Entropy Analysis for the Evaluation of Respiratory Changes Due to Asbestos Exposure and Associated Smoking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.1.1. Respiratory Impedance Complexity
2.1.2. Airflow Pattern Complexity
2.2. Pulmonary Function Analysis
2.3. Complexity Analysis
2.4. Statistics
3. Results
3.1. Demographics, Pulmonary Function and Effect of Smoking in the Respiratory Impedance Analysis
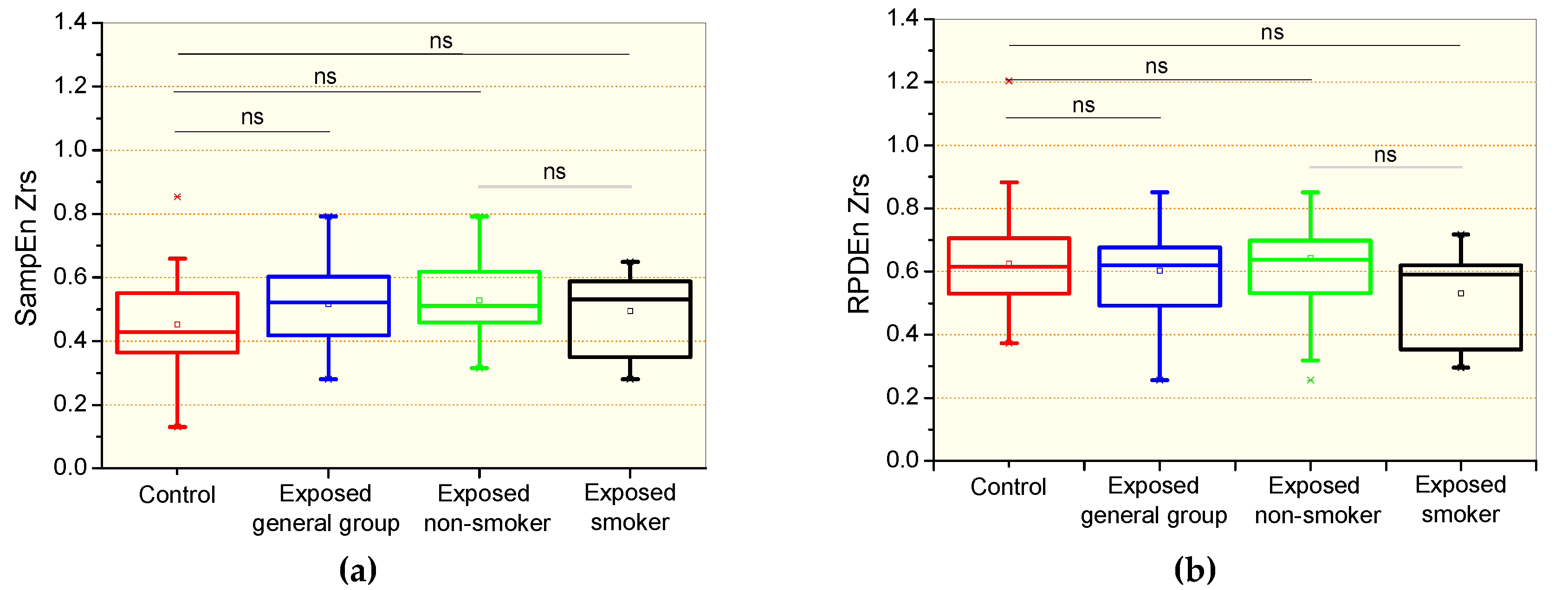
3.2. Complexity Analysis of the Respiratory Impedance Signal
| Non-Smokers (n = 22) | Smokers (n = 12) | p | |
|---|---|---|---|
| Spirometry | |||
| FVC (L) | 2.65 ± 0.69 | 3.08 ± 0.51 | 0.07 |
| FVC (%) | 85.5 ±13.1 | 93 ± 20.6 | 0.37 |
| FEV1 (L) | 2.07 ± 0.50 | 2.04 ± 0.45 | 0.87 |
| FEV1 (%) | 84.6 ± 14.2 | 78.4 ± 15.4 | 0.24 |
| FEV1/FVC | 78.6 ± 6.76 | 66.7 ± 12.4 | <0.004 |
| FEF25–75% (L) | 2.14 ± 0.75 | 1.36 ± 0.81 | <0.009 |
| FEF25–75% (%) | 90.1 ± 32.1 | 51.3 ± 29.2 | <0.004 |
| FEF/FVC | 106.3 ± 38.30 | 57.8 ± 35.5 | <0.002 |
| Plethysmography | |||
| RV (L) | 1.90 ± 0.95 | 2.80 ± 1.05 | <0.02 |
| RV (%) | 116.7 ± 46.87 | 155.1 ± 82.24 | 0.09 |
| TLC (L) | 4.63 ± 1.22 | 5.90 ± 0.91 | <0.004 |
| TLC (%) | 98.6 ±18.0 | 114.7 ± 30.16 | 0.09 |
| RV/TLC (L) | 38.2 ± 10.4 | 46.5 ± 10.3 | <0.04 |
| Raw (cmH2O/L/s) | 5.63 ± 10.4 | 5.17 ± 5.15 | 0.88 |
| Raw (%) | 420.9 ± 776.6 | 372.1 ± 424.0 | 0.84 |
| sGaw (L/s/cmH2O/L) | 0.23 ± 0.37 | 0.09 ± 0.06 | 0.20 |
| sGaw (%) | 90.97 ± 141.8 | 48.4 ± 36.4 | 0.33 |
| Forced Oscillation | |||
| Zm (cmH2O/L/s) | 4.97 ± 1.77 | 5.06 ± 2.26 | 0.90 |
3.3. Demographics, Pulmonary Function and Effect of Smoking in the Airflow Analysis
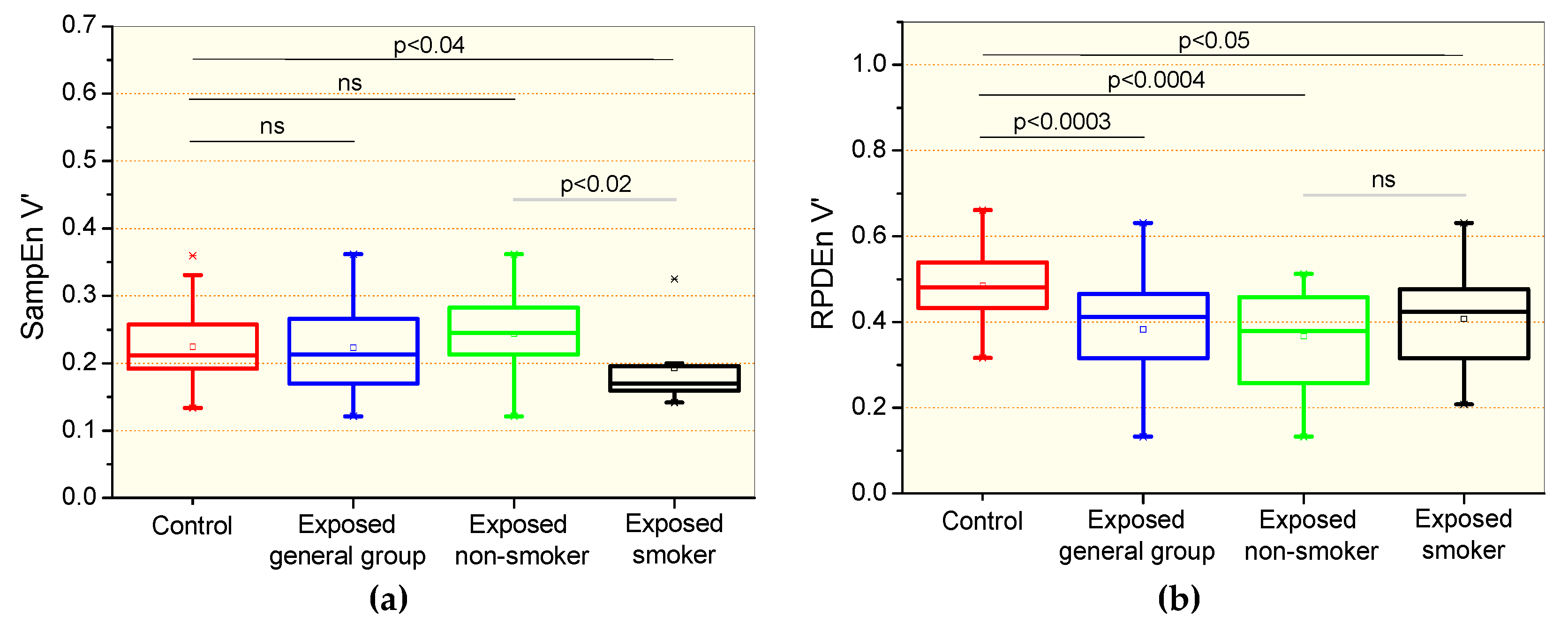
3.4. Complexity Analysis of the Airflow Signal
3.5. Correlation Among the Entropy Analysis and Pulmonary Function Exams
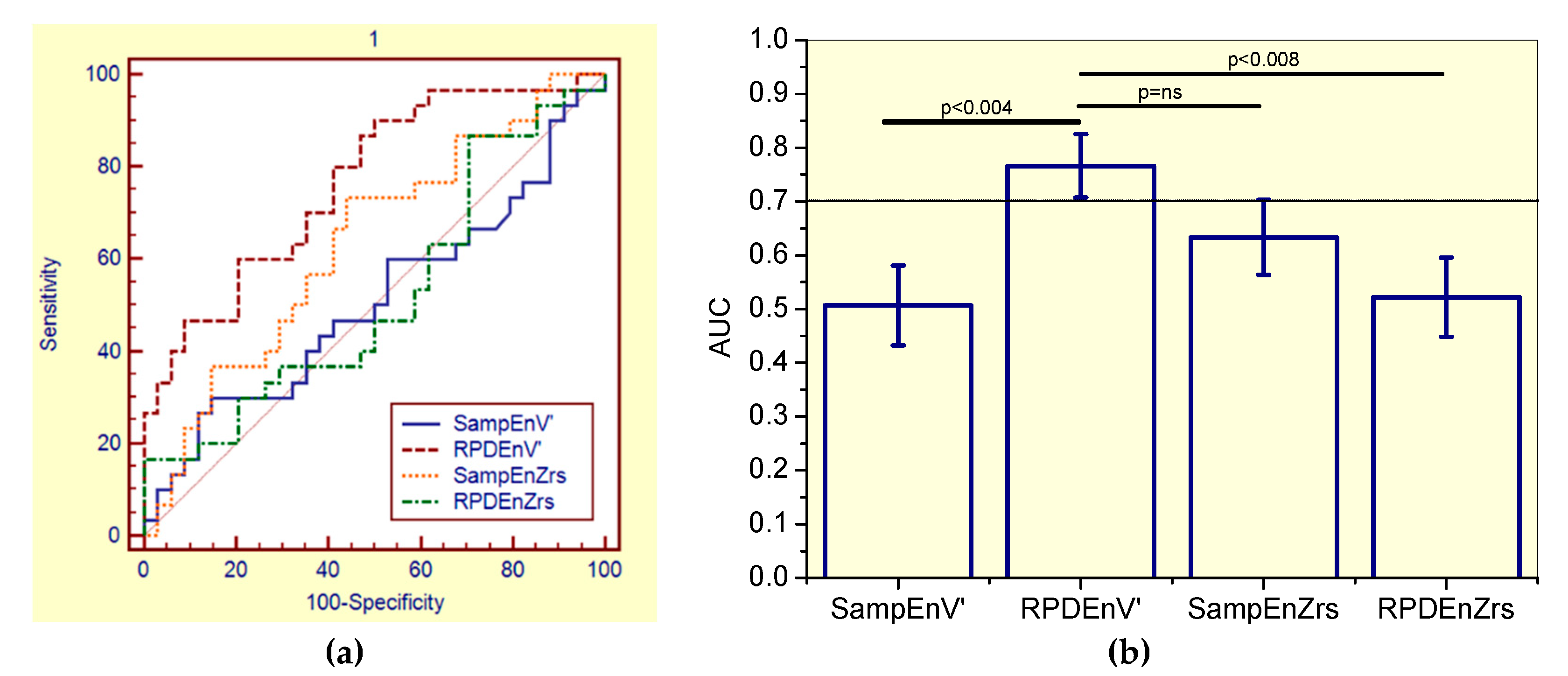
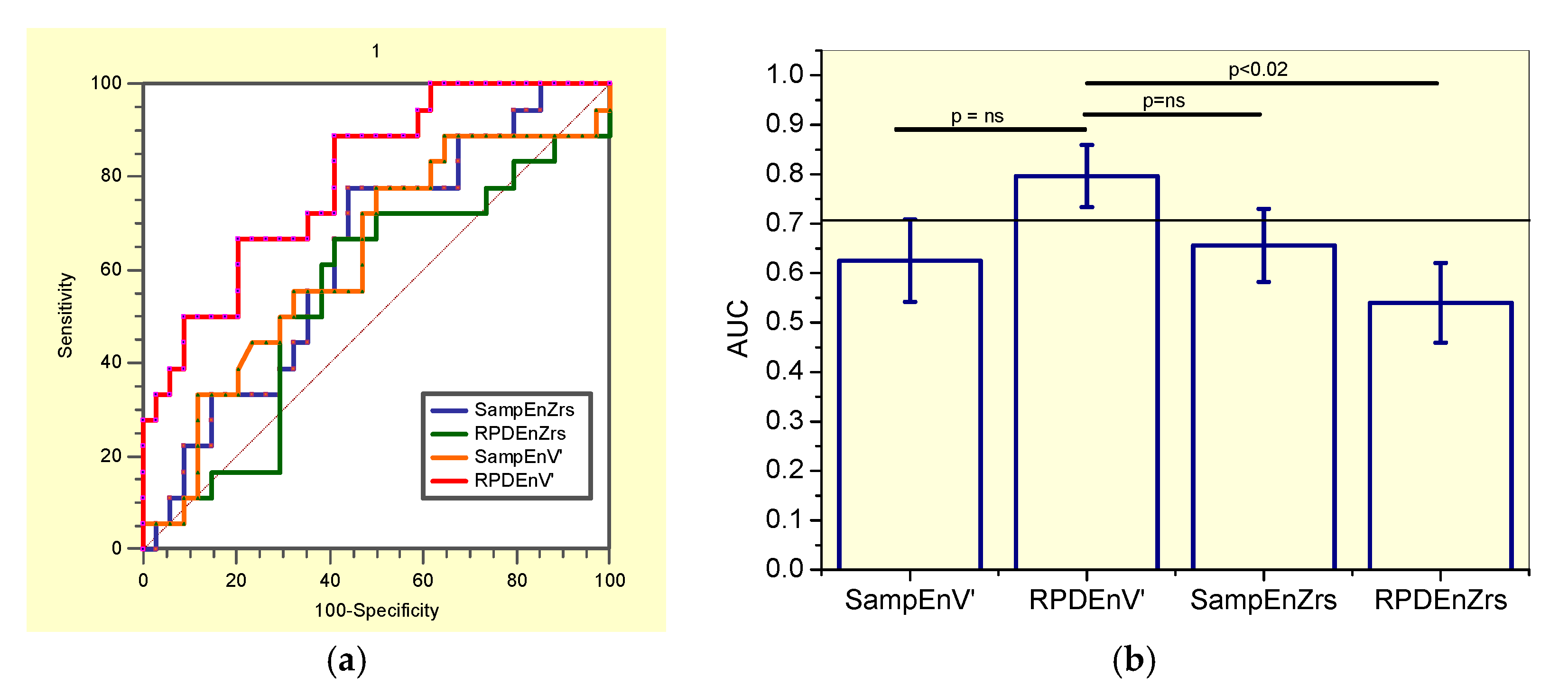
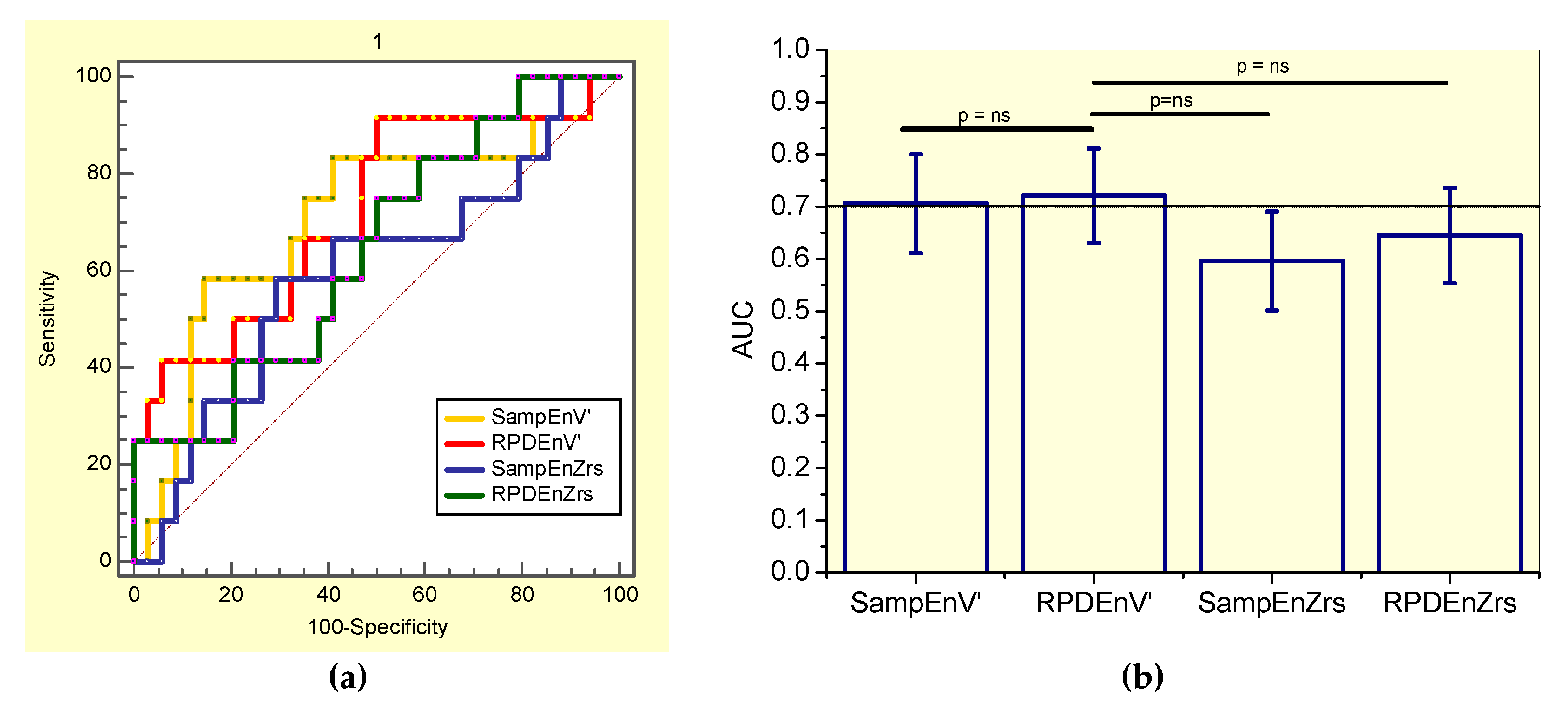
3.6. Diagnostic Accuracy
3.6.1. Analysis including Non-Smokers and Smokers Patients (the General Patient Group)
3.6.2. Analysis including only Non-Smoker Patients
3.6.3. Analysis including only Smoker Patients
4. Discussion
4.1. Biometric and Pulmonary Function
4.2. Effects of Exposition to Asbestos in the Complexity Analysis
4.3. Effect of Exposition to Asbestos Associated with Smoking in Complexity Analysis
4.4. General Discussion
4.5. Diagnostic Use
