Comparison of Both Solutions
For each stream, mass, species, energy, and exergy analyses were applied, in order to obtain the thermodynamic states for the desiccant absorption chiller with the LiCl–H
O solution and, for the refrigeration, the chiller using ammonia and the binary pair LiBr–H
O. The software used was Equation Engineering Solver (EES) [
31], due to its thermodynamic properties.
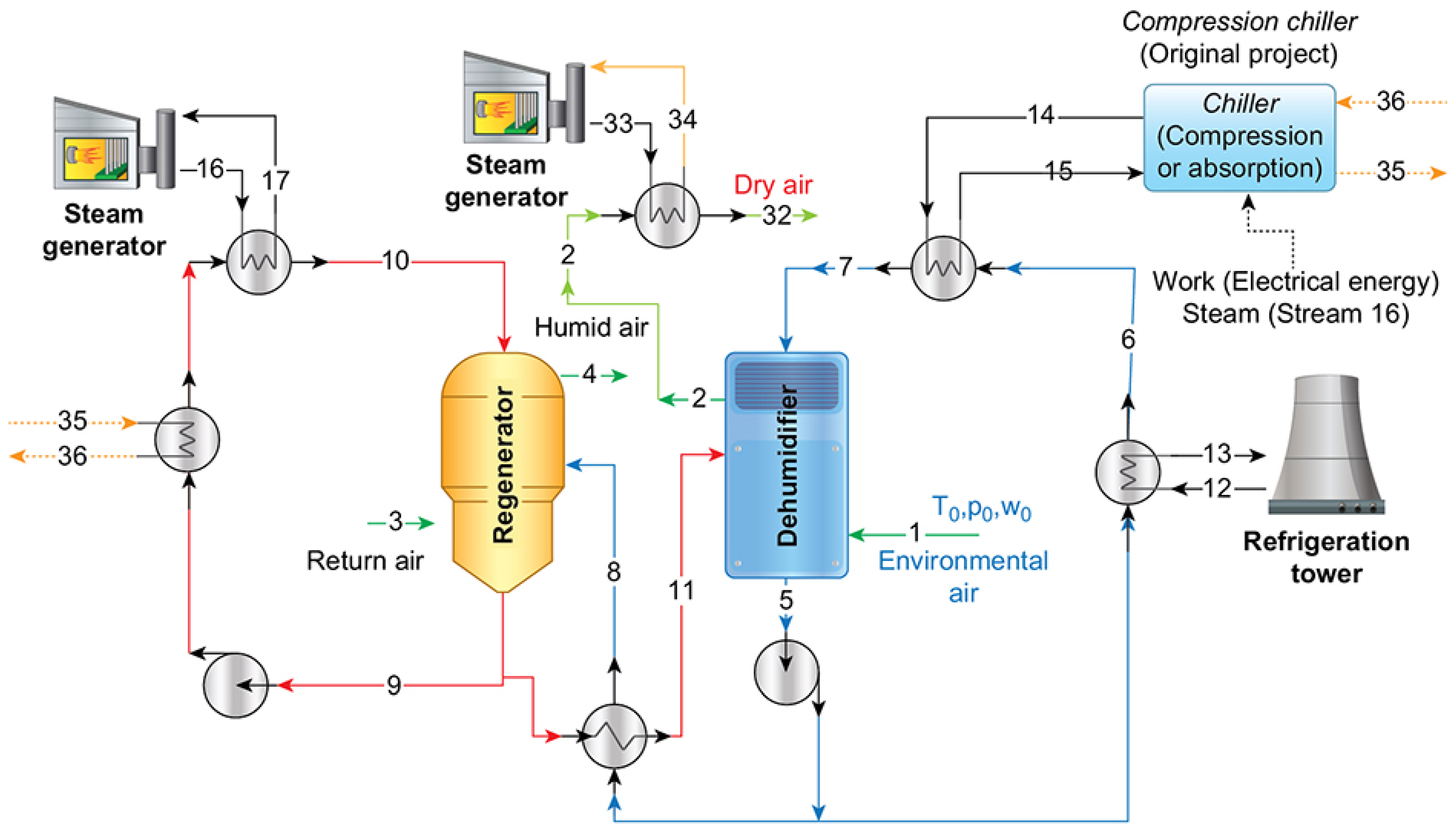
Table 1 indicates the thermodynamic states of the desiccant absorption system and operational data of the plant. This was considered as the reference scenario, regarding
Figure 1 and
Figure 2a,b. Stream 1 is the entering air removed from the environment. The reference conditions are considered with an established temperature, pressure, and humidity ratio (0.016
) for this entire article. Index 0 expresses this thermodynamic state. This fact supports the exergy of the entering air to have negative marks.
