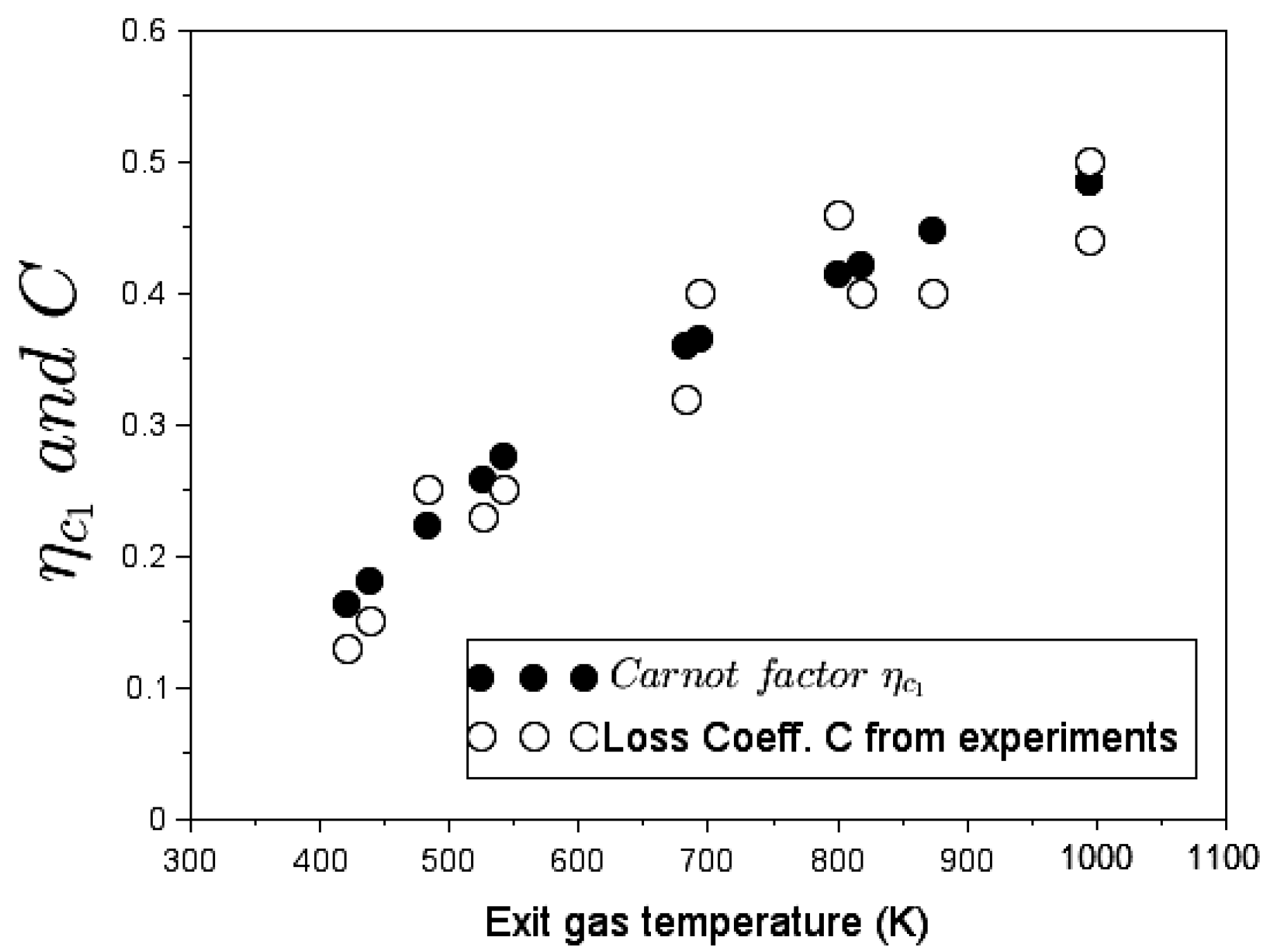
In the stove without pot: Experimental values of the stove loss coefficient at different exhaust-gas temperatures coincide with the heat Carnot factor. Thus, the energy transfer in the cookstove becomes thermodynamically assimilable to a reversible engine that releases its work output into buoyant flow work. This thesis leads to a novel definition of the loss coefficient as a measure of exergy flow.