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caldirola, D.; Bellodi, L.; Caumo, A.; Migliarese, G.; Perna, G. Approximate entropy of respiratory patterns in panic disorder. Am. J. Psychiatry 2004, 161, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Caldirola, D.; Bellodi, L.; Cammino, S.; Perna, G. Smoking and respiratory irregularity in panic disorder. Biol. Psychiatry 2004, 56, 393–398. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, M.F. A diagnostic software tool for determination of complexity in respiratory pattern parameters. Comput. Biol. Med. 2007, 37, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.; Akay, Y.; Curran, A.K.; Akay, M. Complexity measures of the central respiratory networks during wakefulness and sleep. J. Neural Eng. 2008, 5, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Suki, B. In search of complexity. J. Appl. Physiol. 2010, 109, 1571–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, C.M.; Moore, C.; Manickam, K.; Lee, L.; Sykes, A.; Carr, A.; Jones, S.; Jones, J.; Swindell, R.; Homer, J.J.; et al. Electroglottogram approximate entropy: A novel single parameter for objective voice assessment. J. Laryngol. Otol. 2010, 124, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Veiga, J.; Lopes, A.J.; Jansen, J.M.; Melo, P.L. Airflow pattern complexity and airway obstruction in asthma. J. Appl. Physiol. 2011, 111, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Jin, Y.; Chen, C.; Sun, B.; Cao, Z.; Lo, I.L.; Zhao, Q.; Zheng, J.; Shi, Y.; Zhang, X.D. Entropy Change of Biological Dynamics in Asthmatic Patients and Its Diagnostic Value in Individualized Treatment: A Systematic Review. Entropy 2018, 20, 402. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Becklake, M.R.; Bagatin, E.; Neder, J.A. Asbestos-related diseases of the lungs and pleura: Uses, trends and management over the last century. Int. J. Tuberc. Lung Dis. 2007, 11, 356–369. [Google Scholar] [PubMed]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global Asbestos Disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. The tragic legacy of the Sennan asbestos disaster. Lancet. Respir. Med. 2018, 6, 902. [Google Scholar] [CrossRef]
- Abejie, A.B.; Xiaorong, W.; Kales, S.N.; Christiani, C.D. Patterns of pulmonary dysfunction in asbestos workers: A cross-sectional study. J. Occup. Med. Toxicol. 2010, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R. Asbestos and disease: Review of the scientific knowledge and a rationale for urgent change in the current Brazilian policy about this question. Cad. Saude Publ. 2001, 17, 7–29. [Google Scholar] [CrossRef]
