Effect of Ti Content on the Microstructure and Properties of CoCrFeNiMnTix High Entropy Alloy
Abstract
:1. Introduction
2. Experiment Procedures
3. Results and Discussion
3.1. Microstructure Analysis
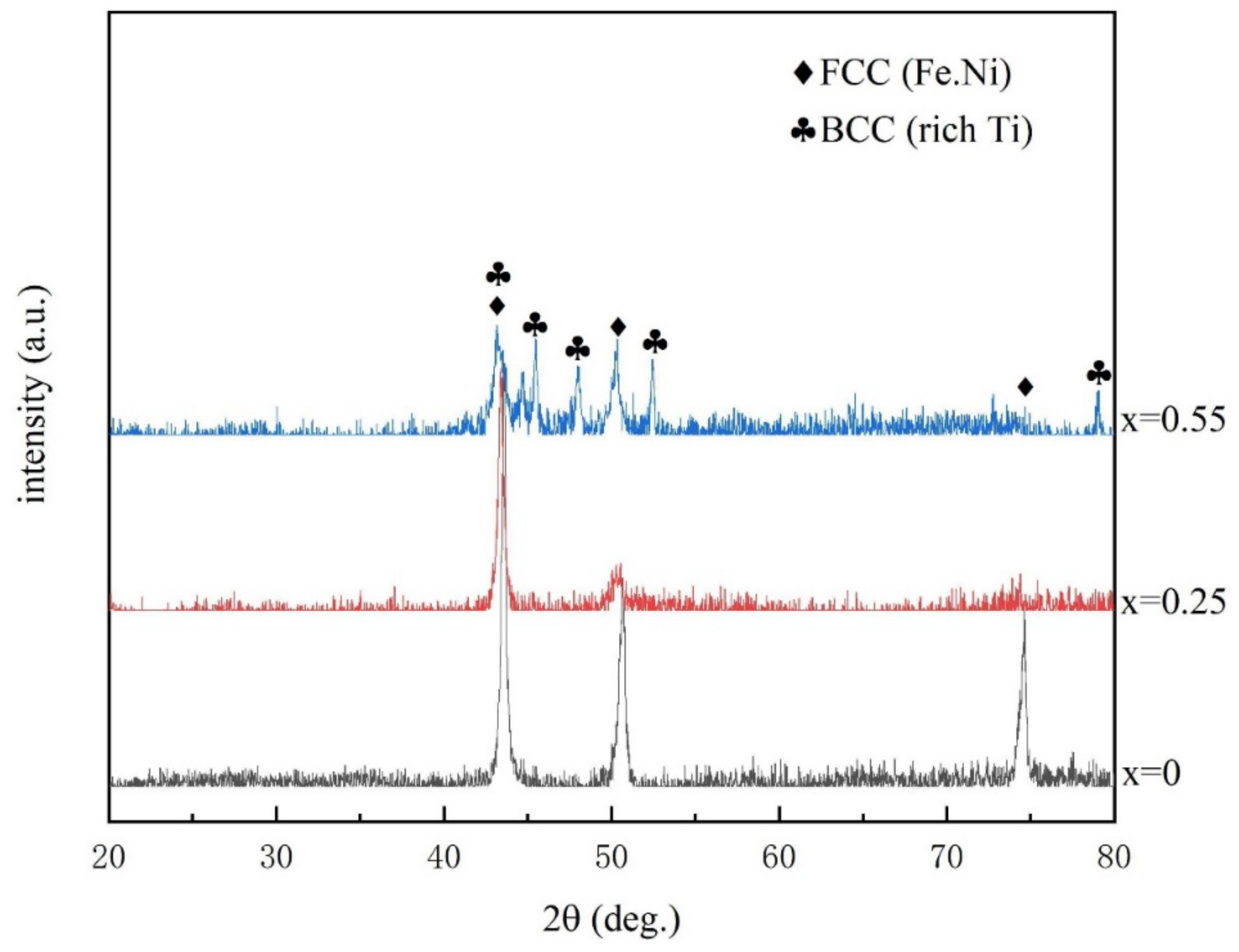
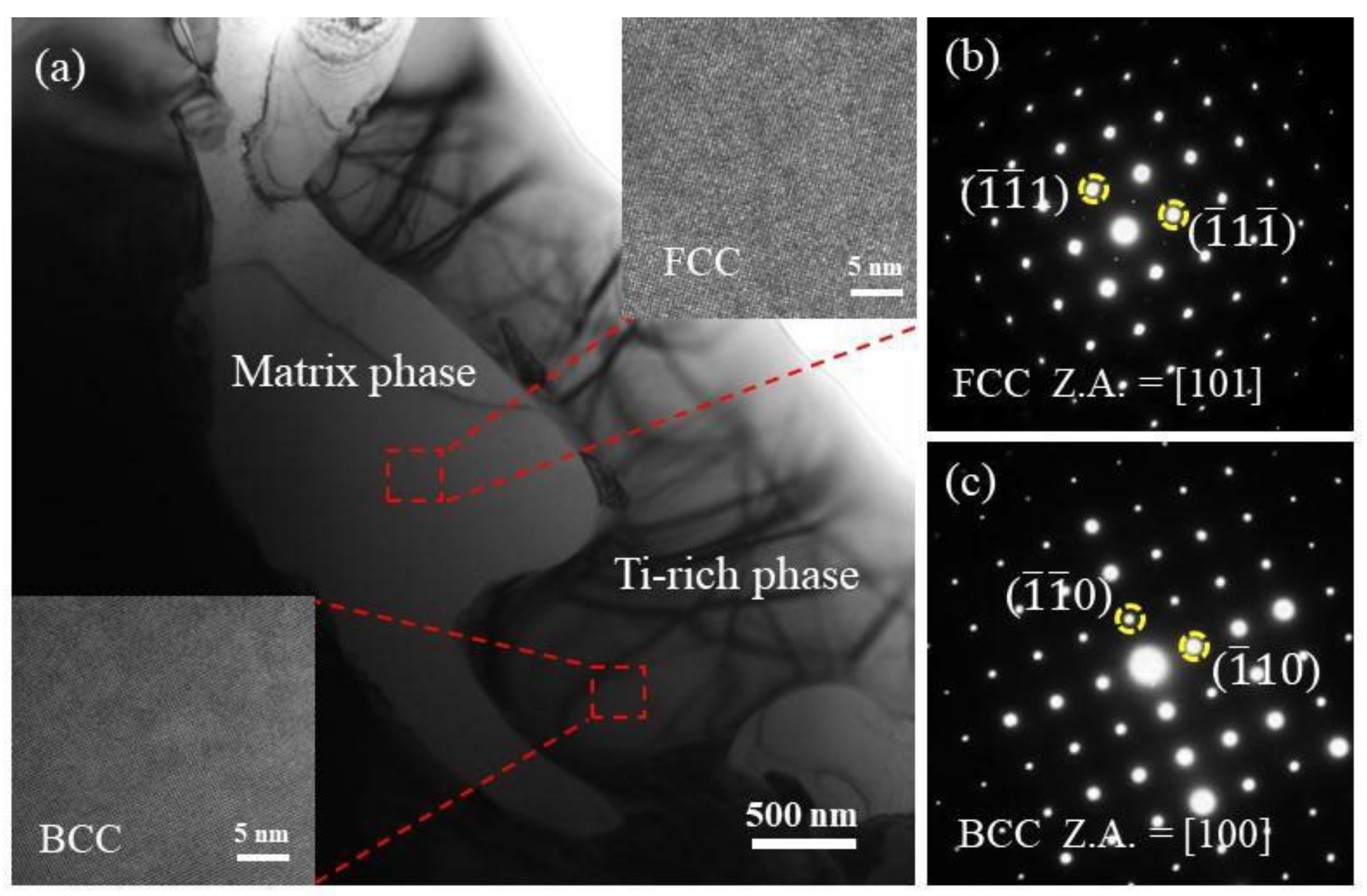
3.2. X-ray Diffraction and TEM Analysis
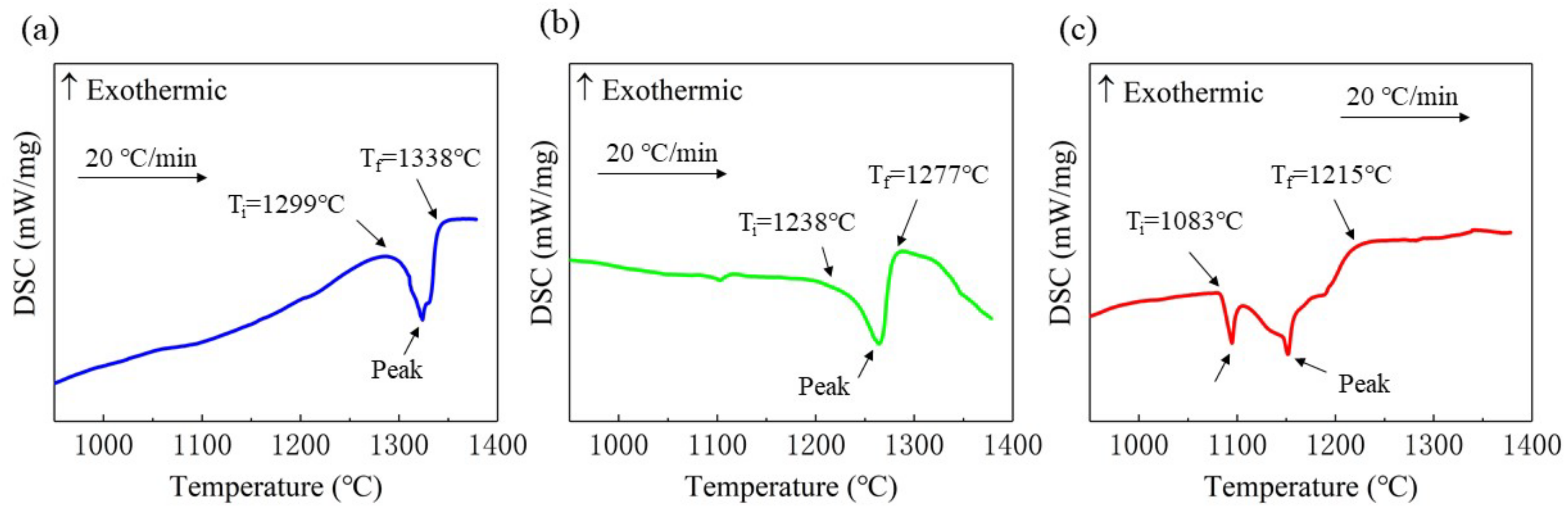
3.3. Analysis of Solid–Liquid Transition Temperature
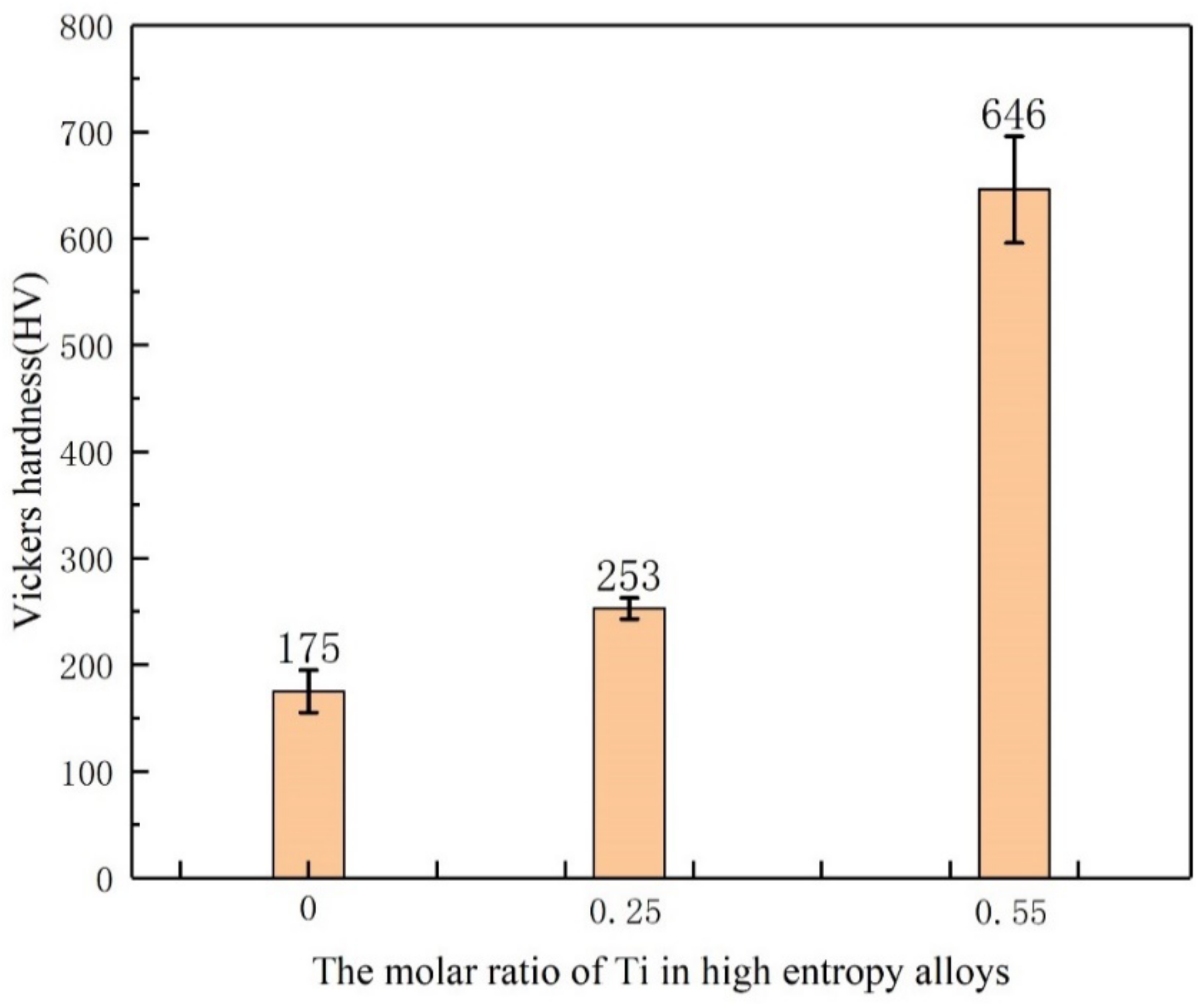
3.4. Micro Vickers Hardness Analysis
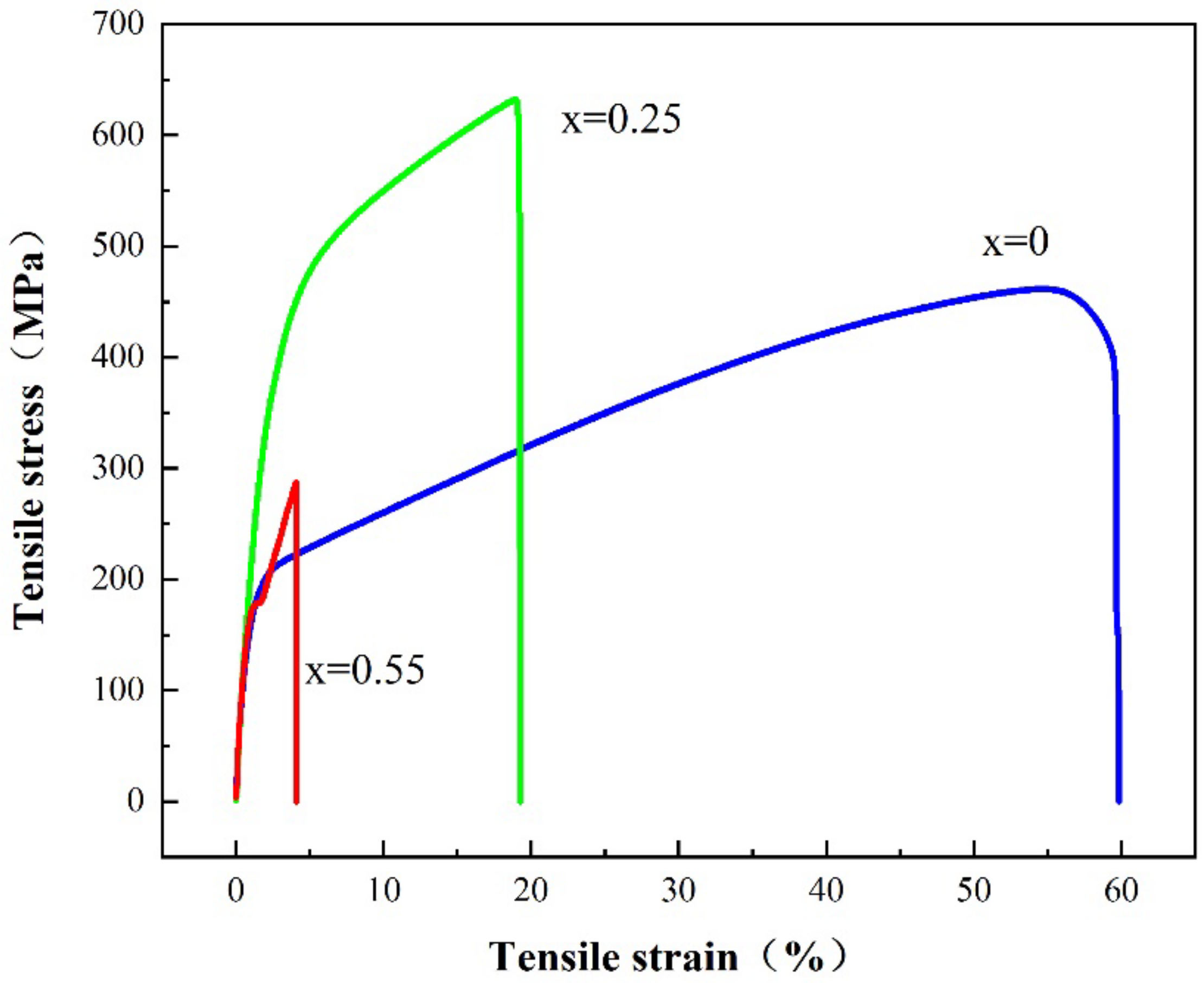
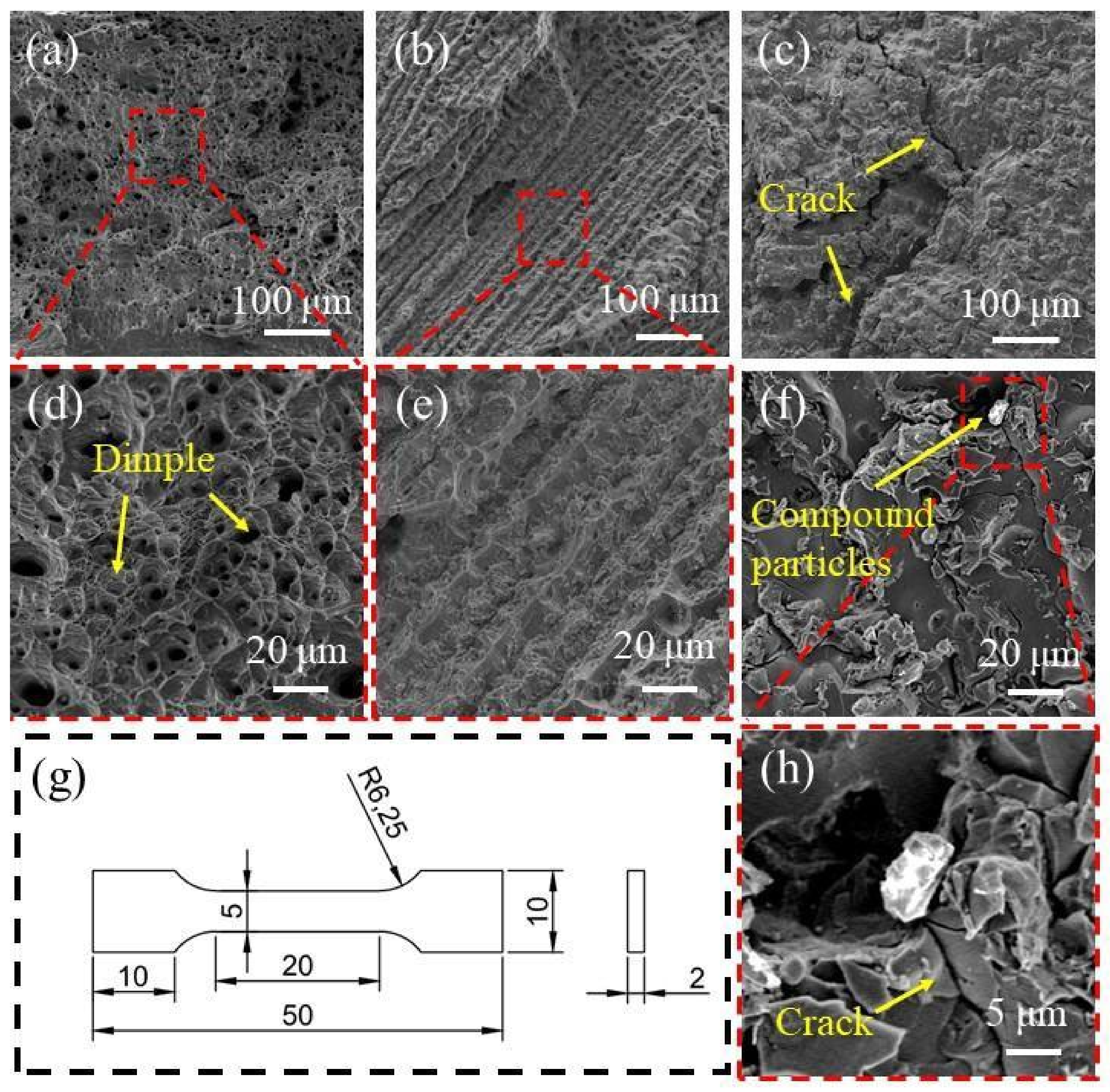
3.5. Tensile Performance Analysis
4. Conclusions
- (1)
- CoCrFeNiMn alloy is a single FCC (Fe.Ni) phase. After adding Ti, the BCC(Ti-rich phase) structure was found. With the increase of Ti content, the Ti-rich phase depicted an increasing trend. When the Ti content is 0.55, the alloy has a structure in which FCC and BCC coexist, and Ti compound particles appeared in some areas.
- (2)