In the last column of
Table 1, the fluid representing the stream is indicated. The specific enthalpy and entropy were obtained directly from Thermodynamic properties and, for the specific exergy of the stream, we considered that the reference condition was 20% (in mass) for LiCl/LiBr–H
O solutions [
17,
18]. It is important to highlight the necessity of the use of a refrigeration tower and, in some conditions, a refrigeration cycle; such as the situations indicated in
Figure 2a,b. In the original configuration of the plant, the vapor compression chiller was used [
32]; herein, we tested the absorption chiller based on the LiBr–H
O pair [
30].
Concerning the Second Law of Thermodynamics, all exergy balances were calculated using Equation (
4). With the exergy defined for all states, irreversibilities were evaluated for each component of the system. In
Table 2, the destroyed exergy of each piece of equipment is given (as well as its relative participation in the total amount of destroyed exergy). It is essential to pronounce here that we did not take into account the irreversibilities of the combustion and the refrigeration systems, which will be further analyzed. When we assessed the irreversibilities of the desiccant system solely, we noticed that the greatest destructions of exergy were by the steam generation heat exchanger and the absorber. It is required to accentuate that the steam heat exchanger represented the largest exergy destroyed (without taking into consideration the combustion process), the main reason for this being the temperature difference between the solution and vapor streams. Concerning the absorber, the leading cause of the exergy destruction rate was the process of mixing between the air and the solution, which occurred inside the chamber. In addition, there were losses in the process related to heat and mass transfer. The overall exergy destruction of the system was 235.50 kW. The exergy input is
kW and the useful exergy is
25,405 kW and the exergy efficiency was 10% (ratio of the exergy of the air to the provided exergy). Note that taking into consideration only the liquid desiccant system, the increase in the exergy from streams 6 to 7 is almost negligible.
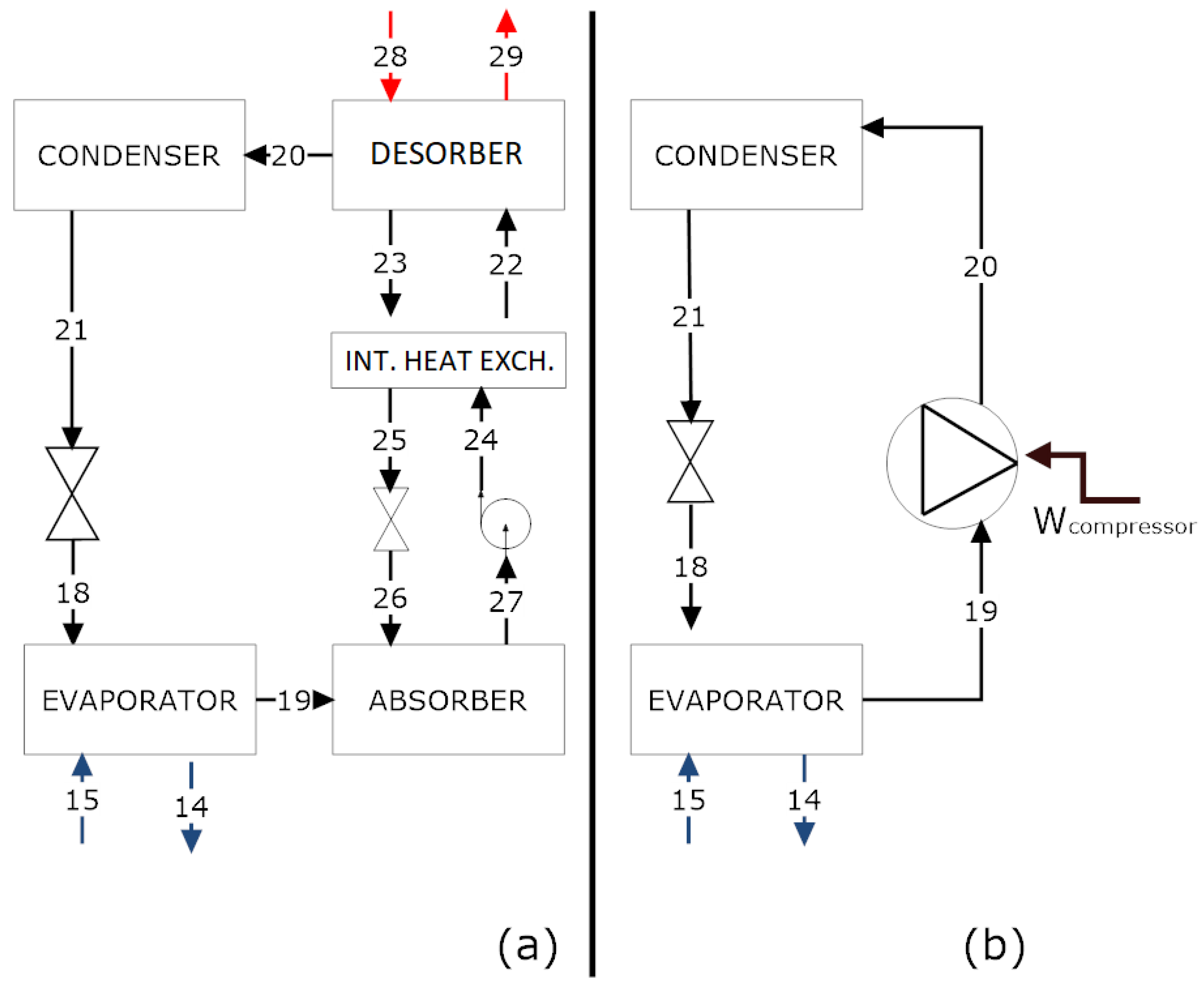
In
Table 3, the thermodynamic states of the steam compression chiller are given. Some parameters were obtained in the factory, such as the steam temperature leaving the compressor. We also gathered the saturation pressures of the condenser and evaporator [