- World Healthy Organization. Outline for the Development of National Programmes for Elimination of Asbestos-Related Diseases; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- International Labor Organization. Outline for the Development of National Programmes for Elimination of Asbestos-Related Diseases; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Dames, K.K.; Lopes, A.J.; de Melo, P.L. Airflow pattern complexity during resting breathing in patients with COPD: Effect of airway obstruction. Respir. Physiol. Neurobiol. 2014, 192, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Veiga, J.; Lopes, A.J.; Jansen, J.M.; Melo, P.L. Fluctuation analysis of respiratory impedance waveform in asthmatic patients: Effect of airway obstruction. Med. Biol. Eng. Comput. 2012, 50, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Raoufy, M.R.; Ghafari, T.; Darooei, R.; Nazari, M.; Mahdaviani, S.A.; Eslaminejad, A.R.; Almasnia, M.; Gharibzadeh, S.; Mani, A.R.; Hajizadeh, S. Classification of Asthma Based on Nonlinear Analysis of Breathing Pattern. PLoS ONE 2016, 11, e0147976. [Google Scholar] [CrossRef] [PubMed]
- Sá, P.M.; Dantas, N.F.; Castro, H.A.; Lopes, A.J.; Melo, P.L. Airflow pattern complexity in asbestos-exposed workers: Effect of smoking and diagnostic accuracy. In Proceedings of the XXVI Congresso Brasileiro de Engenharia Biomédica—CBEB 2018, Rio de Janeiro, Brazil, 21–25 October 2018. The International Federation for Medical and Biological Engineering (IFMBE). [Google Scholar]
- International Labor Organization. Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconiosis; International Labor Organization: Geneva, Switzerland, 2002. [Google Scholar]
- de Melo, P.L.; Lemes, L.N.D. Instrumentation for the analysis of respiratory system disorders during sleep: Design and application. Rev. Sci. Instrum. 2002, 73, 3926–3932. [Google Scholar] [CrossRef]
- Pereira, C.A.C.; Simões, S.P.; Pereira, J.G.; Gerstler, F.W.L.; Nakatani, J.G. Valores de referência para espirometria em uma amostra da população brasileira adulta. J. Bras. Pneumol. 1992, 18, 10–22. [Google Scholar]
- Neder, J.A.; Andreoni, S.; Castelo-Filho, A.; Nery, L.E. Reference values for lung function tests. I. Static volumes. Braz J. Med. Biol Res. 1999, 32, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Estimation of Complexity of Sampled Biomedical Continuous Time Signals Using Approximate Entropy. Front. Physiol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.M.; Nunes, T.P.; Silva, F.H.; Faria, A.C.; Melo, P.L. Analysis of human tremor in patients with Parkinson disease using entropy measures of signal complexity. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 2786–2789. [Google Scholar]