- The solid–liquid transition temperatures of Ti(x=0;0.25;0.55) alloys are 1299 °C–1338 °C, 1238 °C–1277 °C, and 1083 °C–1215 °C, respectively. The solid–liquid transition temperature of the alloy decreased and the melting temperature range extended due to the addition of Ti.
- (3)
- With the addition of Ti element, the hardness of Ti(x=0;0.25 and 0.55) alloy was 175 HV, 253 HV, and 646 HV, respectively. The results show that the hardness of the alloy are obviously improved by Ti.
- (4)
- The tensile strength of the alloy is increased from 461 MPa to 631 MPa, along with Ti(x=0) alloy to Ti(x=0.25) alloy, but the strength of Ti(x=0.25) alloy is reduced to 287 MPa. A certain amount of Ti will improve the tensile strength of the alloy. The addition of excessive Ti will lead to the production of compounds and reduce the tensile strength of the alloy.
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Hsieh, K.-C.; Yu, C.-F.; Hsieh, W.-T.; Chiang, W.-R.; Ku, J.S.; Lai, J.-H.; Tu, C.-P.; Yang, C.C. The microstructure and phase equilibrium of new high performance high-entropy alloys. J. Alloy. Compd. 2009, 483, 209–212. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, L.; Lui, H.-W.; Cai, M.-H.; Yeh, J.-W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Ma, S.G.; Qiao, J.W.; Wang, Z.H.; Yang, H.J.; Zhang, Y. Microstructural features and tensile behaviors of the Al0.5CrCuFeNi2 high-entropy alloys by cold rolling and subsequent annealing. Mater. Des. 2015, 88, 1057–1062. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Sun, Q.; Fang, R.; Lin, H.; Chen, Z.; Zhang, X.; Zhang, J. Microstructure and Properties of AlCoCrCuFeNi High-Entropy Alloy Laser Processing. J. Netshape Form. Eng. 2019, 11, 178–188. [Google Scholar]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloy. Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloy. Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater. Sci. Eng. A 2010, 527, 7210–7214. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Effect of Al on structure and mechanical properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) high entropy alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Gridneva, A.O.; Mishunin, M.V.; Salishchev, G.A.; Zherebtsov, S.V. Effect of Cr and Zr on phase stability of refractory Al-Cr-Nb-Ti-V-Zr high-entropy alloys. J. Alloy. Compd. 2018, 757, 403–414. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys. Mater. Sci. Eng. A 2017, 704, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Maulik, O.; Kumar, D.; Kumar, S.; Fabijanic, D.M.; Kumar, V. Structural evolution of spark plasma sintered AlFeCuCrMgx (x = 0, 0.5, 1, 1.7) high entropy alloys. Intermetallics 2016, 77, 46–56. [Google Scholar] [CrossRef]
- Li, R.; Gao, J.C.; Fan, K. Study to Microstructure and Mechanical Properties of Mg Containing High Entropy Alloys. Mater. Sci. Forum 2010, 938, 265–271. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile properties of high- and medium-entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Dong, X.G.; Wang, Y.H.; Zhang, Z.J.; Zhang, Z.F. Enhanced strength and ductility of bulk CoCrFeMnNi high entropy alloy having fully recrystallized ultrafine-grained structure. Mater. Des. 2017, 133, 122–127. [Google Scholar] [CrossRef]
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Evidence for superplasticity in a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2017, 685, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, X.; Liu, H.; Chen, L.; Liu, Q.; Cao, Y.; Chen, S. Microstructure and Properties of Laser Cladding Co1.5CrFeNi1.5Ti0.75Nbx High-Entropy Alloy. J. Netshape Form. Eng. 2021, 13, 81–86. [Google Scholar]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Qin, G.; Chen, R.R.; Zheng, H.T.; Fang, H.Z.; Wang, L.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Strengthening FCC-CoCrFeMnNi high entropy alloys by Mo addition. J. Mater. Sci. Technol. 2019, 35, 578–583. [Google Scholar] [CrossRef]