32]. It is possible to notice that the temperature of the ammonia in the evaporator was around 10
C, in order to decrease the temperature of the LiCl–H
O solution from 29
C to 23
C, considering the design conditions (25
C). Depending on the environmental circumstances, there was no requirement to utilize a refrigeration cycle.
From the thermodynamic states, it was possible to evaluate the exergy destroyed in each piece of equipment (
Table 4).
For this study, we intended to compare the use of an absorption chiller, as opposed to a vapor compression system, in terms of energy and exergy usage. The absorption chiller can be used as an alternative to the vapor compression system as, in most chemical plants, steam is produced within the factory. Therefore, the use of an absorption chiller was a suitable option. The LiBr–H
O solution obtained the same effect between the input and output states of the vapor compression process. Generally, the energy source used for these systems is steam or water with high temperature; in the present analysis, we considered the prime.
Table 5 indicates the thermodynamic states of the cycle.
Steam was produced inside the considered industrial complex, which increased the appeal of the use of an absorption chiller. As usually displayed in chiller catalogs, the cold water was fixed in a temperature range of 7–12
C (streams 14 and 15), based on the literature. From the energy balance in the evaporator, the result was a temperature of 12
C of the water (refrigerant) before entering the absorber (streams 18 and 19).
Table 5 indicates the thermodynamic states of each stream in the cycle. The concentrations adopted for the solution were 56.7% and 62.4%, according to the literature [
30]. Our intent was to keep the pressure of the fluid and flow rate in values close to that of the few manufacturers found in the literature.
Evaluating the system’s exergy behavior,
Table 6 shows that the greatest irreversibilities were in the desorber, followed by the absorber, with exergy destruction due to the mixing/separation and heat transfer rates as the primary sources. Expanding the dehumidifying system frontiers to include the absorption chiller, the global efficiency was found to be 7.16%. The performance coefficient COP of the refrigeration cycle was 0.7463, while the SMERex was 2.295 kg/kWh. The irreversibility results are shown in
Table 6. The total exergy losses were found to be 331.76 kW (dehumidifier and absorption chiller).