- Graff, B.; Gasecki, D.; Rojek, A.; Boutouyrie, P.; Nyka, W.; Laurent, S.; Narkiewicz, K. Heart rate variability and functional outcome in ischemic stroke: A multiparameter approach. J. Hypertens. 2013, 31, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pham, T.D. Sample entropy and regularity dimension in complexity analysis of cortical surface structure in early Alzheimer’s disease and aging. J. Neurosci. Methods 2013, 215, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Goldberger, A.L. Physiological time-series analysis: What does regularity quantify? Am. J. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A.; McSharry, P.E.; Roberts, S.J.; Costello, D.A.; Moroz, I.M. Exploiting nonlinear recurrence and fractal scaling properties for voice disorder detection. Biomed. Eng. Online 2007, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Muskulus, M.; Slats, A.M.; Sterk, P.J.; Verduyn-Lunel, S. Fluctuations and determinism of respiratory impedance in asthma and chronic obstructive pulmonary disease. J. Appl. Physiol. 2010, 109, 1582–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, M.; Judd, K.; Lowe, M.; Stick, S. Is breathing in infants chaotic? Dimension estimates for respiratory patterns during quiet sleep. J. Appl. Physiol. 1999, 86, 359–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewatia, D.K.; Tolakanahalli, R.P.; Paliwal, B.R.; Tome, W.A. Time series analyses of breathing patterns of lung cancer patients using nonlinear dynamical system theory. Phys. Med. Biol. 2011, 56, 2161–2181. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Judd, K. Comparisons of new nonlinear modeling techniques with applications to infant respiration. Phys. D 1998, 117, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Liebert, W.; Pawelzik, K.; Schuster, H.G. Optimal embeddings of chaotic attractors from topological considerations. Europhys. Lett. 1991, 14, 521–526. [Google Scholar] [CrossRef]
- Goedhart, D.M.; Zanen, P.; Kerstjens, H.A.; Lammers, J.W. Discriminating asthma and COPD based on bronchodilator data: An improvement of the methods. Physiol. Meas. 2005, 26, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Witten, I.H.F.E. Data Mining: Practical Machine Learning Tools and Techniques, 2nd ed.; Morgan Kaufmann Publishers: San Francisico, CA, USA, 1999; p. 560. [Google Scholar]