- Gu, X.Y.; Dong, Y.N.; Zhuang, Y.X.; Wang, J. Microstructure and Mechanical Properties of CoCrFeMnNiSnx High-Entropy Alloys. Met. Mater. Int. 2020, 26, 292–301. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.-H.; Liao, Y.-C.; Li, C.-L.; Jang, J.S.-C.; Hsueh, C.-H. Hardness and strength enhancements of CoCrFeMnNi high-entropy alloy with Nd doping. Mater. Sci. Eng. A 2019, 764, 138192. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, G.; Sui, X.; Xu, Y.; Liu, Y.; Yang, J. Ultra-fine grain TixVNbMoTa refractory high-entropy alloys with superior mechanical properties fabricated by powder metallurgy. J. Alloy. Compd. 2021, 865, 158592. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of TixNbMoTaW refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Kukshal, V.; Patnaik, A.; Bhat, I.K. Corrosion and thermal behaviour of AlCr1.5CuFeNi2Tix high-entropy alloys. Mater. Today: Proc. 2018, 5, 17073–17079. [Google Scholar] [CrossRef]
- Průša, F.; Šenková, A.; Kučera, V.; Čapek, J.; Vojtěch, D. Properties of a high-strength ultrafine-grained CoCrFeNiMn high-entropy alloy prepared by short-term mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2018, 734, 341–352. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of carbon content and annealing on structure and hardness of the CoCrFeNiMn-based high entropy alloys. J. Alloy. Compd. 2016, 687, 59–71. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
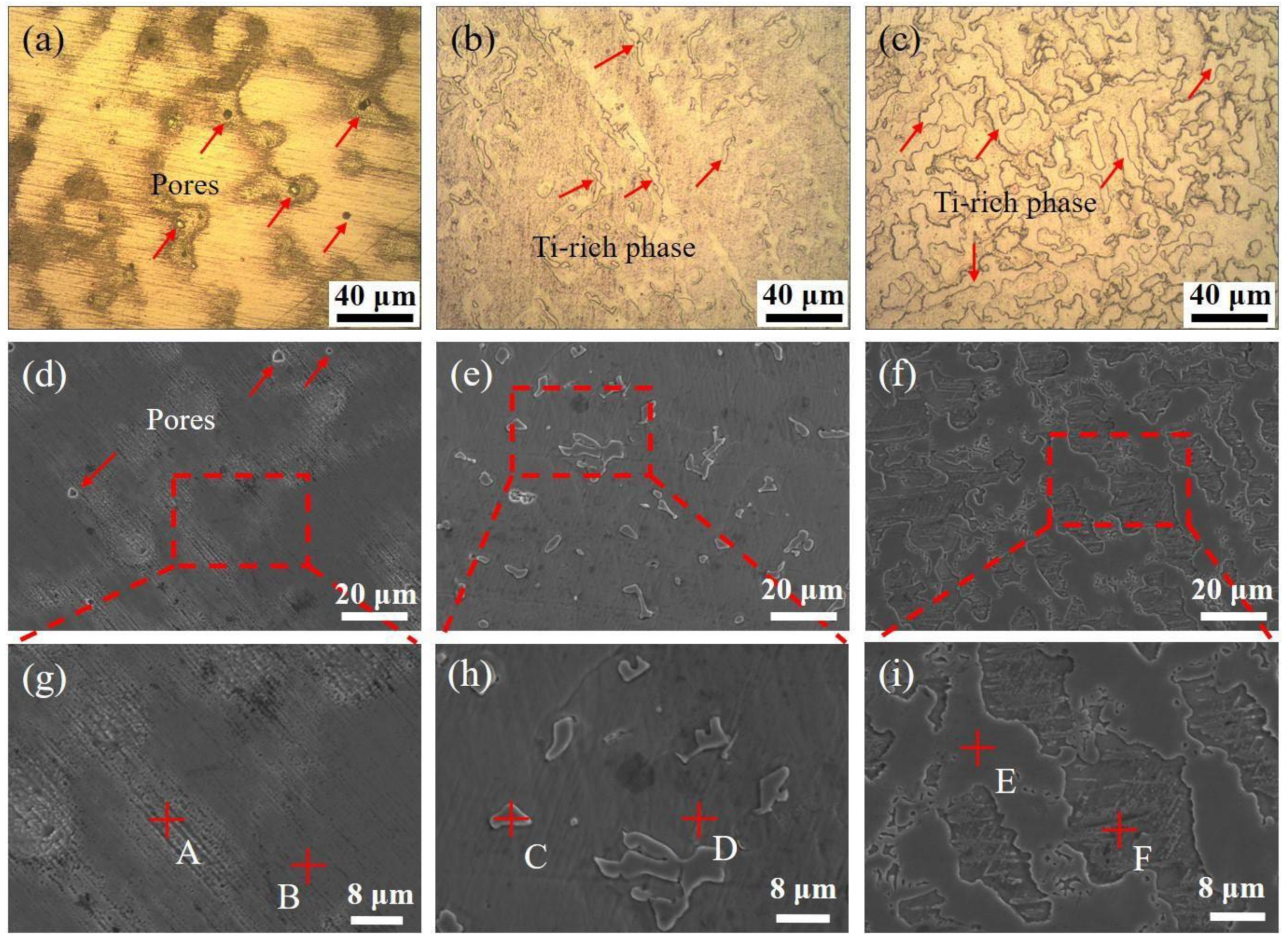
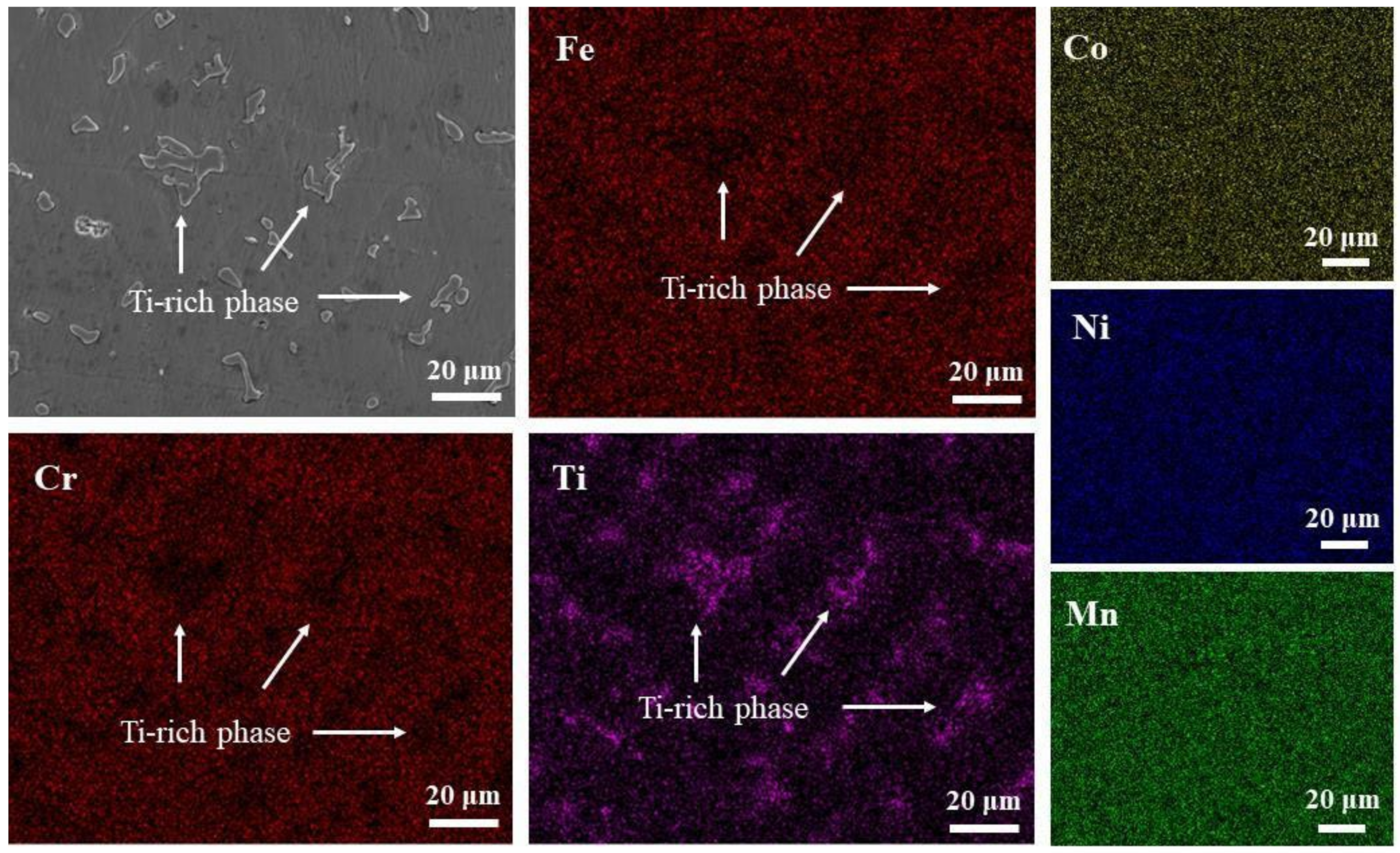
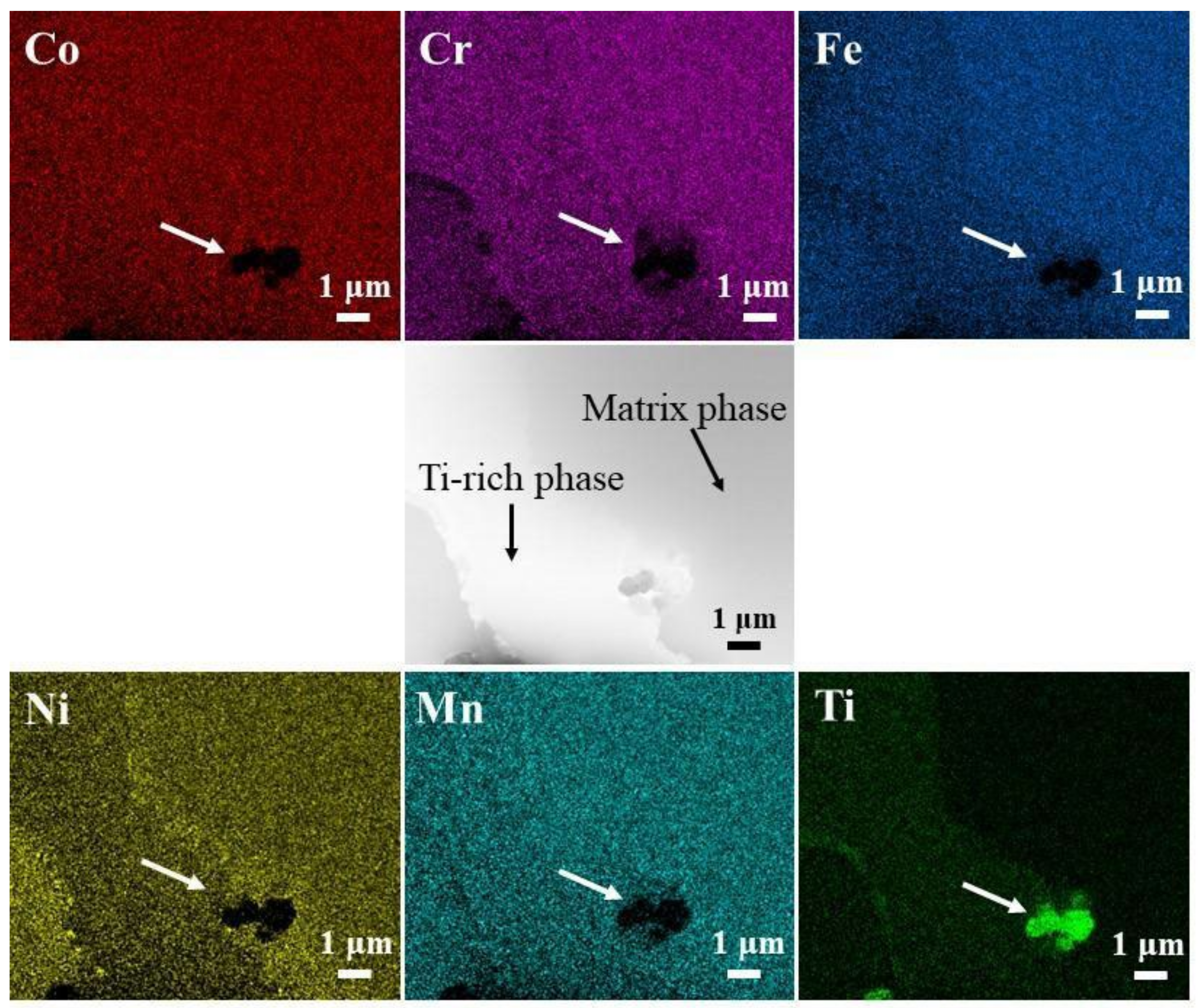
| Collection Point | Co | Cr | Fe | Ni | Mn | Ti |
|---|---|---|---|---|---|---|
| A | 17.34 | 16.83 | 15.22 | 22.30 | 28.30 | |
| B | 21.41 | 22.41 | 22.86 | 17.65 | 15.67 | |
| C | 19.55 | 9.99 | 12.04 | 21.37 | 16.01 | 21.04 |
| D | 19.44 | 21.78 | 21.24 | 16.72 | 17.88 | 2.93 |
| E | 17.64 | 22.34 | 18.72 | 13.11 | 16.88 | 11.31 |
| F | 17.58 | 22.11 | 21.45 | 15.17 | 18.48 | 5.21 |
| The Molar Ratio of Ti | Tensile Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| x = 0 | 212 | 461 | 55 |
| x = 0.25 | 338 | 631 | 19 |