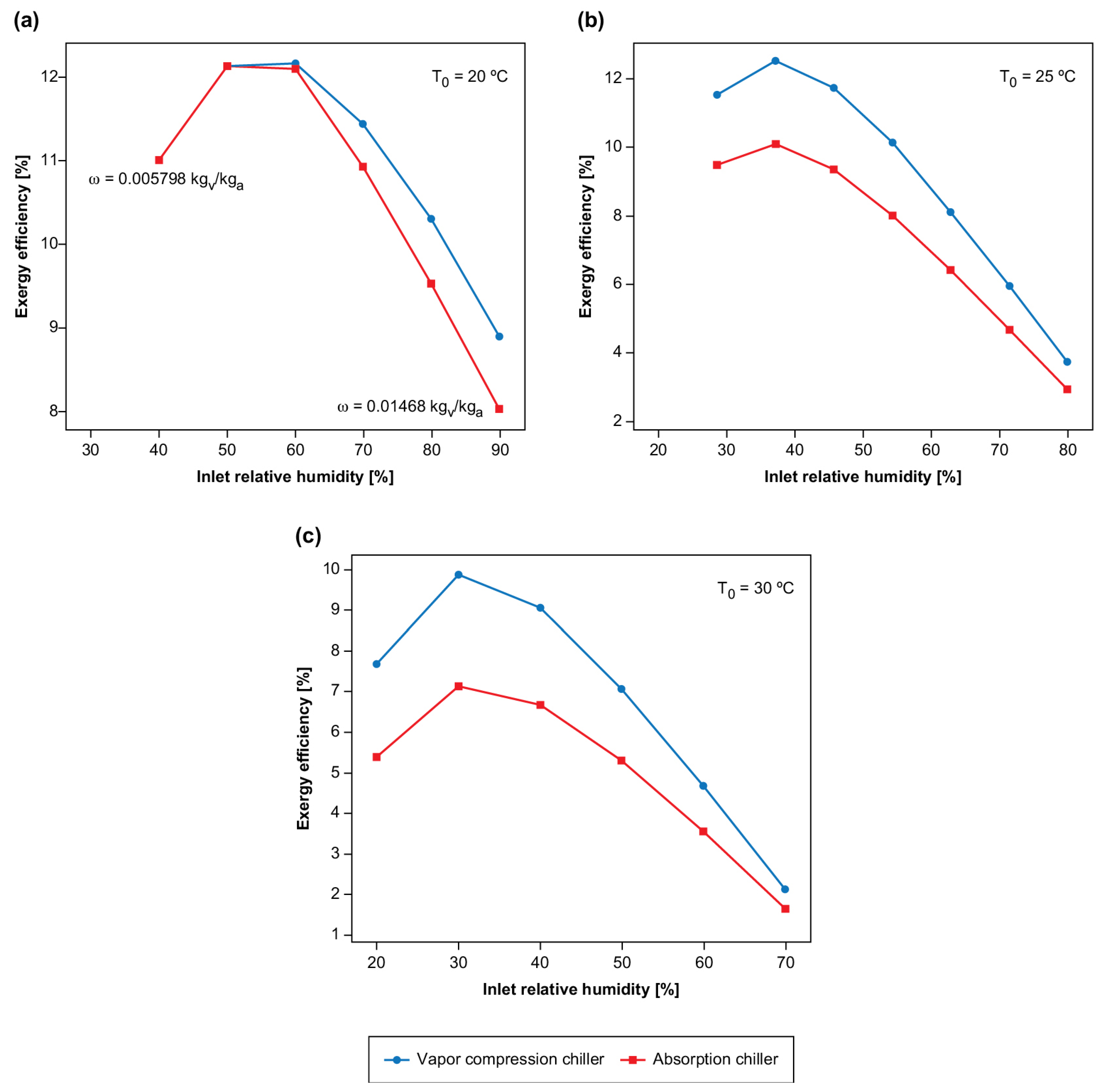
As already demonstrated through the states defined for the inlet air temperature at 25 C and absolute humidity of 0.013 , in this case, the system using a vapor compression chiller showed irreversibility, exergy efficiency, and SMERex index, respectively, of 256.96 kW, 9.05%, and 2.796 kg/kWh. In contrast, those of the absorption chiller were 331.76 kW, 7.16%, and 2.295 kg/kWh. Therefore, the desiccant liquid absorption dehumidifier with the vapor compression chiller proved to be more viable and the best alternative between the two studied. As the system is used throughout all seasons of the year, in different periods of the day, and with the most varied parameters, another evaluation could be done to check whether, under other operating points with different temperatures and air humidities, the absorption chiller would be more efficient. The figures below show the exergy efficiencies at 30 °C, 25 °C, and 20 °C, with different relative humidities for the environmental air.