- Elmes, P.C. Relative importance of cigarette smoking in occupational lung disease. Br. J. Ind. Med. 1981, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vehmas, T.; Kivisaari, L.; Huuskonen, M.S.; Jaakkola, M.S. Effects of tobacco smoking on findings in chest computed tomography among asbestos-exposed workers. Eur. Respir. J. 2003, 21, 866–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberger, A.L. Fractal variability versus pathologic periodicity: Complexity loss and stereotypy in disease. Perspect. Biol. Med. 1997, 40, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Marin Martinez, B.; Clavera, I. Asbestosis. Sistema Sanitario Navarra 2005, 28 (Suppl. 1), 37–44. [Google Scholar] [CrossRef]
- Dujić, Z.; Tocilj, J.; Boschi, S.; Sarić, M.; Eterović, D. Biphasic lung diffusing capacity: Detection of early asbestos induced changes in lung function. Br. J. Ind. Med. 1992, 49, 260–267. [Google Scholar] [PubMed]
- Wang, X.; Wang, M.; Qiu, H.; Yu, I.; Yano, E. Longitudinal changes in pulmonary function of asbestos workers. J. Occup. Health 2010, 52, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, H.S.; Fritschi, L.; de Klerk, N.H.; Ambrosini, G.; Beilby, J.; Olsen, N.; Musk, A.W. Plasma concentrations of retinol, carotene, and vitamin E and mortality in subjects with asbestosis in a cohort exposed to crocidolite in Wittenoom, Western Australia. J. Occup. Environ. Med. 2005, 47, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Arcentales, A.; Giraldo, B.F.; Caminal, P.; Benito, S.; Voss, A. Recurrence quantification analysis of heart rate variability and respiratory flow series in patients on weaning trials. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 2724–2727. [Google Scholar]
- Criner, G.J.; D’Alonzo, G.E. Pulmonary Pathophysiology; Wiley-Blackwell: Madison, CT, USA; Fence Creek, CA, USA, 1999. [Google Scholar]
- Criner, G.J.; Cordova, F.C.; Furukawa, S.; Kuzma, A.M.; Travaline, J.M.; Leyenson, V.; O’Brien, G.M. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Engoren, M. Approximate entropy of respiratory rate and tidal volume during weaning from mechanical ventilation. Crit. Care Med. 1998, 26, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Engoren, M.; Courtney, S.E.; Habib, R.H. Effect of weight and age on respiratory complexity in premature neonates. J. Appl. Physiol. 2009, 106, 766–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khatib, M.; Jamaleddine, G.; Soubra, R.; Muallem, M. Pattern of spontaneous breathing: Potential marker for weaning outcome. Spontaneous breathing pattern and weaning from mechanical ventilation. Intensive Care Med. 2001, 27, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Fiamma, M.N.; Straus, C.; Poon, C.S.; Similowski, T. Chaotic dynamics of resting ventilatory flow in humans assessed through noise titration. Respir. Physiol. Neurobiol. 