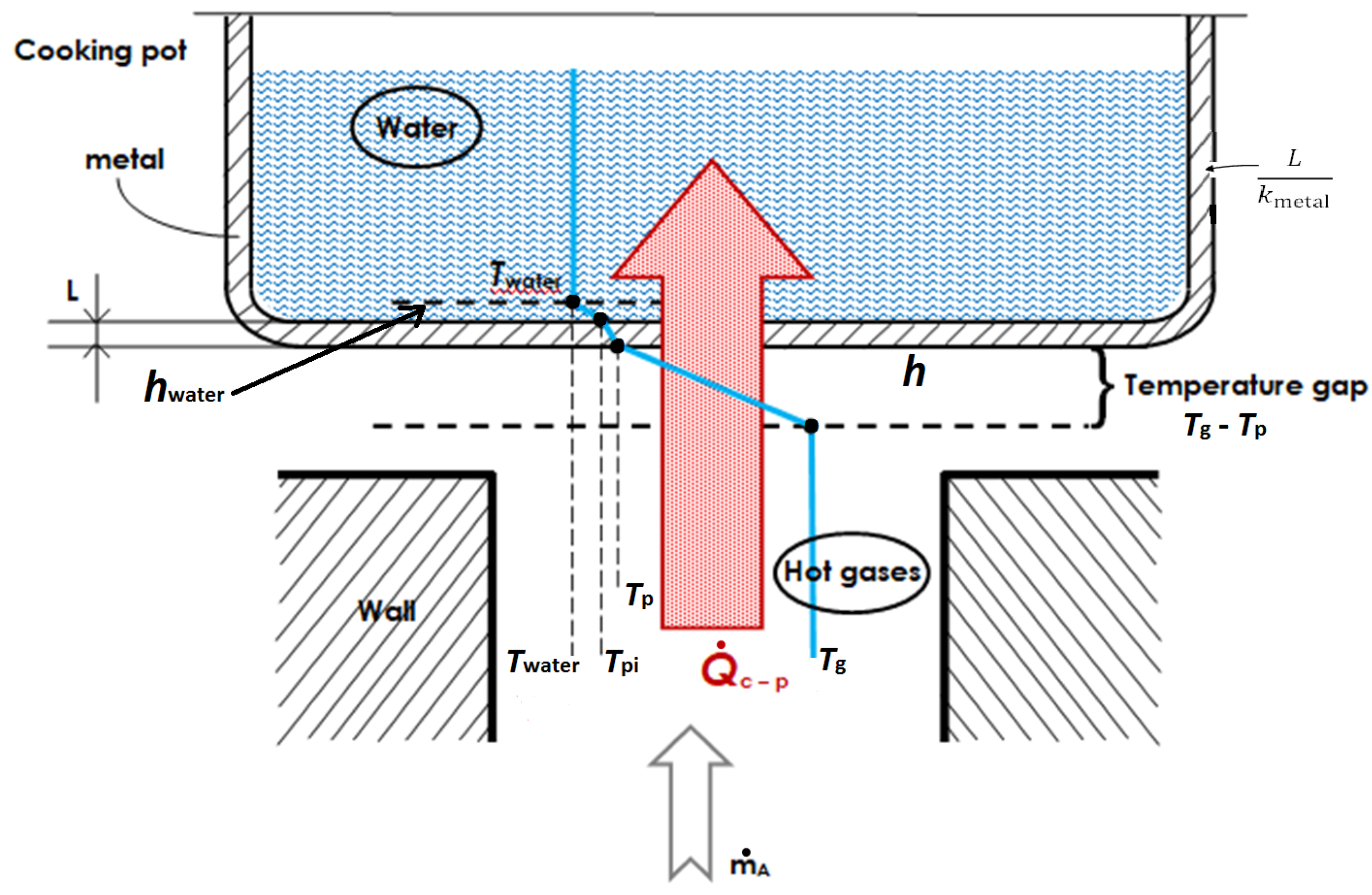
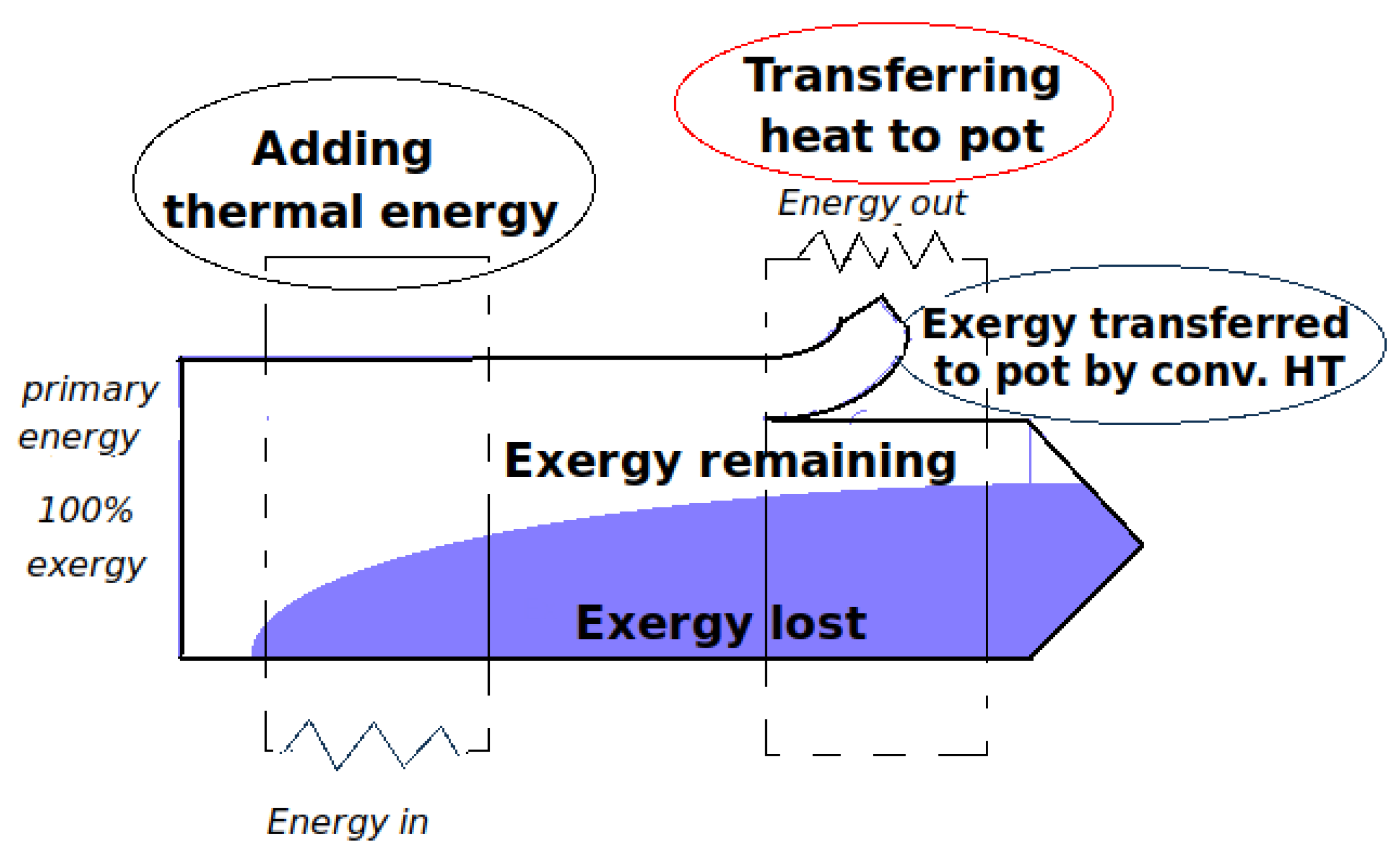
In the stove with a cooking piece (pot) in place: As upward hot gases transfer exergy to pot, both the transferred thermal energy and the needed flow work degrade. Alternative heat-transfer parameters such as exergy Carnot factor and energy-devaluation numbers were introduced to account for the destruction of exergy in the overall process. A clear relationship emerged between devaluated exergy Carnot factor and experimental values of the loss coefficient at different flue gas temperatures.
The second law analysis somewhat changes the paradigm in stove engineering by bringing quite a different perspective to the traditional concept of the so-called loss coefficient. From now, this flow loss coefficient can rather be regarded as the availability of internal energy to generate (buoyant) flow work through the stove. Therefore, the magnitude of this reversible work depends upon operating conditions and consecutive energy-transfer processes undergone following the stove operating at high or low firepower levels. Minimizing entropy generation with a view to optimizing energy-transfer processes in biomass cooking stoves remains a potential application for future works.