Figure 3 shows a comparison of the exergy efficiency as a function of the relative humidity for three inlet temperatures (20, 25, and 30
C). Therefore, from the comparison,
Figure 3a–c show that an increase in the inlet temperature of the air (environmental temperature) caused the chillers to operate using higher power (electric) or enthalpy (steam). It is interesting to note that, at 20
C, both systems had the same efficiency (
%), as there was no necessity for the chiller (i.e., they were turned off), and only the refrigeration tower was operated.
As shown in
Figure 3a–c, for all temperature ranges (30, 25, and 20
C), the system with the vapor compression chiller presented higher exergy efficiency for all values of relative humidity. Therefore, it appears that, for all studied temperature and humidity ranges, the steam absorption chiller, as an energy source, does not offer thermodynamic viability.
It is, however, essential to note that the two systems showed the same efficiencies for the lowest temperature and humidity. This is because, at low humidity and temperatures (temperature and relative humidity below 20 °C and 50%), it was not necessary to use the chiller to cool the inlet solution in the dehumidifier: it was possible to reach humidities below those necessary for the system using only the cooling tower.
Therefore, a possible way to increase the system’s efficiency is to improve the cooling tower’s performance as, the better the thermal exchange, the lower the solution’s mass flow rate needs to be cooled by the chiller. Another point that can be observed in the charts is that the absorption systems, through the liquid desiccant, increase in efficiency as the relative humidity decreases until reaching a maximum point and, then, their efficiency decreases again; that is, this system is more suitable for use in environments that are in “intermediate conditions”. The explanation for this issue is that the thermal load to decrease the air temperature is almost the same (since the air temperature difference would be the same). Although the amount of water removed would be lower for relative humidity reduced, it justifies both systems a decrease in the exergy efficiency. The exergy provided is the same, and the increase in the exergy of the air is lower.
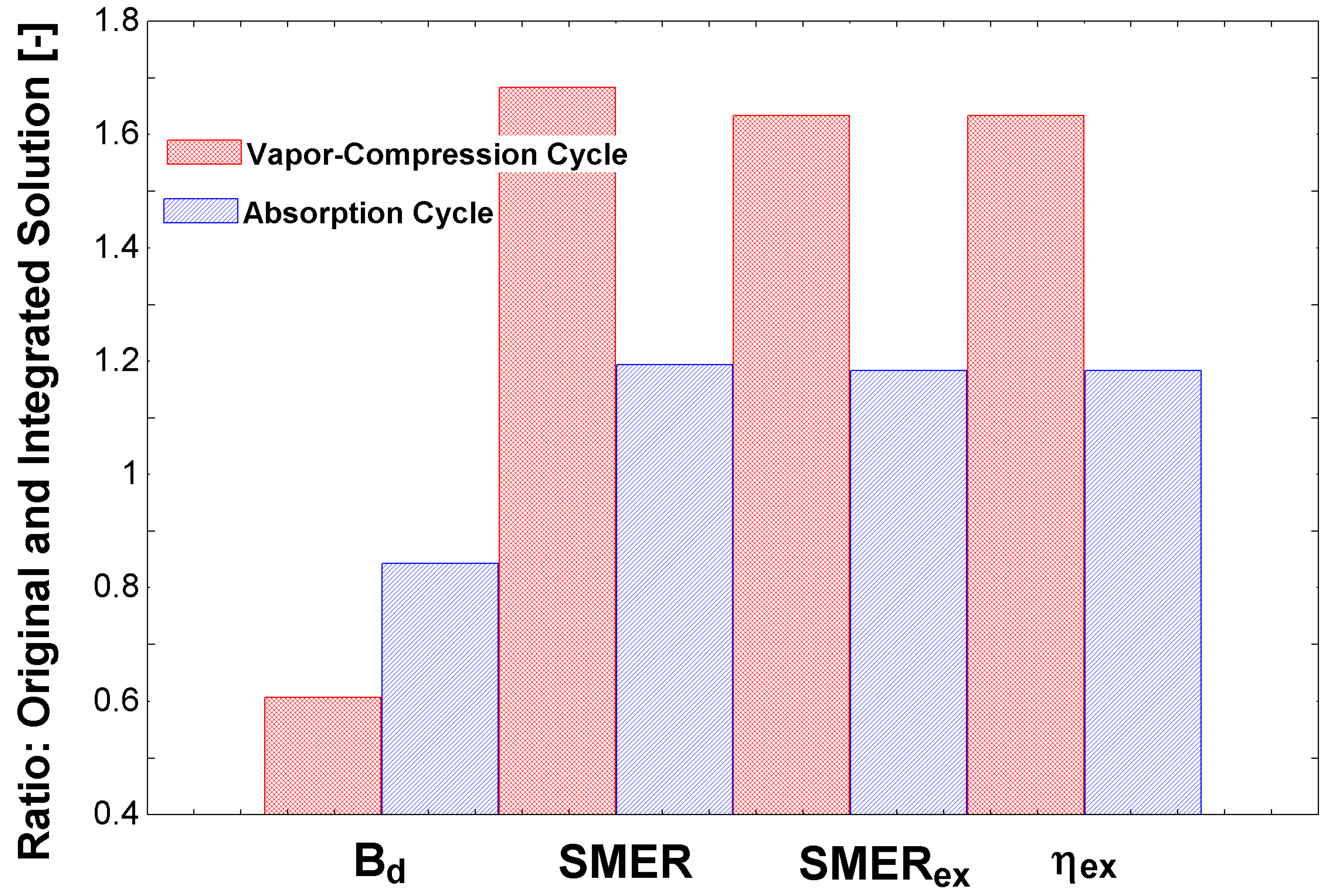