2006, 153, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillancourt, D.E.; Newell, K.M. The dynamics of resting and postural tremor in Parkinson’s disease. Clin. Neurophysiol. 2000, 111, 2046–2056. [Google Scholar] [CrossRef]
- Suki, B.; Bates, J.H.; Frey, U. Complexity and emergent phenomena. Compr. Physiol. 2011, 1, 995–1029. [Google Scholar] [PubMed]
- Frey, U.; Maksym, G.; Suki, B. Temporal complexity in clinical manifestations of lung disease. J. Appl. Physiol. 2011, 110, 1723–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsitz, L.A.; Goldberger, A.L. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA J. Am. Med Assoc. 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Pincus, S.M. Greater signal regularity may indicate increased system isolation. Math. Biosci. 1994, 122, 161–181. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Kadri, N.A.; Suri, J.S.; Yu, W. Automated identification of normal and diabetes heart rate signals using nonlinear measures. Comput. Biol. Med. 2013, 43, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. NonlinearSoft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L. Non-linear dynamics for clinicians: Chaos theory, fractals, and complexity at the bedside. Lancet 1996, 347, 1312–1314. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. 1), 2466–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsitz, L.A. Dynamics of stability: The physiologic basis of functional health and frailty. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B115–B125. [Google Scholar] [CrossRef]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhuang, J.; Yu, W.; Wang, Z. Measuring complexity using FuzzyEn, ApEn, and SampEn. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, Y.; Yuan, C.; Wang, S.; Li, P. Entropy Analysis of Short-Term Heartbeat Interval Time Series during Regular Walking. Entropy 2017, 19, 568. [Google Scholar] [CrossRef]
- Azami, H.; Escudero, J. Amplitude- and Fluctuation-Based Dispersion Entropy. Entropy 2018, 20, 210. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Unakafova, V.A.; Keller, K. Efficiently Measuring Complexity on the Basis of Real-World Data. Entropy 2013, 15, 4392–4415. [Google Scholar] [CrossRef] [Green Version]