| x = 0.55 | 176 | 287 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, W.; Wang, H.; Xie, J.; Zhang, T.; Yin, L.; Huang, Y. Effect of Ti Content on the Microstructure and Properties of CoCrFeNiMnTix High Entropy Alloy. Entropy 2022, 24, 241. https://doi.org/10.3390/e24020241
Chen Y, Liu W, Wang H, Xie J, Zhang T, Yin L, Huang Y. Effect of Ti Content on the Microstructure and Properties of CoCrFeNiMnTix High Entropy Alloy. Entropy. 2022; 24(2):241. https://doi.org/10.3390/e24020241
Chicago/Turabian StyleChen, Yuhua, Wenkuo Liu, Hongwei Wang, Jilin Xie, Timing Zhang, Limeng Yin, and Yongde Huang. 2022. "Effect of Ti Content on the Microstructure and Properties of CoCrFeNiMnTix High Entropy Alloy" Entropy 24, no. 2: 241. https://doi.org/10.3390/e24020241
APA StyleChen, Y., Liu, W., Wang, H., Xie, J., Zhang, T., Yin, L., & Huang, Y. (2022). Effect of Ti Content on the Microstructure and Properties of CoCrFeNiMnTix High Entropy Alloy. Entropy, 24(2), 241. https://doi.org/10.3390/e24020241