Figure 4 shows a comparison of the compression cycle and absorption cycle in the whole system (complete dehumidification system), with and without an usual integration in the air conditioning area of the streams 35 and 36 to preheat the solution before the steam generator. It is essential to highlight that this is not a comprehensive integration, only a solution to reuse the condenser’s exergy content. The results are show in comparison with the project scenario shown in
Table 1. Therefore, using the condenser’s energy to preheat the solution before the boiling entrance (i.e., from stream 9 to 10) increased all performance indicators. It is essential to indicate the similarity of behavior of
and
, as both used exact quantities to propose the associated performance indices. This similar trend was expected. The traditional
index had identical behavior, although at another magnitude. This result shows the importance of comparing these different parameters, in order to conclude the best solution. In these two scenarios, with specific conditions, the original compression cycle would be more appropriate.
It is important to discuss that, in this figure, the usage of the condenser’s energy (which would be transferred to the environment as an exergy loss) is now used as an input to the process. Another critical point is the higher exergy performance of the compression cycle using ammonia. Future research may indicate which energy input would be better to invest in, e.g., photovoltaic for the compressors or solar thermal energy for the absorption cycle, or another process that requires steam. From the comparison of both refrigeration systems, it is possible to conclude that there was a considerable discrepancy between the chillers: The vapor compression system had an advantage concerning the performance coefficients. Therefore, there was an unfavorable point hidden in these production indicators. The absorption chiller, in this specific case, used steam produced in the plant’s boiler. Therefore, it was considered a high-quality energy input, which may be suppressed by other energy sources of lesser quality, such as residual heat, natural gas, or renewable energy. Of course, the quality of the energy input to the compression chiller was high; nevertheless, it may not use other production types, other than photovoltaic cells. A comparison of both may be carried out in a future study.