- Porta, A.; Baselli, G.; Liberati, D.; Montano, N.; Cogliati, C.; Gnecchi-Ruscone, T.; Malliani, A.; Cerutti, S. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol. Cybern. 1998, 78, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Li, K.; Zheng, D.; Liu, C.; Hou, Y. Assessing the complexity of short-term heartbeat interval series by distribution entropy. Med. Biol. Eng. Comput. 2015, 53, 77–87. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 34) | Exposed (n = 34) | p | |
|---|---|---|---|
| Biometric data | |||
| Age (years) | 59.8 ± 13.1 | 64.0 ± 8.17 | 0.06 |
| Weight (kg) | 75.6 ± 13.1 | 76.8 ± 14.7 | 0.76 |
| Height (cm) | 164.3 ± 7.85 | 163.6 ± 10.08 | 0.75 |
| BMI (kg/m2) | 28.4 ± 4.65 | 28.6 ± 4.47 | 0.86 |
| Spirometry | |||
| FVC (L) | 3.24 ± 0,83 | 2.80 ± 0.66 | <0.02 |
| FVC (%) | 93.6 ± 13.7 | 88.1 ± 16.2 | 0.07 |
| FEV1 (L) | 2.59 ± 0.76 | 2.06 ± 0.48 | <0.003 |
| FEV1 (%) | 92.4 ± 13.6 | 82.4 ± 14.7 | <0.006 |
| FEV1/FVC | 78.4 ± 6.17 | 74.4 ± 10.6 | 0.17 |
| FEF25–75% (L) | 2.39 ± 0.94 | 1.87 ± 0.85 | <0.02 |
| FEF25–75% (%) | 86.6 ± 25.1 | 76.4 ± 36.0 | 0.18 |
| FEF/FVC | 82.7 ± 22.3 | 89.2 ± 43.6 | 0.44 |
| Forced oscillation | |||
| Zm (cmH2O/L/s) | 3.93 ± 1.17 | 5.00 ± 1.93 | <0.02 |
| Control (n = 34) | Exposed (n = 30) | p | |
|---|---|---|---|
| Biometric data | |||
| Age (years) | 60.9 ± 11.7 | 63.8 ± 8.25 | 0.36 |
| Weight (kg) | 73.4 ± 12.1 | 76.7 ± 14.5 | 0.38 |
| Height (cm) | 163.3 ± 8.43 | 164.6 ± 9.90 | 0.72 |
| BMI (kg/m2) | 27.8 ± 4.81 | 28.3 ± 4.55 | 0.65 |
| Spirometry | |||
| FVC (L) | 3.15 ± 0.86 | 2.84 ± 0.66 | <0.03 |
| FVC (%) | 93.8 ±15.4 | 87.4 ± 17.1 | 0.10 |
| FEV1 (L) | 2.51 ± 0.77 | 2.08 ± 0.48 | <0.004 |
| FEV1 (%) | 93.1 ± 15.2 | 81.4 ± 15.0 | <0.002 |
| FEV1/FVC | 78.2 ± 5.78 | 74.0 ±10.1 | 0.09 |
| FEF25–75% (L) | 2.32 ± 0.95 | 1.98 ± 1.27 | 0.30 |
| FEF25–75% (%) | 90.5 ± 28.3 | 73.2 ± 33.8 | 0.83 |
| FEF/FVC | 83.9 ± 25.1 | 84.9 ± 42.4 | 0.94 |
| Forced oscillation | |||
| Zm (cmH2O/L/s) | 3.92 1.46 | 4.83 2.16 | 0.08 |
| Non-Smokers (n = 18) | Smokers (n = 12) | p | |
|---|---|---|---|
| Spirometry | |||
| FVC (L) | 2.68 ± 0.74 | 3.11 ± 0.48 | 0.16 |
| FVC (%) | 85.3 ± 13.7 | 92.3 ± 21.3 | 0.40 |
| FEV1 (L) | 2.08 ± 0.53 | 2.04 ± 0.45 | 0.83 |
| FEV1 (%) | 83.5 ± 13.9 | 77.2 ± 15.7 | 0.26 |
| FEV1/FVC | 78.2 ± 7.38 | 66.0 ± 11.6 | <0.002 |
| FEF25-75% (L) | 2.15 ± 0.80 | 1.82 ± 2.01 | <0.05 |
| FEF25-75% (%) | 88.3 ± 33.8 | 50.9 ± 28.8 | <0.004 |
| FEF/ CVF | 105.1 ± 41.6 | 53.6 ± 32.3 | <0.002 |
| Plethysmography | |||
| RV (L) | 1.97± 1.01 | 2.83 ± 1.04 | <0.02 |
| RV (%) | 122.4 ± 51.4 | 154.2 ± 82.6 | 0.66 |
| TLC (L) | 4.73 ± 1.31 | 5.90 ± 0.91 | <0.02 |
| TLC (%) | 100.5 ± 20.0 | 112.7 ± 31.3 | 0.43 |
| RV/TLC (L) | 38.3 ± 11.1 | 47.1 ± 10.3 | <0.04 |
| Raw (cmH2O/L/s) | 5.98 ± 11.4 | 5.11 ± 5.19 | 0.80 |
| Raw (%) | 445.1 ± 855.0 | 368.8 ± 426.0 | 0.77 |
| sGaw (L/s/cmH2O/L) | 0.25 ± 0.41 | 0.09 ± 0.07 | 0.20 |
| sGaw (%) | 98.6 ± 156.2 | 50.2 ± 38.5 | 0.30 |
| Forced oscillation | |||
| ZmV’ (cmH2O/L/s) | 4.74 ± 2.01 | 4.97 ± 2.43 | 0.77 |
| SampEnV’ | RPDEnV’ | SampEnZrs | RPDEnZrs | ||
|---|---|---|---|---|---|
| FEV1 (%) | r | 0.10 | 0.15 | -0.12 | 0.21 |
| p | 0.42 | 0.24 | 0.32 | 0.08 | |
| FVC (%) | r | 0.04 | 0.15 | -0.20 | 0.02 |
| p | 0.74 | 0.42 | 0.09 | 0.89 | |
| FEF25-75% (%) | r | 0.23 | 0.01 | 0.03 | 0.26 |
| p | 0.07 | 0.93 | 0.78 | <0.03 | |
| Zrs (cmH2O/L/s) | r | −0.10 | 0.01 | −0.33 | −0.26 |
| p | 0.40 | 0.93 | <0.006 | <0.04 | |
| SampEnZrs | RPDEnZrs | SampEnV’ | RPDEnV’ | |
|---|---|---|---|---|
| AUC | 0.633 | 0.522 | 0.507 | 0.766 |
| Se (%) | 56.7 | 46.7 | 50.0 | 70.0 |
| Sp (%) | 64.7 | 47.1 | 50.0 | 64.7 |
| Cut-off | >0.505 | ≤0.621 | ≤0.213 | ≤0.446 |
| SampEnZrs | RPDEnZrs | SampEnV’ | RPDEnV’ | |
|---|---|---|---|---|
| AUC | 0.656 | 0.540 | 0.625 | 0.796 |
| Se (%) | 77.3 | 63.64 | 77.8 | 88.9 |
| Sp (%) | 55.9 | 58.8 | 50.0 | 58.8 |
| Cut-off | >0.451 | >0.626 | >0.212 | ≤0.470 |
| SampEnZrs | RPDEnZrs | SampEnV’ | RPDEnV’ | |
|---|---|---|---|---|
| AUC | 0.596 | 0.645 | 0.706 | 0.721 |
| Se (%) | 66.7 | 58.3 | 75.0 | 66.7 |
| Sp (%) | 58.8 | 58.8 | 64.7 | 64.7 |
| Cut-off | >0.468 | ≤0.600 | ≤0.195 | ≤0.443 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, P.M.; Castro, H.A.; Lopes, A.J.; Melo, P.L. Entropy Analysis for the Evaluation of Respiratory Changes Due to Asbestos Exposure and Associated Smoking. Entropy 2019, 21, 225. https://doi.org/10.3390/e21030225
Sá PM, Castro HA, Lopes AJ, Melo PL. Entropy Analysis for the Evaluation of Respiratory Changes Due to Asbestos Exposure and Associated Smoking. Entropy. 2019; 21(3):225. https://doi.org/10.3390/e21030225
Chicago/Turabian StyleSá, Paula M., Hermano A. Castro, Agnaldo J. Lopes, and Pedro L. Melo. 2019. "Entropy Analysis for the Evaluation of Respiratory Changes Due to Asbestos Exposure and Associated Smoking" Entropy 21, no. 3: 225. https://doi.org/10.3390/e21030225
APA StyleSá, P. M., Castro, H. A., Lopes, A. J., & Melo, P. L. (2019). Entropy Analysis for the Evaluation of Respiratory Changes Due to Asbestos Exposure and Associated Smoking. Entropy, 21(3), 225. https://doi.org/10.3390/e21030225






